Effect of Salinity and Nitrogen Form in Irrigation Water on Growth, Antioxidants and Fatty Acids Profiles in Halophytes Salsola australis, Suaeda maritima, and Enchylaena tomentosa for a Perspective of Biosaline Agriculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Seedling Collect, Plant Cultivation and Multiplication
2.3. Combined Salinity and Nitrogen Ratio Experiment
2.4. Determination of Radical Scavenging Activity and Total Phenolic Compound Contents
2.5. Determination of Fatty Acid Profiles
2.6. Statistical Analysis
3. Results
3.1. Mean Growth and Functional Values for Fatty Acids and Antioxidants
3.2. Influence of Salinity and NO3−-N:NH4+-N Ratio on Growth and Functional Values for Fatty Acids and Antioxydants
3.2.1. Salsola australis
- -
- moderate salinity combined with 75:25 or 25:75 N-ratio and high salinity combined with all rich NO3−-N ratios tends to decrease the RGR versus control (ranging from ~57.0% to ~86.9%).
- -
- high salinity combined with 100:0, 75:25 or 25:75 tends to decrease the water content versus control (of ~27.7%, ~34.8% or ~21.1% respectively).
- -
- high salinity combined with 0:100 N-ratio increases significantly the AAI (of ~212.5%; p < 0.05) and the TPC content (of ~105.2%; p < 0.01) versus control. This combined treatment increases also significantly the AAI versus 50:50 N-ratio (of ~354.0%; p < 0.001) and the TPC content versus 100:0 N-ratio (of ~76.0%; p < 0.05). Further, high salinity combined with 100:0 N-ratio increases also significantly the AAI versus 50:50 N-ratio (of ~218.2%; p < 0.01). In addition, moderate salinity combined with 100:0 N-ratio increases significantly the TPC content versus control (of ~53.2%; p < 0.05).
- -
- high salinity combined with 100:0, 50:50 or 0:100 N-ratio tends to increase the n − 6:n − 3 ratio versus 75:25 N-ratio (of ~86.3%, ~34.2% or ~48.2% respectively).
- -
- high salinity combined with 50:50 or 0:100 N-ratio tends to decrease the PUFA:SFA ratio versus control (of 6.9% or ~15.7% respectively).
- -
- high salinity combined with 75:25, 50:50 or 25:75 N-ratio tends to decreases the TFA content versus control (of ~30.1%, ~23.9% or ~25.4% respectively) and when combined with 100:0 N-ratio, it tends to increase the TFA content versus all other ratios (ranging from ~30.3% to ~45.2%).
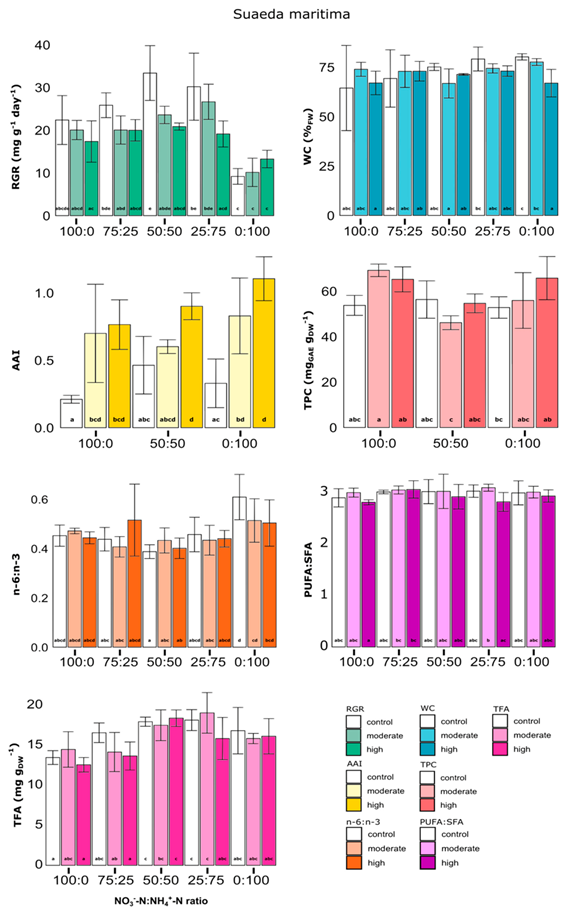
3.2.2. Suaeda maritima
- -
- high salinity combined with 50:50 or 25:75 N-ratio tends to decrease the RGR versus control (of ~37.4% and ~36.7% respectively). In addition, control and moderate salinities combined with 0:100 N-ratio tends to decrease the RGR versus 100:0, 75:25, 50:50 or 25:75 N-ratio (ranging from ~58.9% to ~72.4% at control salinity from ~49.4% to ~61.9% at moderate salinity and from ~23.6% to ~36.4% at high salinity).
- -
- high salinity combined with 0:100 N-ratio tends to decrease the water content versus control (of ~16.6%).
- -
- moderate salinity combined with 100:0 or 0:100 N-ratio as well as high salinity combined with all N-ratios tend to increase the AAI versus control (ranging from ~94.4% to ~262.6%).
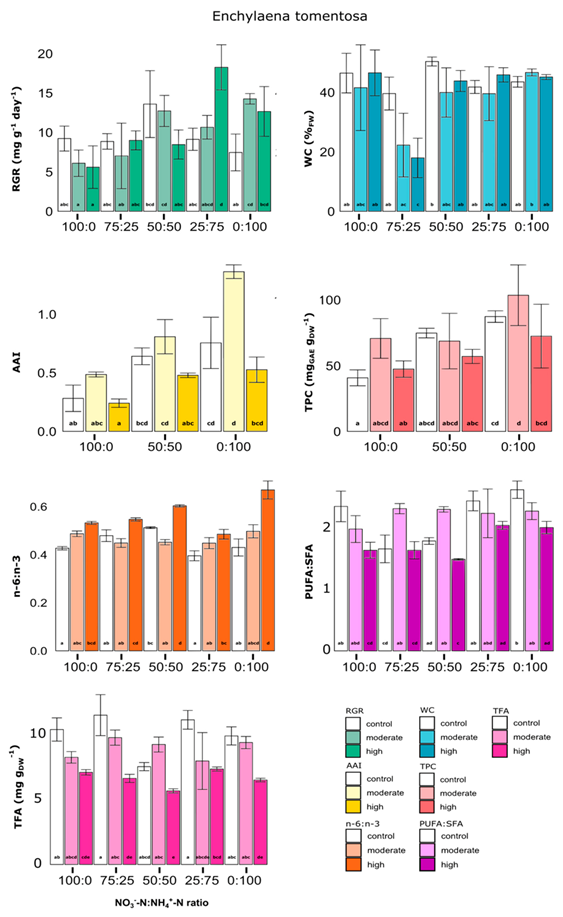
3.2.3. Enchylaena tomentosa
- -
- moderate salinity combined with 0:100 N-ratio tends to increase the RGR of ~91.1% versus control and that high salinity combined with 25:75 N-ratio tends to increase the RGR of ~100.0% versus control. Further, high salinity combined with 25:75 N-ratio tends to increase the RGR versus 100:0 ratio (of ~226.2%), versus 75:25 ratio (of ~102.9%) or versus 50:50 N-ratio (of~115.7%).
- -
- high salinity combined with 75:25 N-ratio tends to decrease the water content versus control (of ~54.6%) and versus 100:0, 50:50, 25:75 or 0:100 N-ratio (ranging from ~59.0% to ~61.4%).
- -
- all salinities combined with 0:100 N-ratio tend to increase the AAI versus 100:0 N-ratio (ranging from ~118.3% to ~180.6%).
- -
- high salinity combined with all N-ratios tends to increase the n − 6:n − 3 ratio versus control (ranging from ~14.2% to ~55.7%).
- -
- high salinity combined with 100:0, 50:50 or 0:100 N-ratio tends to decrease the PUFA:SFA ratio versus control (ranging from ~16.3% to ~30.4%). Further, moderate salinity combined with 75:25 or 50:50 N-ratio tends to increase the PUFA:SFA ratio versus control (of ~39.5% or ~28.7% respectively)
- -
- high salinity combined with all N-ratios tends to decrease the TFA content versus control (ranging from ~24.8% to ~42.3%).
4. Discussion
4.1. Mean Growth and Functional Values for Antioxidants and Fatty Acids
4.2. Influence of Salinity and NO3−-N:NH4+-N Ratio on Salt-Tolerance and N-Nutrition
4.2.1. Salsola australis
4.2.2. Suaeda maritima
4.2.3. Enchylaena tomentosa
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carillo, P.; Grazia, M.; Pontecorvo, G.; Fuggi, A.; Woodrow, P. Salinity stress and salt tolerance. In Abiotic Stress in Plants—Mechanisms and Adaptations; IntechOpen: London, UK, 2011. [Google Scholar]
- Rozema, J.; Flowers, T. Ecology. Crops for a salinized world. Science 2008, 322, 1478–1480. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S.S.; Saad-Allah, K.; Fareed, M.; Ali, A.; El-Badry, A.; El-Zawawy, N.A.; Wu, J.; Sun, J.; Mao, G.-H.; et al. Phytochemical analysis and assessment of antioxidant and antimicrobial activities of some medicinal plant species from Egyptian flora. J. Appl. Biomed. 2018, 16, 289–300. [Google Scholar] [CrossRef]
- Oueslati, S.; Ksouri, R.; Falleh, H.; Pichette, A.; Abdelly, C.; Legault, J. Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Suaeda fruticosa Forssk. Food Chem. 2012, 132, 943–947. [Google Scholar] [CrossRef]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2011, 32, 289–326. [Google Scholar] [CrossRef] [PubMed]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Revell, D.K.; Norman, H.C.; Vercoe, P.E.; Phillips, N.; Toovey, A.; Bickell, S.; Hulm, E.; Hughes, S.; Emms, J. Australian perennial shrub species add value to the feed base of grazing livestock in low- to medium-rainfall zones. Anim. Prod. Sci. 2013, 53, 1221–1230. [Google Scholar] [CrossRef]
- Centofanti, T.; Bañuelos, G. Evaluation of the halophyte Salsola soda as an alternative crop for saline soils high in selenium and boron. J. Environ. Manag. 2015, 157, 96–102. [Google Scholar] [CrossRef]
- Song, J.; Wang, B. Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann. Bot. 2014, 115, 541–553. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Nishiyama, Y.; Suzuki, I.; Tasaka, Y.; Murata, N. Genetic engineering of the unsaturation of fatty acids in membrane lipids alters the tolerance of Synechocystis to salt stress. Proc. Natl. Acad. Sci. USA 1999, 96, 5862–5867. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, T.V.; Myasoedov, N.A.; Pchelkin, V.P.; Tsydendambaev, V.D.; Vereshchagin, A.G. Increased content of very-long-chain fatty acids in the lipids of halophyte vegetative organs. Russ. J. Plant Physiol. 2009, 56, 787–794. [Google Scholar] [CrossRef]
- Tsydendambaev, V.D.; Ivanova, T.V.; Khalilova, L.A.; Kurkova, E.B.; Myasoedov, N.A.; Balnokin, Y.V. Fatty acid composition of lipids in vegetative organs of the halophyte Suaeda altissima under different levels of salinity. Russ. J. Plant Physiol. 2013, 60, 661–671. [Google Scholar] [CrossRef]
- Alhdad, G.M.; Seal, C.E.; Al-Azzawi, M.J.; Flowers, T.J. The effect of combined salinity and waterlogging on the halophyte Suaeda maritima: The role of antioxidants. Environ. Exp. Bot. 2013, 87, 120–125. [Google Scholar] [CrossRef]
- Gourguillon, L.; Destandau, É.; Lobstein, A.; Lesellier, É. Comparaison de différentes méthodes d’extraction d’acides dicaféoylquiniques à partir d’une plante halophile. Comptes Rendus Chim. 2016, 19, 1133–1141. [Google Scholar] [CrossRef]
- Slama, I.; M’Rabet, R.; Ksouri, R.; Talbi, O.; Debez, A.; Abdelly, C. Effects of salt treatment on growth, lipid membrane peroxidation, polyphenol content, and antioxidant activities in leaves of Sesuvium portulacastrum L. Arid Land Res. Manag. 2017, 31, 404–417. [Google Scholar] [CrossRef]
- Sui, N.; Han, G. Increases of unsaturated fatty acids in membrane lipids protects photosystem II from photoinhibition under salinity in different halophytes. J. Agric. Sci. 2014, 6, 623–629. [Google Scholar] [CrossRef]
- Camalle, M.; Standing, D.; Jitan, M.; Muhaisen, R.; Bader, N.; Bsoul, M.; Ventura, Y.; Soltabayeva, A.; Sagi, M. Effect of salinity and nitrogen sources on the leaf quality, biomass, and metabolic responses of two ecotypes of Portulaca oleracea. Agronomy 2020, 10, 656. [Google Scholar] [CrossRef]
- Fontana, E.; Hoeberechts, J.; Nicola, S.; Cros, V.; Palmegiano, G.B.; Peiretti, P.G. Nitrogen concentration and nitrate/ammonium ratio affect yield and change the oxalic acid concentration and fatty acid profile of purslane (Portulaca oleracea L.) grown in a soilless culture system. J. Sci. Food Agric. 2006, 86, 2417–2424. [Google Scholar] [CrossRef]
- Kudo, N.; Fujiyama, H. Responses of halophyte Salicornia bigelovii to different forms of nitrogen source. Pedosphere 2010, 20, 311–317. [Google Scholar] [CrossRef]
- Szalai, G.; Dai, N.; Danin, A.; Dudai, N.; Barazani, O. Effect of nitrogen source in the fertilizing solution on nutritional quality of three members of the Portulaca oleracea aggregate. J. Sci. Food Agric. 2010, 90, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Hessini, K.; Ben Hamed, K.; Gandour, M.; Mejri, M.; Abdelly, C.; Cruz, C. Ammonium nutrition in the halophyte Spartina alterniflora under salt stress: Evidence for a priming effect of ammonium? Plant Soil 2013, 370, 163–173. [Google Scholar] [CrossRef]
- Ushakova, S.A.; Kovaleva, N.P.; Tikhomirova, N.A.; Gribovskaya, I.V.; Kolmakova, A.A. Effect of photosynthetically active radiation, salinization, and type of nitrogen nutrition on growth of Salicornia europaea plants. Russ. J. Plant Physiol. 2006, 53, 785–792. [Google Scholar] [CrossRef]
- Hessini, K.; Gandour, M.; Megdich, W.; Soltani, A.; Abdely, C. How does ammonium nutrition influence salt tolerance in Spartina alterniflora loisel? In Salinity and Water Stress. Tasks for Vegetation Sciences; Ashraf, M., Ozturk, M., Athar, H., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 44. [Google Scholar] [CrossRef]
- Karakaş, S.; Çullu, M.A.; Dikilitaş, M. Comparison of two halophyte species (Salsola soda and Portulaca oleracea) for salt removal potential under different soil salinity conditions. Turk. J. Agric. For. 2017, 41, 183–190. [Google Scholar] [CrossRef]
- Heidari-Sharifabad, H.; Mirzaie-Nodoushan, H. Salinity-induced growth and some metabolic changes in three Salsola species. J. Arid Environ. 2006, 67, 715–720. [Google Scholar] [CrossRef]
- Pyankov, V.I.; Ziegler, H.; Akhani, H.; Deigele, C.; Lüttge, U. European plants with C4 photosynthesis: Geographical and taxonomic distribution and relations to climate parameters. Bot. J. Linn. Soc. 2010, 163, 283–304. [Google Scholar] [CrossRef]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Kucukoduk, M. The role of antioxidant responses on the tolerance range of extreme halophyte Salsola crassa grown under toxic salt concentrations. Ecotoxicol. Environ. Saf. 2014, 110, 21–30. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Zakery-Asl, M.A.; Bolandnazar, S.; Oustan, S. Effect of salinity and nitrogen on growth, sodium, potassium accumulation, and osmotic adjustment of halophyte Suaeda aegyptiaca (Hasselq.) Zoh. Arch. Agron. Soil Sci. 2014, 60, 785–792. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Li, W.; Li, C.; Duan, D.; Tadano, T. Interactive effects of sodium chloride and nitrogen on growth and ion accumulation of a halophyte. Commun. Soil Sci. Plant Anal. 2005, 35, 2111–2123. [Google Scholar] [CrossRef]
- Yuan, J.-F.; Feng, G.; Ma, H.-Y.; Tian, C.-Y. Effect of nitrate on root development and nitrogen uptake of Suaeda physophora Under NaCl salinity. Pedosphere 2010, 20, 536–544. [Google Scholar] [CrossRef]
- Jefferson, L. The Biology and Ecology of Species of Maireana and Enchylaena: Intra- and Inter-Specific Competition in Plant Communities in the Eastern Goldfields of Western Australia. Ph.D. Thesis, Curtin University, Bentley, Australia, 2001. [Google Scholar]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Zidane Djerroudi, O. Caractérisation Morpho-Physiologique d’une Halophyte, Atriplex, aux Conditions Arides. Ph.D. Thesis, Université d’Oran, Oran, Algeria, 2017. [Google Scholar]
- Hunt, R. Relative growth rates BT—Basic growth analysis: Plant growth analysis for beginners. In Basic Growth Analysis; Springer: Dordrecht, The Netherlands, 1990; ISBN 978-94-010-9117-6. [Google Scholar]
- Surget, G.; Roberto, V.P.; Le Lann, K.; Mira, S.; Guérard, F.; Laizé, V.; Poupart, N.; Cancela, M.L.; Stiger-Pouvreau, V. Marine green macroalgae: A source of natural compounds with mineralogenic and antioxidant activities. J. Appl. Phycol. 2016, 29, 575–584. [Google Scholar] [CrossRef]
- Azabou, S.; Sebii, H.; Ben Taheur, F.; Abid, Y.; Jridi, M.; Nasri, M. Phytochemical profile and antioxidant properties of tomato by-products as affected by extraction solvents and potential application in refined olive oils. Food Biosci. 2020, 36, 100664. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Azmir, J.; Zaidul, I.; Rahman, M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Popovici, C.; Saykova, I. Evaluation de l’activité antioxydant des composés phénoliques par la réactivité avec le radical libre DPPH. Génie Ind. 2009, 4, 25–39. [Google Scholar]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry to total phenolics with phosphomolybdic acid reagents. Am. J. Enol. Vinic. 1965, 16, 144–158. [Google Scholar]
- Camacho, A.P.; Delgado, M. Histological study of the gonadal development of Ruditapes decussates (L.) (Mollusca: Bivalvia) and its relationship with available food. Sci. Mar. 2005, 69, 87–97. [Google Scholar] [CrossRef]
- Le Grand, F. Study of The Relationships between Membrane Lipid Compositions and Cell Functions: Case of Hemocytes of Bivalves Affected by Disseminated Neoplasia. Ph.D. Thesis, Université de Bretagne Occidentale, Brest, France, 2010. [Google Scholar]
- National Academy of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005; ISBN 978-0-309-08525-0. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; ISBN 3-900051-07-01. [Google Scholar]
- Pearson, K. Note on regression and inheritance in the case of two parents. Proc. R. Soc. Lond. 1895, 58, 240–242. [Google Scholar]
- Iman, R.L. A power study of a rank transform for the two-way classification model when interaction may be present. Can. J. Stat. 1974, 2, 227–239. [Google Scholar] [CrossRef]
- Hollander, M.; Wolfe, D.; Chicken, E. Nonparametric Statistical Methods; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2014; ISBN 978-0-470-38737-5. [Google Scholar]
- Dunn, O.J. Multiple comparisons using rank sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar] [CrossRef]
- Ben Rejeb, K.; Ghnaya, T.; Zaier, H.; Benzarti, M.; Baioui, R.; Ghabriche, R.; Wali, M.; Lutts, S.; Abdelly, C. Evaluation of the Cd2+ phytoextraction potential in the xerohalophyte Salsola kali L. and the impact of EDTA on this process. Ecol. Eng. 2013, 60, 309–315. [Google Scholar] [CrossRef]
- Xing, J.; Cai, M.; Chen, S.; Chen, L.; Lan, H. Seed germination, plant growth and physiological responses of Salsola ikonnikovii to short-term NaCl stress. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2013, 147, 285–297. [Google Scholar] [CrossRef]
- Padilla, F.M.; Miranda, J.D.; Jorquera, M.J.; Pugnaire, F.I. Variability in amount and frequency of water supply affects roots but not growth of arid shrubs. Plant Ecol. 2009, 204, 261–270. [Google Scholar] [CrossRef]
- Hanif, Z.; Ali, H.; Rasool, G.; Tanveer, A.; Chauhan, B.S. Genus Salsola: Its benefits, uses, environmental perspectives and future aspects—A review. J. Rangel. Sci. 2018, 8, 315–328. [Google Scholar]
- Song, J.; Chen, M.; Feng, G.; Jia, Y.; Wang, B.; Zhang, F. Effect of salinity on growth, ion accumulation and the roles of ions in osmotic adjustment of two populations of Suaeda salsa. Plant Soil 2008, 314, 133–141. [Google Scholar] [CrossRef]
- Bankaji, I.; Caçador, I.; Sleimi, N. Physiological and biochemical responses of Suaeda fruticosa to cadmium and copper stresses: Growth, nutrient uptake, antioxidant enzymes, phytochelatin, and glutathione levels. Environ. Sci. Pollut. Res. 2015, 22, 13058–13069. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Cambrollé, J.; Luque, T.; Figueroa, M.E.; Davy, A.J. Carry-over of differential salt tolerance in plants grown from dimorphic seeds of Suaeda splendens. Ann. Bot. 2008, 102, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Hastwell, G.T.; Facelli, J.M. Differing effects of shade-induced facilitation on growth and survival during the establishment of a chenopod shrub. J. Ecol. 2003, 91, 941–950. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Le Floch, G.; Magné, C. Radical scavenging, antioxidant and antimicrobial activities of halophytic species. J. Ethnopharmacol. 2008, 116, 258–262. [Google Scholar] [CrossRef]
- Urquiaga, I.; Leighton, F. Plant polyphenol antioxidants and oxidative stress. Biol. Res. 2000, 33, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K.; Pękal, A. Application of free radical diphenylpicrylhydrazyl (DPPH) to estimate the antioxidant capacity of food samples. Anal. Methods 2013, 5, 4288–4295. [Google Scholar] [CrossRef]
- Liu, L.; Howe, P.; Zhou, Y.-F.; Hocart, C.; Zhang, R. Fatty acid profiles of leaves of nine edible wild plants: An Australian study. J. Food Lipids 2002, 9, 65–71. [Google Scholar] [CrossRef]
- Prakash, D.; Nath, P.; Pal, M. Composition, variation of nutritional contents in leaves, seed protein, fat and fatty acid profile ofchenopodium species. J. Sci. Food Agric. 1993, 62, 203–205. [Google Scholar] [CrossRef]
- Vidrih, R.; Filip, S.; Hribar, J. Content of higher fatty acids in green vegetables. Czech J. Food Sci. 2009, 27, S125–S129. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-3 fatty acids and antioxidants in edible wild plants. Biol. Res. 2004, 37, 263–277. [Google Scholar] [CrossRef]
- Wijendran, V.; Hayes, K. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu. Rev. Nutr. 2004, 24, 597–615. [Google Scholar] [CrossRef]
- FAO-WHO. Fats and Fatty Acids in Human Nutrition—Report of an Expert Consultation, Joint FAO/WHO expert consultation, Geneva, Switzerland, 10–14 november 2008; World Health Organization: Geneva, Switzerland, 2010; ISBN 978-92-5-106733-8. [Google Scholar]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef]
- Boucaud, J.; Billard, J.P. Nitrogen nutrition in the estuarine zone: The case of Suaeda maritima var. macrocarpa. Ecol. Coast. Veg. 1985, 62, 303–308. [Google Scholar] [CrossRef]
- Song, J.; Ding, X.; Feng, G.; Zhang, F. Nutritional and osmotic roles of nitrate in a euhalophyte and a xerophyte in saline conditions. New Phytol. 2006, 171, 357–366. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Wen, W.; Yang, G. The antioxidant system in Suaeda salsa under salt stress. Plant Signal. Behav. 2020, 15, 1771939. [Google Scholar] [CrossRef]
- Mallik, S.; Nayak, M.; Sahu, B.B.; Panigrahi, A.K.; Shaw, B.P. Response of antioxidant enzymes to high NaCl concentration in different salt-tolerant plants. Biol. Plant. 2011, 55, 191–195. [Google Scholar] [CrossRef]
- Kant, S.; Kant, P.; Lips, H.; Barak, S. Partial substitution of NO3− by NH4+ fertilization increases ammonium assimilating enzyme activities and reduces the deleterious effects of salinity on the growth of barley. J. Plant Physiol. 2007, 164, 303–311. [Google Scholar] [CrossRef]
- Reginato, M.A.; Castagna, A.; Furlán, A.; Castro, S.; Ranieri, A.; Luna, V. Physiological responses of a halophytic shrub to salt stress by Na2SO4 and NaCl: Oxidative damage and the role of polyphenols in antioxidant protection. AoB PLANTS 2014, 6, plu042. [Google Scholar] [CrossRef]

| (a) Salinity | |||||
| Control | Moderate | High | |||
| S. australis | 0 | 60 | 449 | ||
| S. maritima | 0 | 150 | 500 | ||
| E. tomentosa | 0 | 35 | 300 | ||
| (b) NO3−-N:NH4+-N | |||||
| 0:100 | 25:75 | 50:50 | 75:25 | 100:0 | |
| Ca(NO3)2 | 0.0 | 1.8 | 3.6 | 2.7 | 4.3 |
| KNO3 | 0.0 | 0.0 | 0.0 | 5.4 | 5.7 |
| MgSO4 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| NH4H2PO4 | 1.0 | 1.0 | 1.0 | 1.0 | 0.0 |
| KH2PO4 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 |
| KCl | 7.7 | 7.7 | 7.7 | 2.3 | 1.0 |
| NH4Cl | 13.0 | 9.7 | 6.1 | 2.5 | 0.0 |
| CaCl2 | 4.3 | 2.4 | 0.7 | 1.5 | 0.0 |
| 16:0 | 18:0 | 18:1n − 9 | 18:2n − 6 | 18:3n − 3 | SFA | MUFA | PUFA | Unknown | TFA | |
|---|---|---|---|---|---|---|---|---|---|---|
| S. australis | 14.8 ± 1.9 | 2.8 ± 0.5 | 11.0 ± 5.5 | 26.0 ± 3.1 | 34.9 ± 7.8 | 21.8 ± 2.0 | 14.1 ± 5.6 | 61.8 ± 4.6 | 2.4 ± 0.8 | 13.0 ± 2.5 |
| S. maritima | 14.6 ± 0.7 | 4.2 ± 0.6 | 4.8 ± 1.0 | 21.2 ± 2.3 | 45.9 ± 2.8 | 22.9 ± 0.9 | 6.9 ± 1.0 | 67.8 ± 1.3 | 2.4 ± 0.6 | 16.0 ± 2.5 |
| E. tomentosa | 17.0 ± 1.4 | 3.0 ± 0.3 | 9.4 ± 0.9 | 18.3 ± 1.4 | 37.3 ± 4.4 | 27.9 ± 3.8 | 12.3 ± 0.9 | 56.1 ± 4.4 | 3.6 ± 0.8 | 8.6 ± 2.1 |
| (a)Salsola australis | ||||||||
| Salinity Factor | N-Ratio Factor | |||||||
| Control | Moderate | High | 100:0 | 75:25 | 50:50 | 25:75 | 0:100 | |
| RGR | 31.1 ± 5.1 a | 21.8 ± 6.7 b | 10.6 ± 6.4 c | N.S | N.S | N.S | N.S | N.S |
| WC | 70.6 ± 11.1 a | 68.8 ± 13.6 a | 57.1 ± 11.6 b | N.S | N.S | N.S | N.S | N.S |
| AAI | N.S | N.S | N.S | 0.26 ± 0.08 a | N.D | 0.15 ± 0.05 b | N.D | 0.30 ± 0.18 a,b |
| TPC | N.S | N.S | N.S | N.S | N.D | N.S | N.D | N.S |
| TFA | 14.0 ± 2.3 a | 13.7 ± 2.3 a | 11.3 ± 1.9 b | 15.2 ± 1.5 a | 13.5 ± 2.6 a,b | 13.4 ± 1.9 a,b | 11.5 ± 2.9 b,c | 11.4 ± 1.2 c |
| PUFA:SFA | 2.93 ± 0.15 a | 2.94 ± 0.31 a | 2.65 ± 0.18 b | N.S | N.S | N.S | N.S | N.S |
| n − 6:n − 3 | 0.65 ± 0.19 a | 0.70 ± 0.29 a | 1.02 ± 0.26 b | N.S | N.S | N.S | N.S | N.S |
| (b) Suaeda maritima | ||||||||
| Salinity Factor | N-Ratio Factor | |||||||
| Control | Moderate | High | 100:0 | 75:25 | 50:50 | 25:75 | 0:100 | |
| RGR | 25.3 ± 9.2 a | 20.8 ± 5.8 a,b | 18.1 ± 3.7 b | 19.9 ± 4.5 a | 22.0 ± 3.9 a | 26.0 ± 6.6 a | 25.3 ± 6.8 a | 11.2 ± 2.8 b |
| WC | 73.7 ± 11.9 a | 73.2 ± 5.8 a,b | 70.3 ± 5.0 b | N.S | N.S | N.S | N.S | N.S |
| AAI | 0.33 ± 0.18 a | 0.71 ± 0.25 b | 0.92 ± 0.2 b | N.S | N.D | N.S | N.D | N.S |
| TPC | N.S | N.S | N.S | 62.7 ± 7.9 a | N.D | 52.4 ± 6.8 b | N.D | 58.2 ± 10.0 a,b |
| TFA | N.S | N.S | N.S | 13.5 ± 1.5 a | 14.8 ± 2.1 a,b | 17.9 ± 1.2 c | 17.7 ± 2.4 c | 16.3 ± 1.9 b,c |
| PUFA:SFA | N.S | N.S | N.S | N.S | N.S | N.S | N.S | N.S |
| n − 6:n − 3 | N.S | N.S | N.S | 0.46 ± 0.03 a,b | 0.45 ± 0.09 a,c | 0.41 ± 0.04 c | 0.44 ± 0.05 a,c | 0.54 ± 0.09 b |
| (c) Enchylaena tomentosa | ||||||||
| Salinity Factor | N-Ratio Factor | |||||||
| Control | Moderate | High | 100:0 | 75:25 | 50:50 | 25:75 | 0:100 | |
| RGR | N.S | N.S | N.S | 7.0 ± 2.5 a | 8.3 ± 2.4 a,b | 11.3 ± 3.2 b,c | 13.1 ± 4.7 c | 11.5 ± 3.7 b,c |
| WC | N.S | N.S | N.S | 44.9 ± 15.5 a | 26.6 ± 15.5 b | 44.0 ± 9.4 a | 42.4 ± 8.8 a | 45.1 ± 2.4 a |
| AAI | 0.55 ± 0.26 a,b | 0.89 ± 0.39 b | 0.42 ± 0.14 a | 0.34 ± 0.13 a | N.D | 0.64 ± 0.17 b | N.D | 0.88 ± 0.39 b |
| TPC | N.S | N.S | N.S | 53.0 ± 16.2 a | N.D | 65.9 ± 14.0 a | N.D | 87.8 ± 21.6 b |
| TFA | 10.3 ± 1.9 a | 8.9 ± 1.7 a | 6.6 ± 0.7 b | N.S | N.S | N.S | N.S | N.S |
| PUFA:SFA | 2.21 ± 0.48 a | 2.23 ± 0.34 a | 1.77 ± 0.27 b | N.S | N.S | N.S | N.S | N.S |
| n − 6:n − 3 | 0.45 ± 0.05 a | 0.47 ± 0.04 a | 0.57 ± 0.07 b | N.S | N.S | N.S | N.S | N.S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Certain, C.; Della Patrona, L.; Gunkel-Grillon, P.; Léopold, A.; Soudant, P.; Le Grand, F. Effect of Salinity and Nitrogen Form in Irrigation Water on Growth, Antioxidants and Fatty Acids Profiles in Halophytes Salsola australis, Suaeda maritima, and Enchylaena tomentosa for a Perspective of Biosaline Agriculture. Agronomy 2021, 11, 449. https://doi.org/10.3390/agronomy11030449
Certain C, Della Patrona L, Gunkel-Grillon P, Léopold A, Soudant P, Le Grand F. Effect of Salinity and Nitrogen Form in Irrigation Water on Growth, Antioxidants and Fatty Acids Profiles in Halophytes Salsola australis, Suaeda maritima, and Enchylaena tomentosa for a Perspective of Biosaline Agriculture. Agronomy. 2021; 11(3):449. https://doi.org/10.3390/agronomy11030449
Chicago/Turabian StyleCertain, Cassandre, Luc Della Patrona, Peggy Gunkel-Grillon, Audrey Léopold, Philippe Soudant, and Fabienne Le Grand. 2021. "Effect of Salinity and Nitrogen Form in Irrigation Water on Growth, Antioxidants and Fatty Acids Profiles in Halophytes Salsola australis, Suaeda maritima, and Enchylaena tomentosa for a Perspective of Biosaline Agriculture" Agronomy 11, no. 3: 449. https://doi.org/10.3390/agronomy11030449
APA StyleCertain, C., Della Patrona, L., Gunkel-Grillon, P., Léopold, A., Soudant, P., & Le Grand, F. (2021). Effect of Salinity and Nitrogen Form in Irrigation Water on Growth, Antioxidants and Fatty Acids Profiles in Halophytes Salsola australis, Suaeda maritima, and Enchylaena tomentosa for a Perspective of Biosaline Agriculture. Agronomy, 11(3), 449. https://doi.org/10.3390/agronomy11030449







