Abstract
The main purpose of this study was to examine the impact of different phosphorus (P) fertilizers and organic manures alone and in combination with Bacillus sp. MN-54 on growth, yield, nutrient uptake, chlorophyll (SPAD value) and crude proteins content of chickpea. The simple manure (SM), processed manure (PM), single super phosphate (SSP), and rock phosphate (RP) were applied individually and in different combinations to the soil in pots, and the chickpea seeds treated with Bacillus sp. MN-54 were sown in the selective pots. Results showed that individual use of SM, PM, SSP, RP, and strain MN-54 significantly increased (P ≤ 0.05) the nutrient uptake, growth, yield, and protein content of chickpea as compared to control treatments. While the combined use of SM or PM, SSP or RP, and MN-54 further enhanced this effect. Among different treatments, combined use of RP, PM and MN-54 proved the most effective treatment showing increase of 37.5 and 42.6% in shoot and root lengths, 43.4 and 38.3% in fresh and dry shoot weights, 36.1 and 36.5% in fresh and dry root weights, 45.8% in no. of pods, 43.6% in nodules counts, 16.0% in 100-grain weight and 31–36% nutrient uptake over control treatments. Our findings suggest that the co-addition of organic manures and P fertilizers along with plant growth promoting bacteria (i.e., Bacillus sp. MN-54) not only increases the growth and yield but also improves nodulation, nutrient uptake, and crude proteins content in chickpea.
1. Introduction
Leguminous crops are considered as an important component of all types of farming systems in agriculture-based countries. Chickpea (Cicer arietinum L.) ranks third among leguminous crops after pea (Pisum sativum L.) and beans (Phaseolus vulgaris L.) [1]. Nutritionally, chickpea is a good source of proteins and can serve as an alternative to meat [2]. Being a leguminous crop, chickpea has very high requirement of phosphorus (P). The P plays important roles in different processes of metabolism such as macromolecular biosynthesis, energy transfer, respiration and photosynthesis reaction [3]. Therefore, an optimum amount of P is required by the plants from early seedling stage to maturity [4].
The P is the 2nd most important macronutrient, and mineral P fertilizers and manures are commonly used as main sources of P for agricultural crops. The P deficiency is considered as a main hurdle in crop production, affecting soil fertility and productivity throughout the world [5]. Mostly P is available at a soil pH 6.5–7.0 [6] and its availability to plants becomes low in calcareous soil due to its fixation on either lime and clay surfaces or precipitation with calcium (Ca) in saline soils or aluminum and iron and in acidic soils [7]. Being alkaline, alluvial and calcareous in nature, approximately 90% of Pakistani soils have P deficiency and show low P availability to plants [8]. Although many plants have adapted certain modifications in morphological (growth escalation in root hairs length, number of root hairs, changes in diameter and lateral secondary root formation) and biological (such as proton and organic acids secretion) processes to alleviate the negative impacts of P deficiency [9]; however, the negative influence of P deficiency on plants still need to be explored. Phosphatic fertilizers application is a common practice to maintain and improve P status of soils [10]. However, consistent use of these fertilizers has caused many environmental issues all around the world [11]. Hence, there is a dire need to explore the eco-friendly options to nourish the soil without compromising the sustainability of agroecosystems and quality of the environment [12].
Use of plant growth-promoting microorganisms is believed to be a potential option to diminish the bad impacts of chemical fertilizers to the environment. Use of microbes to improve plants ability to extract nutrients from the soil is being widely accepted [13]. Phosphate solubilizing capability of microbes is increasingly used to change P from its inaccessible to readily available forms for the plants [14]. These beneficial microbes help plants by enhancing P availability and uptake from P-deficient soils and proved effective in achieving sustainability of farms and have lessened the chemical fertilizers usage to some extent [15,16]. The P solubilizing bacteria (PSB) solubilize P by excreting organic acids and enzymes and make it available for plants uptake [17,18]. Thus, beneficial microbes have a good potential to maintain and improve quality and fertility status of the soils [19].
Other alternatives to synthetic chemical fertilizers are the use of organic amendments such as composts, animal and poultry manures. Organic amendments improve soil fertility by improving soil porosity as well as by adding essential nutrients within the soil upon decomposition, which also alters distribution and amount of different soil P fractions [20]. Sources of organic amendments can increase the P availability from the existing P directly; however, indirect methods include release of organic acids [21], blockage of P fixation sites [22], and speeding up of microbial activity [23]. Recently, the use of processed manure (PM) to enhance crop production and to sustain soil fertility is being highly recommended [24,25]. Application of rock phosphate (RP) in combination with other amendments such as manures and PSB in basic soils is considered as a better option for normal plants growth [26].
In order to develop an eco-friendly approach to nourish soils, it is better to substitute the chemical fertilizers usage with different combinations of natural or organic sources along with microbial inoculations to enhance their efficiency within agriculture cropping systems. For this purpose, Bacillus sp. MN-54, a previously isolated strain from maize (Zea mays L.) rhizosphere [27] and having ability to enhance growth, yield and physiology of various crops [28,29], was used along with single super phosphate (SSP), rock phosphate (RP), simple manure (SM), and processed manure (PM) to improve growth and yield attributes of chickpea. It is hypothesized that inoculation of strain MN-54 along with integrated use of different P fertilizers and organic manures can be utilized to promote growth, yield attributes and nutrient content of chickpea.
2. Materials and Methods
2.1. Experimental Setup
A pot trial was carried out at the wire house of Institute of Soil and Environmental Sciences (ISES), University of Agriculture, Faisalabad (UAF), Pakistan (31°26′2.18″N, 73°3′53.6″E) to evaluate the effect of different P fertilizers and manures alone and in combination with Bacillus sp. MN-54 on growth, yield and crude protein content of chickpea. Seeds of desi chickpea (Cicer arietinum L.) cultivar (NIAB-CH-2016) were purchased from the Nuclear Institute for Agriculture and Biology (NIAB), Faisalabad. Seeds were surface sterilized by soaking them first in a 5% sodium hypochlorite solution for two minutes and then in a 70% ethanol solution for another two minutes. Finally, seeds were rinsed thoroughly with autoclaved distilled water before further use. Soil collection and preparation was done following standard protocols [30]. The soil (8 kg) was added into each pot. Recommended doses of nitrogen (N) (15 kg ha−1), P (50 kg ha−1) and potassium (K) (14 kg ha−1) were applied using urea, rock phosphate (RP) and/or single super phosphate (SSP), and murate of potash (MOP) in the respective treatments. Manures (simple and processed) prepared from animal waste were applied to the allocated treatments (at the rate of 10 g kg−1). Six surface-sterilized chickpea seeds, un-inoculated and inoculated with Bacillus sp. MN-54, were sown in each pot. After germination and seedling establishment, only three plants were maintained in each pot. The experiment was performed following a completely randomized design containing 18 different treatments with six replicates each, comprised of two different P fertilizers (i.e., RP and SSP) applied individually and in combination with two different organic manures (i.e., SM and PM) with and without inoculation of Bacillus sp. MN-54.
2.2. Physicochemical Characteristics of Soil
Initially, the soil was analyzed for physicochemical properties (Table 1). The soil texture was determined following hydrometer method using 400 g air-died soil mixed with 600 mL sodium (hexa-meta) phosphate [(4:1; (NaPO3)13: Na2CO3)] dispersion solution [31]. The pH, electrical conductivity (EC), cation exchange capacity (CEC), soluble Ca2++ Mg2+, CO32− and HCO31− were determined using appropriate instruments following standard procedures [32]. To determine soil organic matter (SOM) content, Walkley-Black method was followed [33]. Contents of N, P, and K were measured using Kjeldahl apparatus, spectrophotometer (T60 (UV-Vis), PG instruments limited, UK), and flame photometer (Jenway, PFP-7, England), respectively, following standard procedures [34,35,36].

Table 1.
Physio-chemical characteristics of soil, simple, and processed manure used in this study.
2.3. Preparation and Nutrient Analysis of SM and PM
The SM (animal dung) was collected from the Directorate of Farms, UAF, Pakistan, and was used without any amendment, while PM was prepared by composting SM [37]. For nutrient analysis, 0.5 g samples of SM and PM were digested with H2SO4 following standard method [38] and heated till clear solution. Finally, nutrient (i.e., N, P, and K) content (Table 1) was determined using suitable apparatus and instruments as described above.
2.4. Preparation and Use of Inoculum
The rhizospheric plant growth-promoting bacterium Bacillus sp. MN-54 (previously isolated, sequenced and deposited in the GenBank database under accession number KT375574) was obtained from Soil and Environmental Microbiology Lab, UAF. The strain MN-54 was grown in tryptic soy broth medium at 28 ± 1 °C and 180 rpm for 3 d in a 250 mL Erlenmeyer flask [29]. The culture optical density was determined at 600 nm with spectrophotometer to assure a uniform bacterial population (108–109 CFU mL−1). Before seed coating, MN-54 strain suspension was added into sterile peat (100 mL kg−1). Surface sterilized chickpea seeds were inoculated with inoculum at 1:1 (w/w) seed to peat ratio before further use.
2.5. Seedling Emergence, Agronomic and Physiological Attributes of Chickpea Plants
Emergence of seedlings in each pot was recorded daily till constant numbers (i.e., 10 d after sowing). The chlorophyll content (SPAD value) were taken after 55 d of sowing and measured using chlorophyll meter (SPAD-502 Konica, Minolta, Japan) [39]. Shoot and root lengths and dry and fresh weights of shoot and root were taken after 70 d of sowing by harvesting three of six replicates for each treatment. For dry weights, fresh shoots and roots were dried in the oven at 75 °C till constant weight [40]. After 120 d crop plants were harvested and number of pods and root nodules present on each plant were recorded and the crude protein content were calculated by multiplying N value by factor 6.25 [41].
2.6. Analyses of Nutrient Content in Chickpea Plants
The N, P and K content (%) in chickpea straw and grains were determined by digesting and analyzing respective plant parts following method reported by Wolf [42]. Briefly, after oven drying, 0.5 g plant samples were ground and subjected to digestion using a mixture of H2SO4 and 30% concentrated hydrogen peroxide (H2O2) in digestion tubes. The digests were filtered and N, P, and K content were determined using suitable instruments as described above.
2.7. Statistical Analysis
The analysis of variance was performed to estimate the variations from the mean (n = 3) values by standard errors, while F-test was used to evaluate the significance differences among the treatments and least significant difference test was applied to compare means (p ≤ 0.05) using Statistix 8.1 [43].
3. Results
3.1. Seedling Emergence and Growth Attributes of Chickpea
In general, all treatments with Bacillus sp. MN-54 showed better response in terms of seedling emergence as compared to their respective control treatments (Table 2). The co-addition of different organic and inorganic amendments with MN-54 further improved this effect on seedling emergence (Table 2), suggesting the positive impact of MN-54 on early seedling establishment. Results showed that individual use of each of organic, inorganic amendments or MN-54 showed promotive effects on shoot or root lengths of chickpea (Table 2). The use of MN-54 showed 4.50–9.90% and 13.8–19.0% higher shoot and root lengths, respectively, than that of control treatments. However, the application of organic manures together with MN-54 and fertilizers significantly enhanced the shoot and root lengths when compared with their individual treatments or respective controls (Table 2). For instance, the co-addition of RP, PM and MN-54 showed 21.4–37.5% and 18.1–42.6% higher shoot and root lengths, respectively, than that of their individual or control treatments. Overall, treatments with combined use of fertilizers, manures and strain MN-54 proved effective in improving the emergence of seedling, and root and shoot lengths of chickpea.

Table 2.
Effect of phosphorus fertilizers, organic manures, and Bacillus sp. MN-54 addition on seedling emergence and growth of chickpea.
Similarly, all treatments with Bacillus sp. MN-54 showed better response in terms of dry and fresh weights of roots and shoots compared to their respective control treatments, while the addition of different P fertilizers and manures with MN-54 further improved the effect of MN-54 on fresh and dry weights of chickpea (Table 2). The use of MN-54 showed 10.1–14.7% and 9.50–13.6% increased fresh and dry shoot weights, respectively, than that of control treatments. However, the addition of manures together with MN-54 and fertilizers significantly enhanced the root and shoot dry and fresh weights when compared with their individual treatments or respective controls (Table 2). The co-addition of RP, PM and MN-54 showed 16.3–43.4% and 20.1–38.3% higher fresh and dry shoot weights, respectively, than that of their individual or control treatments. Likewise, the combined use of RP, PM and MN-54 showed 19.8–36.1% and 19.7–36.5% higher fresh and dry root weights, respectively, than that of their individual or control treatments. In general, treatments with combined use of fertilizers, manures and MN-54 proved very effective in improving the dry and fresh weights of chickpea. Furthermore, regardless of MN-54 addition, SSP and PM showed better results than RP and SM, respectively, in improving growth attributes of chickpea (Table 2).
3.2. Yield Attributes of Chickpea
Obtained results showed that individual application of MN-54 significantly improved the yield attributes of chickpea, with 13.8–19.3%, 21.3–28.7% and 22.9–26.0% increase in pods count, nodules count and 100-grain weight, respectively, than that of their respective controls (Table 3). While the combined application of inorganic and organic amendments with MN-54 further improved the yield attributes of chickpea (Table 3). The co-addition of RP, PM and MN-54 significantly enhanced the values of pods count, nodules count and 100-grain weight up to 23.3–45.8%, 19.5–43.6% and 9.6–16.0%, respectively, than that of control treatments. In addition, regardless of MN-54 addition, SSP and PM showed better results than RP and SM, respectively. Overall, co-addition of P fertilizers and manures with strain MN-54 proved very effective in improving yield attributes showing RP, PM and MN-54 integrated application as a best one among all the other treatments.

Table 3.
Effect of phosphorus fertilizers, organic manures, and Bacillus sp. MN-54 addition on yield attributes of chickpea.
3.3. N, P, and K Content in Chickpea
Interestingly, all treatments containing Bacillus sp. MN-54 showed higher P, N, and K amounts in straw and grains of chickpea that that of their control treatments (Table 4). The use of MN-54 showed 6.8–10.6% and 11.6–16.8% higher N, 14.5–18.8% and 11.3–16.4% higher P, and 7.5–11.8% and 8.9–15.0% higher K content in the grains and straw of chickpea, respectively, than that of control treatments. Furthermore, the application of MN-54 in combination with manures and fertilizers significantly enhanced the N, P, and K content when compared with their individual treatments or controls (Table 4). The combined use of RP, PM and MN-54 showed 16.9–31.2% and 21.3–39.3% higher N, 18.5–36.1% and 18.6–39.8% higher P, and 19.6–36.4% and 19.6–40.5% higher K content in grains and straw, respectively, than that of their individual or control treatments. Particularly, treatments with co-addition of P fertilizers, manures and MN-54 were found to be the most effective in increasing P, N, and K content of chickpea.

Table 4.
Effect of phosphorus fertilizers, organic manures, and Bacillus sp. MN-54 addition on N, P, and K content in chickpea.
3.4. Chlorophyll (SPAD value) and Crude Protein Content in Chickpea
Our results showed that individual use of MN-54 resulted in substantial increase in chlorophyll (SPAD value) (Figure 1) and crude protein content of chickpea (Figure 2). Application of only MN-54 showed 2.2–10.0% and 6.1–10.6% higher chlorophyll and crude protein content of chickpea, respectively, than that of their control treatments (Figure 1 and Figure 2). Combined use of P fertilizers and manures with MN-54 further enhanced the chlorophyll and crude protein content in chickpea. For instance, the combined use of RP, PM and MN-54 showed 24.5–45.3% and 16.9–31.2% higher chlorophyll and crude protein content, respectively, than that of their control treatments (Figure 1 and Figure 2). Overall, treatments with co-addition of P fertilizers, manures and MN-54 were found to be the most effective in improving the chlorophyll and protein content of chickpea. Likewise, regardless of MN-54 addition, SSP and PM showed better results than RP and SM, respectively.
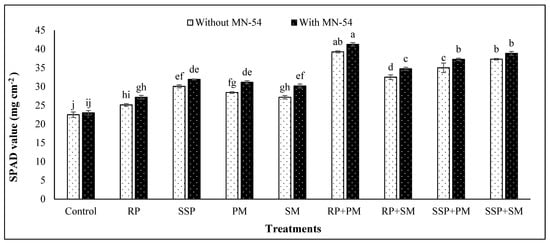
Figure 1.
Effect of phosphorus fertilizers, organic manures, and Bacillus sp. MN-54 addition on chlorophyll content (SPAD value) of chickpea measured at 55 d after sowing. Column and error bars represent means and standard errors of triplicate values, respectively. Means having different letters differ significantly according to LSD test at p ≤ 0.05. (MN-54, Bacillus sp. MN-54; RP, rock phosphate; SSP, single super phosphate; PM, processed manure; SM, simple manure).

Figure 2.
Effect of phosphorus fertilizers, organic manures, and Bacillus sp. MN-54 addition on crude protein content of chickpea measured at 120 d after sowing. Column and error bars represent means and standard errors of triplicate values, respectively. Means having different letters differ significantly according to LSD test at p ≤ 0.05. (MN-54, Bacillus sp. MN-54; RP, rock phosphate; SSP, single super phosphate; PM, processed manure; SM, simple manure).
4. Discussion
4.1. Seedling Emergence and Growth Attributes of Chickpea
Generally, the emergence of seedlings and growth attributes of a crop are considered as important indicators to monitor overall growth and productivity. Our results showed that addition of P fertilizers and manures with strain MN-54 resulted in a great increase in chickpea growth which is quite consistent with previous reports [28,44]. Similarly, our results are in agreement with those of [45] who concluded that combined use of RP, farmyard manure and PSB was effective in promoting canola (Brassica napus L.) growth. In this study, the enhanced growth of chickpea can be explained by the fact that manures help in maintaining nutritional status and availability of nutrients by supplying macro and micronutrients, improving soil chemical and physical properties, and increasing SOM content, and promoting overall plant growth [46,47]. Besides providing nutrients, manures also provide organic carbon which strengthens the overall status of SOM and boosts microbial activities [48,49]. Furthermore, improved growth of chickpea on inoculation of MN-54 may be associated with the plant growth-promoting traits of strain MN-54 such as 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase activity, siderophores and indole acetic acid (IAA) production, and phosphate solubilization [28,50].
4.2. Yield Attributes of Chickpea
Our findings related to yield attributes of chickpea are consistent with the previous results reported by [51] proving the effectiveness of different combinations of RP-enriched organic fertilizers with PSB in improving nodulation and yield of chickpea. Similarly, our results are also consistent with those of [52,53] reporting that combined use of organic and inorganic fertilizer inputs with PSB gives maximum net returns. The PSB such as strain MN-54 could be effective in increasing growth and yield attributes of legume crops including chickpea [54,55]. The improved yield of chickpea in our study can be explained by the reason that the organic manures and P fertilizers maintain soil fertility and enhance nutrient availability (particularly of P) to plants which eventually improve growth and productivity of plants [56,57]. Moreover, plant growth promoting rhizobacteria (PGPR) such as Bacillus sp. MN-54 has ability to enhance uptake and availability of nutrients to plants and thus overall yield by nodulation and production of growth hormones [58,59].
4.3. N, P, and K Content in Chickpea
For improving available nutrients particularly within the rhizosphere, use of organic and inorganic amendments with microbial inoculation is considered to be the best possible eco-friendly option [60]. Our results support the findings of [61] who reported enhanced uptake and total nutrients concentrations in chickpea and wheat, respectively, on applying RP with organic amendments. The increased P, N, and K concentrations in chickpea plants could be due to the fact that the acidification process by the production of CO2 and different organic acids in manures might influence soil pH and nutrient availability to plants. It is fact that the organic sources of P such as manures and composts are mineralized by phosphatase enzymes and improve the available P content within the rhizosphere of crop plants [62]. In addition, the PSB such as Bacillus sp. MN-54 may increase P accumulation thus have abilities to enhance plant growth and overall nutrient concentrations in plants [63,64]. The increased nutrients availability and uptake in chickpea on inoculation of strain MN-54 might be due to the effect of acidic exudates (produced by the MN-54) on solubilization of nutrients. Furthermore, the strain MN-54 may have influence on soil pH which enhanced mobilization and uptake of nutrients and eventually their accumulation in plant tissues [13, 16).
4.4. Chlorophyll (SPAD value) and Crude Protein Content in Chickpea
In this study, addition of strain MN-54 with organic and inorganic amendments was found very effective in increasing chlorophyll (SPAD value) and crude protein content in chickpea. These results are in a good agreement with those reported by [65,66] using chickpea and [67] using pea (Pisum sativum L.) plants. Increased chlorophyll and protein content might be due to the fact that use of P fertilizers and organic manures may serve as an adequate nutrition that is prerequisite in chlorophyll and protein biosynthesis.
Similarly, optimum availability of P, N, and K nutrients can enhance plant growth by increasing photosynthesis rates that can improve chlorophyll and protein content [68]. Besides, owing plant growth promoting characteristics, Bacillus sp. MN-54 may have increased nutrient availability and uptake by chickpea plants through colonization in the plant rhizosphere and thus increase chlorophyll and protein content, because nutrients availability is crucially important in increasing chlorophyll biosynthesis and protein content [69,70,71]. Although, in this study, we did not analyze the population of strain MN-54 in the plant rhizosphere, our previous studies showed clear evidence of survival and colonization of this strain in the plant rhizosphere under normal or stressed conditions [28,72]. In general, the interactive effect of SSP, RP, SM, PM, and strain MN-54 was found to be helpful in improving growth, nutrients uptake, yield and crude protein content of chickpea. Particularly, the combined use of RP, PM and strain MN-54 was very effective in increasing chickpea productivity and thus can serve as an efficient combination for achieving maximum productivity goals. To our knowledge, this is the first study reporting the interactive effect of inorganic fertilizers (i.e., RP and SSP), organic manures (i.e., PM and SM) and microbes (i.e., Bacillus sp. MN-54) in enhancing chickpea productivity. Our findings provide a greater insight of bacterium-plant interactions in the absence or presence of fertilizers and manures, providing a novel information on the combined use of beneficial bacteria, fertilizers and manures for improving crop productivity in eco-friendly way.
5. Conclusions
In this study, we examined the effects of different P fertilizers and organic manures addition with and without Bacillus sp. MN-54 on nutrient uptake, growth, yield, chlorophyll (SPAD value), and protein content of chickpea. All the treatments with Bacillus sp. MN-54 showed better response in improving all the tested growth and yield attributes of chickpea. While the addition of strain MN-54 with P fertilizers and manures further enhanced the promotive effect of MN-54 on chickpea growth. Combined use of PM, RP and MN-54 was the most effective treatment in enhancing growth and yield attributes, and nutrient content of chickpea. Furthermore, the combination of SM, SSP and MN-54 also found to be at par with that of PM, RP and MN-54 in improving growth and yield of chickpea. Our findings suggest that the combined use of P fertilizers and manures along with growth promoting bacterium (i.e., Bacillus sp. MN-54) could be effective in improving growth and yield of chickpea and serve as an alternate eco-friendly option to the excessive usage of single source fertilizers inputs for sustainable agriculture and environmental stewardship.
Author Contributions
Conceptualization, M.I.K. and Z.C.; Data curation, M.J.A., S.A. and M.I.K.; Formal analysis, M.J.A., S.B., Z.C. and S.A.; Funding acquisition, M.I.K.; Investigation, M.J.A., S.A. and M.I.K.; Methodology, S.A. and M.J.A.; Project administration, M.I.K.; Resources, M.I.K., M.N. and S.B.; Software, S.A. and M.J.A.; Supervision, M.I.K.; Writing-original draft, M.I.K. and M.J.A.; Writing—review and editing, M.I.K., M.H.A., M.N., S.B., Z.C., A.W., M.S., and S.A.C. All authors read and approved the submission of the manuscript to the journal.
Funding
This study was partially funded by the Higher Education Commission (HEC) of Pakistan (project number NRPU-7730).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Authors are grateful to all members of Environmental Science Lab (ESL-6), UAF for their assistance and cooperation in this research work.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Kafadar, F.N.; Özkan, A.; Can, C.; Kar, Y.; Mart, D.; Ceyhan, E. Genetic and biochemical properties of Cicer spp reveal distinction between wild and cultivated chickpea genotypes. Legume Res. Int. J. 2019, 42, 1–9. [Google Scholar] [CrossRef]
- Rokhzadi, A.; Toashih, V. Nutrient uptake and yield of chickpea (Cicer arietinum L.) inoculated with plant growth promoting rhizobacteria. Aust. J. Crop Sci. 2011, 5, 44–48. [Google Scholar]
- Anand, K.; Kumari, B.; Mallick, M.A. Phosphate solubilizing microbes: An effective and alternative approach as bio-fertilizers. Int. J. Pharm. Pharm. Sci. 2016, 8, 37–40. [Google Scholar]
- Saleem, M.; Ali, H.; Rehman, S.; Rana, M.; Rizwan, M.S.; Kamran, M.; Liu, L. Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 2020, 248, 126032. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Y.; Zhao, X.; Jin, X.; Hou, L.; Shi, Y.; Ahammed, G.J. Silicon compensates phosphorus deficit-induced growth inhibition by improving photosynthetic capacity, antioxidant potential, and nutrient homeostasis in tomato. Agronomy 2019, 9, 733. [Google Scholar] [CrossRef]
- Malla, R.; Shrestha, S.; Khadka, D.; Bam, C.R. Soil fertility mapping and assessment of the spatial distribution of Sarlahi District, Nepal. Am. J. Agric. Sci. 2020, 7, 8–16. [Google Scholar]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Ahmad, A.; Arif, M.S.; Yasmeen, T.; Riaz, M.; Rizwan, M.; Shahzad, S.M.; Sarosh, M. Seasonal variations of soil phosphorus and associated fertility indicators in wastewater-irrigated urban aridisol. Chemosphere 2020, 239, 124725. [Google Scholar] [CrossRef]
- Luo, J.; Liu, Y.; Zhang, H.; Wang, J.; Chen, Z.; Luo, L.; Liu, P. Metabolic alterations provide insights into stylosanthes roots responding to phosphorus deficiency. BMC Plant Biol. 2020, 20, 85. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, E.M.; de Lima, W.; Oliveira-Longatti, S.M.; de Souza, F.M. Phosphate-solubilising bacteria enhance Oryza sativa growth and nutrient accumulation in an oxisol fertilized with rock phosphate. Ecol. Eng. 2015, 83, 380–385. [Google Scholar] [CrossRef]
- Biswakarma, B.; Verma, H.; Sarkar, N.C. Effect of phosphate solubilizing bacteria on yield of transplanted rice under lateritic belt of West Bengal. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3192–3204. [Google Scholar] [CrossRef]
- Kumar, R.; Saurabh, K.; Kumawat, N.; Mishra, J.S.; Hans, H.; Krishna, B.; Hazra, K.K.; Meena, R.S.; Kumar, S.; Singh, B.J.; et al. Conservation Agriculture: Perspectives on soil and environmental management in Indo-Gangetic plains of South Asia. In Sustainable Management of Soil and Environment; Springer: Singapore, 2019; pp. 123–168. [Google Scholar] [CrossRef]
- Qiu, Z.; Egidi, E.; Liu, H.; Kaur, S.; Singh, B.K. New frontiers in agriculture productivity: Optimised microbial inoculants and in situ microbiome engineering. Biotechnol. Adv. 2019, 37, 107371. [Google Scholar] [CrossRef]
- Bargaz, A.; Lyamlouli, K.; Chtouki, M.; Zeroual, Y.; Dhiba, D. Soil microbial resources for improving fertilizers efficiency in an integrated plant nutrient management system. Front. Microbiol. 2018, 9, 1606. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.J.; Fraaije, B.A.; Clark, I.M.; Jackson, R.W.; Hirsch, P.R.; Mauchline, T.H. Endophytic bacterial community composition in wheat (Triticum aestivum) is determined by plant tissue type, developmental stage and soil nutrient availability. Plant Soil 2016, 405, 381–396. [Google Scholar] [CrossRef]
- Wu, C.; Li, B.; Wei, Q.; Pan, R.; Zhang, W. Endophytic fungus Serendipita indica increased nutrition absorption and biomass accumulation in Cunninghamia lanceolata seedlings under low phosphate. Acta Ecol. Sin. 2019, 39, 21–29. [Google Scholar] [CrossRef]
- Singh, A.V.; Chandra, R.; Goel, R. Phosphate solubilization by Chryseobacterium sp. and their combined effect with N and P fertilizers on plant growth promotion. Arch. Agron. Soil Sci. 2013, 59, 641–651. [Google Scholar] [CrossRef]
- Prabhu, N.; Borkar, S.; Garg, S. Phosphate Solubilization by Microorganisms: Overview, Mechanisms, Applications and Advances; Meena, S.N., Naik, M., Eds.; Advances in Biological Science Research Academic Press: London, UK, 2019; pp. 161–176. [Google Scholar] [CrossRef]
- Paredes, S.H.; Lebeis, S.L. Giving back to the community: Microbial mechanisms of plant–soil interactions. Funct. Ecol. 2016, 30, 1–10. [Google Scholar] [CrossRef]
- Niamat, B.; Naveed, M.; Ahmad, Z.; Yaseen, M.; Ditta, A.; Mustafa, A.; Xu, M. Calcium-enriched animal manure alleviates the adverse effects of salt stress on growth, physiology and nutrients homeostasis of Zea mays L. Plants 2019, 8, 480. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, B.; Bolan, N.; Naidu, R.; Mora, M.D.L.L. Phosphorus in organic waste-soil systems. J. Soil Sci. Plant Nutr. 2006, 6, 64–83. [Google Scholar] [CrossRef]
- Chakraborty, K.; Chakrabarti, A.; Chakraborty, A.; Ghosh, S. Effect of long-term fertilizers and manure application on microbial biomass and microbial activity of a tropical agricultural soil. Biol. Fertil. Soils 2011, 47, 227–233. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Renella, G.; Puglisi, E.; Ceccanti, B.; Masciandaro, G.; Fornasier, F.; Moscatelli, M.C.; Marinari, S. Soil enzymology: Classical and molecular approaches. Biol. Fertil. Soils 2012, 48, 743–762. [Google Scholar] [CrossRef]
- Pagliari, P.H. Variety and solubility of phosphorus forms in animal manure and their effects on soil test phosphorus. In Applied Manure and Nutrient Chemistry for Sustainable Agriculture and Environment; Springer: Dordrecht, The Nrtherlands, 2014; pp. 141–161. [Google Scholar] [CrossRef]
- Leenstra, F.; Vellinga, T.; Neijenhuis, F.; de Buisonjeé, F.E. Manure: A Valuable Resource; Wageningen UR Livestock Research: Lelystad, The Netherlands, 2019; Available online: https://library.wur.nl/WebQuery/wurpubs/451354 (accessed on 26 February 2021).
- Ben Zineb, A.; Trabelsi, D.; Ayachi, I.; Barhoumi, F.; Aroca, R.; Mhamdi, R. Inoculation with elite strains of phosphate-solubilizing bacteria enhances the effectiveness of fertilization with rock phosphates. Geomicrobiol. J. 2020, 2020 37, 22–30. [Google Scholar] [CrossRef]
- Naveed, M. Maize Endophytes-Diversity, Functionality and Application Potential. Ph.D. Thesis, AIT Austrian Institute of Technology GmbH, Bioresources Unit, Seibersdorf, Austria, 2013. [Google Scholar]
- Samreen, T.; Zahir, Z.A.; Naveed, M.; Asghar, M. Boron tolerant phosphorus solubilizing Bacillus sp. strain MN-54 improved canola growth in alkaline calcareous soils. Int. J. Agric. Biol. 2019, 21, 538–546. [Google Scholar] [CrossRef]
- Afzal, M.J.; Khan, M.I.; Cheema, S.A.; Hussain, S.; Anwar-ul-Haq, M.; Ali, M.H.; Naveed, M. Combined application of Bacillus sp. MN-54 and phosphorus improved growth and reduced lead uptake by maize in the lead-contaminated soil. Environ. Sci. Pollut. Res. 2020, 27, 44528–44539. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.G.; Salami, S.J.; Gushit, J.S.; Dalen, M.B. Accumulation of Polychlorinated Biphenyls (PCBS) in Soil and Water from Electrical Transformers Installation Sites in Selected Locations in Jos Metropolis, Plateau State, Nigeria. J. Environ. Anal. Toxicol. 2018, 8, 1–6. [Google Scholar] [CrossRef]
- Strickland, T.C.; Kerle, E.A.; Sollins, P.; Schimel, D.S. Aggregation and aggregate stability in forest and range soils. Soil Sci. Soc. Am. J. 1988, 52, 829–833. [Google Scholar] [CrossRef]
- Estefan, G.; Sommer, R.; Ryan, R. Methods of Soil, Plant, and Water Analysis: A Manual for the West Asia and North Africa Region, 3rd ed.; International Center for Agricultural Research in the Dry Areas (ICARDA): Beirut, Lebanon, 2013; pp. 170–176. Available online: https://hdl.handle.net/20.500.11766/7512 (accessed on 26 February 2021).
- Storer, D.A. A simple high sample volume ashing procedure for determination of soil organic matter. Commun. Soil Sci. Plant Anal. 1984, 15, 759–772. [Google Scholar] [CrossRef]
- Buondonno, A.; Rashad, A.A.; Coppola, E. Comparing tests for soil fertility. II. The hydrogen peroxide/sulfuric acid treatment as an alternative to the copper/selenium catalyzed digestion process for routine determination of soil nitrogen-kjeldahl. Commun. Soil Sci. Plant Anal. 1995, 26, 1607–1619. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis Part 2 Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Stanford, G.; English, L. Use of the flame photometer in rapid soil tests for K and Ca. Agron. J. 1949, 41, 446–447. [Google Scholar] [CrossRef]
- Naveed, M.; Sajid, H.; Mustafa, A.; Niamat, B.; Ahmad, Z.; Yaseen, M.; Chen, J.T. Alleviation of salinity-induced oxidative stress, improvement in growth, physiology and mineral nutrition of canola (Brassica napus L.) through calcium-fortified composted animal manure. Sustainability 2020, 12, 846. [Google Scholar] [CrossRef]
- Wolf, P. Aerobic thermophilic stabilization of sludge versus anaerobic digestion and other kinds of sludge treatment at middle-sized plants with respect to power conservation and economy. Water Sci. Technol. 1982, 14, 727–738. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, A.R.; Del Campillo, M.C.; Torrent, J.; Jones, D.L. Organic acids alleviate iron chlorosis in chickpea grown on two P-fertilized soils. J. Soil Sci. Plant Nutr. 2014, 14, 292–303. [Google Scholar] [CrossRef]
- Sattar, A.; Sher, A.; Ijaz, M.; Kashif, M.; Suleman, M.; Ali, M.U.; Mahboob, A. Exogenous application of potassium improves the drought tolerance in chickpea. J. Arable Crop. Mark. 2017, 1, 31–34. [Google Scholar] [CrossRef]
- Zhang, Q.; Qi, D.; Dong, X.; Li, X.; Cheng, L.; Liu, H.; Liu, G. Amino acid composition, protein content and accurate nitrogen-to-protein conversion factor for sheepgrass (Leymus chinensis). Botany 2020, 98, 137–146. [Google Scholar] [CrossRef]
- Lowther, J.R. Use of a single sulphuric acid-hydrogen peroxide digest for the analysis of Pinus radiata needles. Commun. Soil Sci. Plant Anal. 1980, 11, 175–188. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dicky, D.A. Principles and Procedures of Statistics—A Biometric Approach, 3rd ed.; Subsequent Edition; McGraw-Hill College: New York, NY, USA, 1997. [Google Scholar]
- Hussain, M.; Mehboob, N.; Naveed, M.; Shehzadi, K.; Yasir, T.A. Optimizing boron seed coating level and boron-tolerant bacteria for improving yield and biofortification of chickpea. J. Soil Sci. Plant Nutr. 2020, 20, 2471–2478. [Google Scholar] [CrossRef]
- Awaad, M.S.; Rashad, A.A.; Bayoumi, M.A. Effect of farmyard manure combined with some phosphate sources on the productivity of canola plants grown on a sandy soil. Res. J. Agric. Biol. Sci. 2009, 5, 1176–1181. [Google Scholar]
- Agegnehu, G.; Nelson, P.N.; Bird, M.I. Crop yield, plant nutrient uptake and soil physicochemical properties under organic soil amendments and nitrogen fertilization on nitisols. Soil Tillage Res. 2016, 160, 1–13. [Google Scholar] [CrossRef]
- Singh, L.; Sukul, P. Impact of vermicompost, farmyard manure, fly ash and inorganic fertilizers on growth and yield attributing characters of maize (Zea mays L.). Plant Arch. 2019, 19, 2193–2200. [Google Scholar]
- Lu, H.; Lashari, M.S.; Liu, X.; Ji, H.; Li, L.; Zheng, J.; Kibue, G.W.; Joseph, S.; Pan, G. Changes in soil microbial community structure and enzyme activity with amendment of biochar-manure compost and pyroligneous solution in a saline soil from Central China. Eur. J. Soil Biol. 2015, 70, 67–76. [Google Scholar] [CrossRef]
- Singh, T.B.; Ali, A.; Prasad, M.; Yadav, A.; Shrivastav, P.; Goyal, D.; Dantu, P.K. Role of Organic Fertilizers in Improving Soil Fertility. In Contaminants in Agriculture; Naeem, M., Ansari, A.A., Gill, S.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 61–77. [Google Scholar] [CrossRef]
- Pandey, S.; Gupta, S.; Ramawat, N. Unravelling the potential of microbes isolated from rhizospheric soil of chickpea (Cicer arietinum) as plant growth promoter. 3 Biotech 2019, 9, 277. [Google Scholar] [CrossRef]
- Ditta, A.; Muhammad, J.; Imtiaz, M.; Mehmood, S.; Qian, Z.; Tu, S. Application of rock phosphate enriched composts increases nodulation, growth and yield of chickpea. Int. J. Recycl. Org. Waste Agric. 2018, 7, 33–40. [Google Scholar] [CrossRef]
- Niazi, M.T.H.; Kashif, S.U.R.; Asghar, H.N.; Saleem, M.; Khan, M.Y.; Zahir, Z.A. Phosphate solubilizing bacteria in combination with pressmud improve growth and yield of mash bean. J. Anim. Plant Sci. 2015, 25, 1049–1054. [Google Scholar]
- Pincus, L.; Margenot, A.; Six, J.; Scow, K. On-farm trial assessing combined organic and mineral fertilizer amendments on vegetable yields in central Uganda. Agric. Ecosyst. Environ. 2016, 225, 62–71. [Google Scholar] [CrossRef]
- Gull, M.; Hafeez, F.Y.; Saleem, M.; Malik, K.A. Phosphorus uptake and growth promotion of chickpea by co-inoculation of mineral phosphate-solubilizing bacteria and a mixed rhizobial culture. Aust. J. Exp. Agric. 2004, 44, 623–628. [Google Scholar] [CrossRef]
- Wani, P.A.; Khan, M.S.; Zaidi, A. Synergistic effects of the inoculation with nitrogen-fixing and phosphate-solubilizing rhizobacteria on the performance of field-grown chickpea. J. Plant Nutr. Soil Sci. 2007, 170, 283–287. [Google Scholar] [CrossRef]
- Yadav, J.K.; Sharma, M.; Yadav, R.N.; Yadav, S.K.; Yadav, S. Effect of different organic manures on growth and yield of chickpea (Cicer arietinum L.). J. Pharmacogn. Phytochem. 2017, 6, 1857–1860. [Google Scholar]
- Moe, K.; Htwe, A.Z.; Thu, T.T.P.; Kajihara, Y.; Yamakawa, T. Effects on NPK status, growth, dry matter and yield of rice (Oryza sativa) by organic fertilizers applied in field condition. Agriculture 2019, 9, 109. [Google Scholar] [CrossRef]
- Kenneth, O.C.; Nwadibe, E.C.; Kalu, A.U.; Unah, U.V. Plant growth promoting rhizobacteria (PGPR): A novel agent for sustainable food production. Am. J. Agric. Biol. Sci. 2019, 14, 35–54. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, I.; Altaf, M.; Khan, M.; Shouche, Y.S. Characterization of Paenibacillus durus (PNF16) a new isolate and its synergistic interaction with other isolated rhizobacteria in promoting growth and yield of chickpea. J. Microbiol. Biotechnol. Food Sci. 2020, 5, 345–350. [Google Scholar] [CrossRef]
- Shahzad, S.M.; Arifm, M.S.; Riaz, M.; Ashraf, M.; Yasmeen, T.; Zaheer, A.; Bragazza, L.; Buttler, A.; Robroek, B.J.M. Interaction of compost additives with phosphate solubilizing rhizobacteria improved maize production and soil biochemical properties under dryland agriculture. Soil Tillage Res. 2017, 174, 70–80. [Google Scholar] [CrossRef]
- Rabari, K.V.; Patel, K.M.; Chaudhary, H.L. Effect of rock phosphate enriched different organic manures and chemical fertilizers on growth and yield of wheat. Crop Res. 2020, 55, 6–9. [Google Scholar] [CrossRef]
- Tazisong, I.A.; Senwo, Z.N.; He, Z.Q. Phosphatase hydrolysis of organic phosphorus compounds. Adv. Enzym. Res. 2015, 3, 39–51. [Google Scholar] [CrossRef]
- Kaur, G.; Reddy, M.S. Role of phosphate-solubilizing bacteria in improving the soil fertility and crop productivity in organic farming. Arch. Agron. Soil Sci. 2014, 60, 549–564. [Google Scholar] [CrossRef]
- Solanki, K.D.; Sakarvadia, H.L.; Purohit, M.P. Influence of nutrients accumulation by various levels of phosphorus and phosphorus solubilizing bacteria in summer groundnut. Int. J. Chem. Stud. 2020, 8, 983–989. [Google Scholar] [CrossRef]
- Patil, S.V.; Halikatti, S.I.; Hiremath, S.M.; Babalad, H.B.; Sreenivasa, M.N.; Hebsur, N.S.; Somanagouda, G. Effect of organic manures and rock phosphate on growth and yield of chickpea (Cicer arietinum L.) in vertisols. Karnataka J. Agric. Sci. 2012, 24, 636–638. [Google Scholar]
- Pingoliya, K.K.; Mathur, A.K.; Dotaniya, M.L.; Dotaniya, C.K. Impact of phosphorus and iron on protein and chlorophyll content in chickpea (Cicer arietinum L.). Legume Res. 2015, 38, 558–560. [Google Scholar] [CrossRef]
- Khafagy, H.; Ahmed, M.; Abdel-Azeem, S. Impact of mineral fertilizers with or without bio fertilizers or potassium humate on some soil properties, yield and quality of pea plants under salt affected soil conditions. J. Agric. Chem. Biotechnol. 2019, 10, 19–27. [Google Scholar] [CrossRef]
- El-Azab, M.E. Effects of foliar NPK spraying with micronutrients on yield and quality of cowpea plants. Asian J. Appl. Sci. 2016, 4. Available online: https://ajouronline.com/index.php/AJAS/article/view/3595 (accessed on 26 February 2021).
- Yildirim, E.; Karlidag, H.; Turan, M.; Dursun, A.; Goktepe, F. Growth, nutrient uptake, and yield promotion of broccoli by plant growth promoting rhizobacteria with manure. Am. Soc. Hortic. Sci. 2011, 46, 932–936. [Google Scholar] [CrossRef]
- Ejaz, S.; Batool, S.; Anjum, M.A.; Naz, S.; Qayyum, M.F.; Naqqash, T.; Ali, S. Effects of inoculation of root associative Azospirillum and Agrobacterium strains on growth, yield and quality of pea (Pisum sativum L.) grown under different nitrogen and phosphorus regimes. Sci. Hortic. 2020, 270, 109401. [Google Scholar] [CrossRef]
- Ali, M.H.; Sattar, M.T.; Khan, M.I.; Naveed, M.; Rafique, M.; Alamri, S.; Siddiqui, M.H. Enhanced growth of mungbean and remediation of petroleum hydrocarbons by Enterobacter sp. MN17 and biochar addition in diesel contaminated soil. Appl. Sci. 2020, 10, 8548. [Google Scholar] [CrossRef]
- Yang, A.; Akhtar, S.S.; Iqbal, S.; Amjad, M.; Naveed, M.; Zahir, Z.A.; Jacobsen, S.E. Enhancing salt tolerance in quinoa by halotolerant bacterial inoculation. Funct. Plant Biol. 2016, 43, 632–642. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).