Constitutive and Induced Salt Tolerance Mechanisms and Potential Uses of Limonium Mill. Species
Abstract
1. Introduction
2. Limonium, an Infra-Utilised Reservoir of Species with Great Potential as New, Non-Conventional Crops
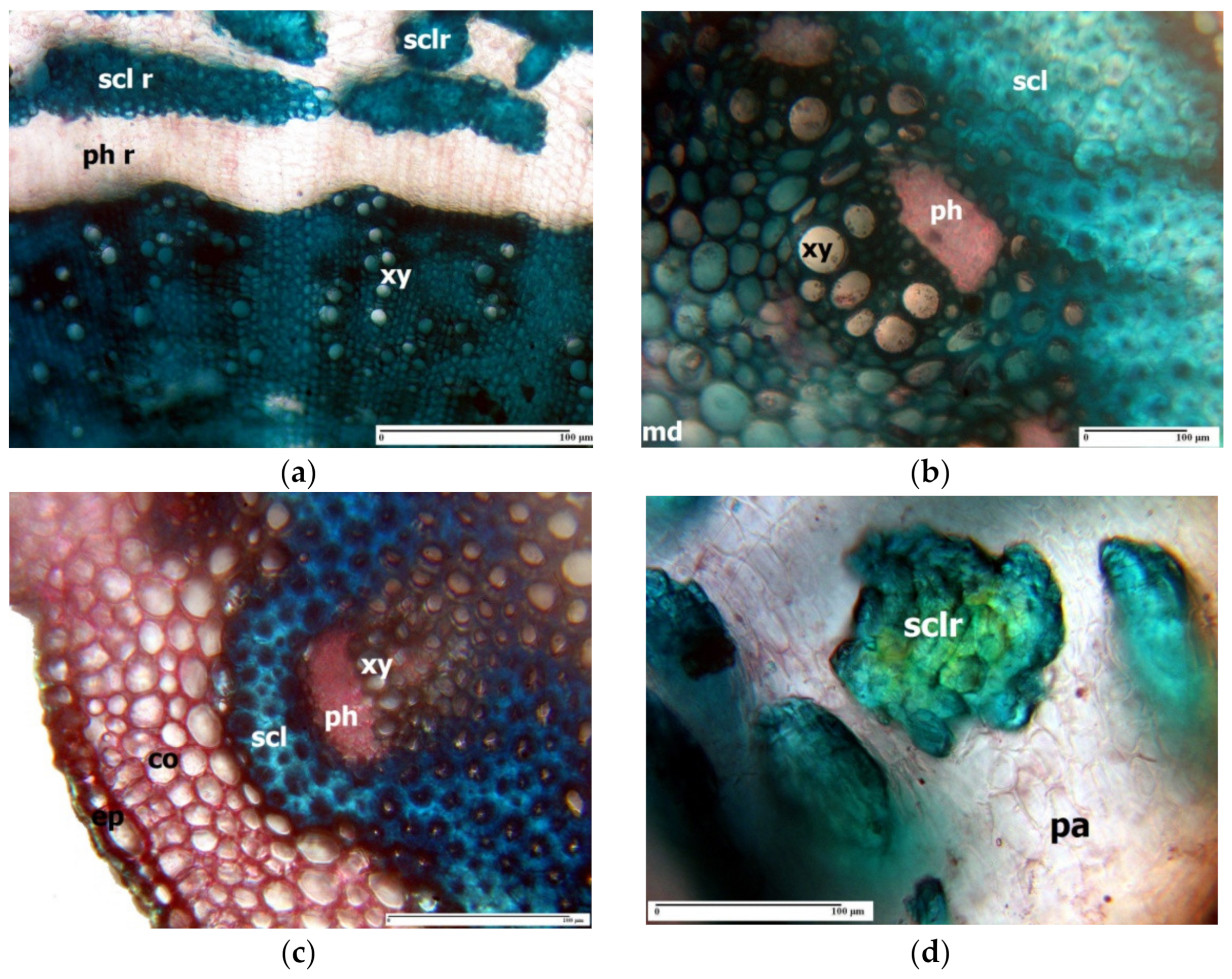
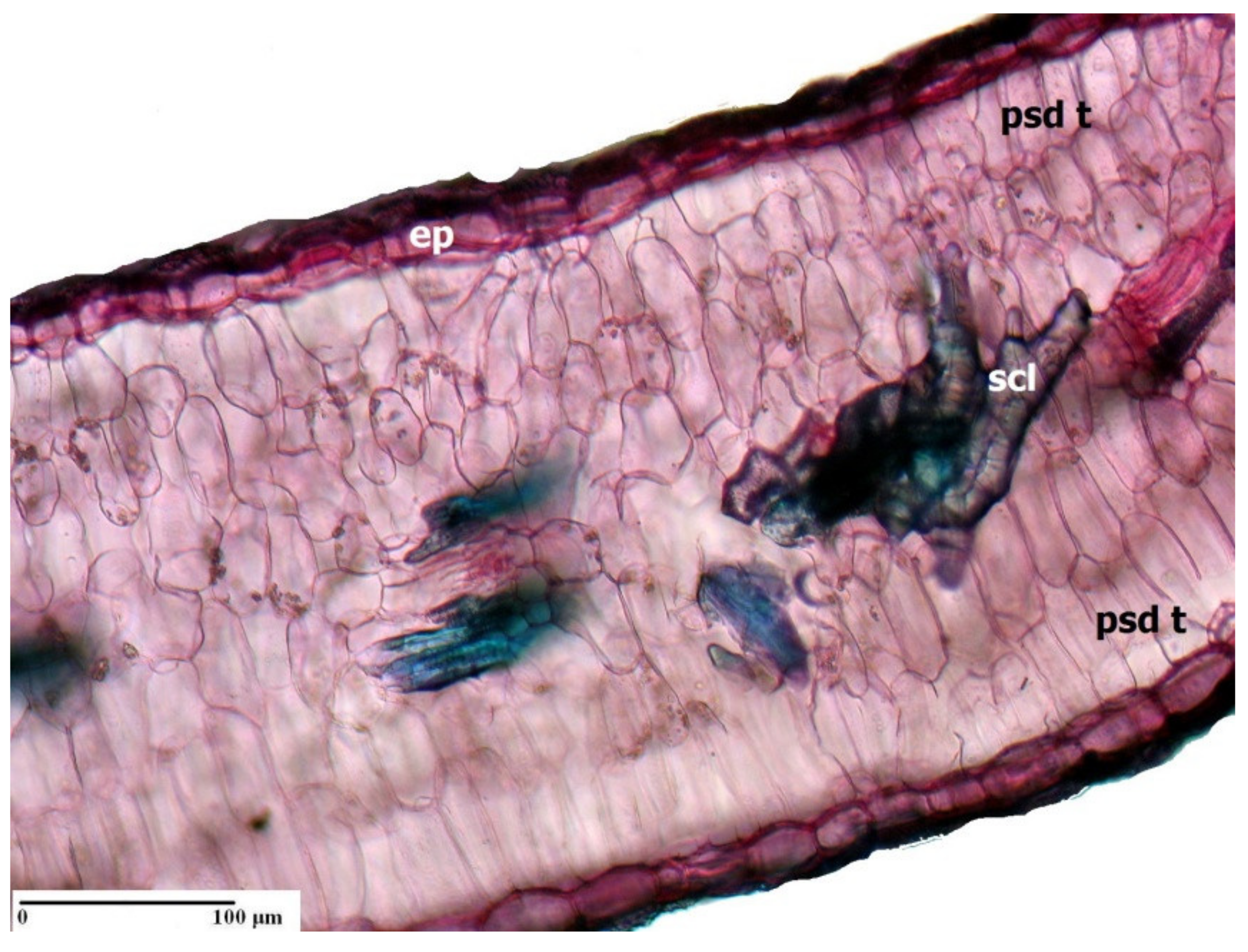
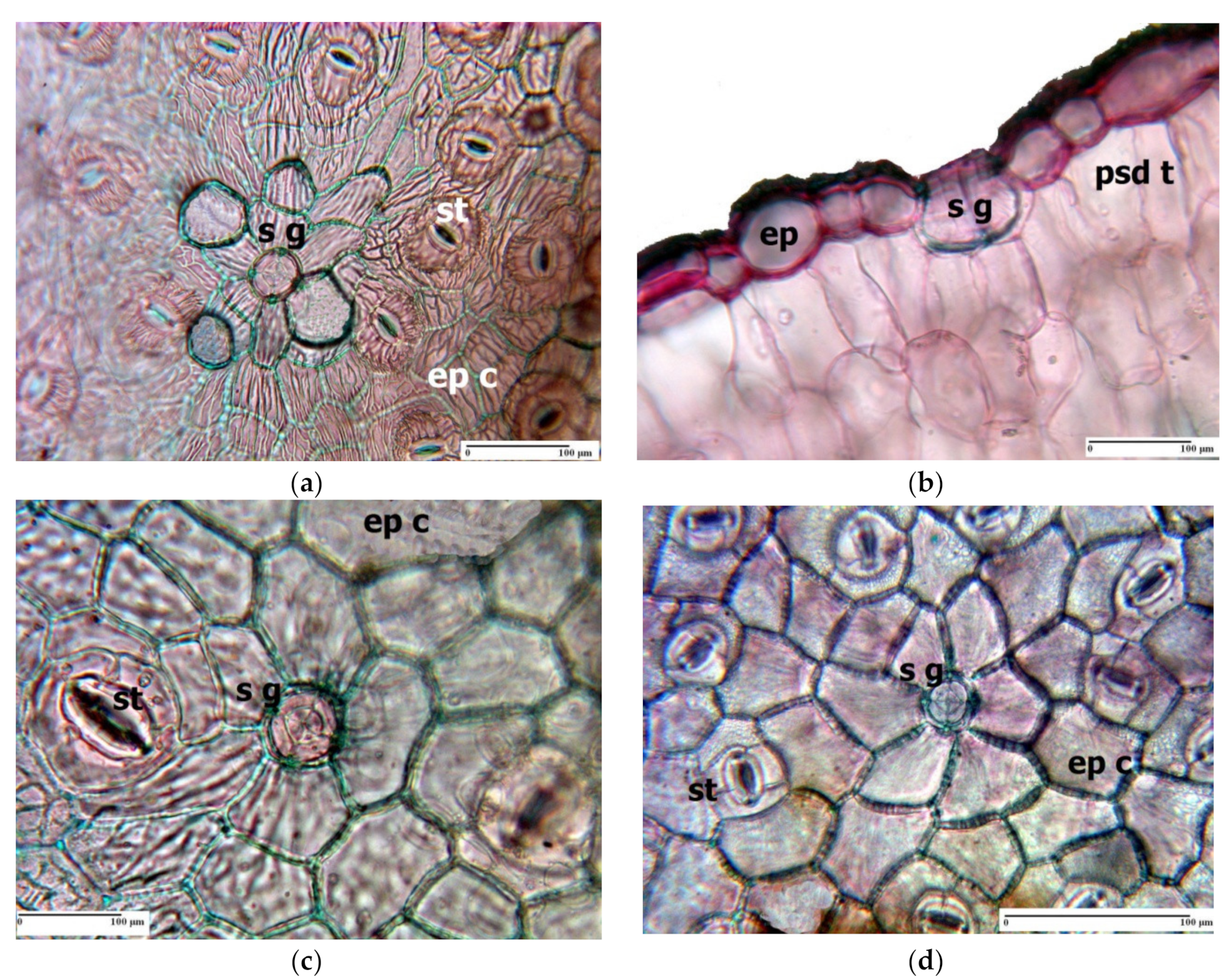
3. Morpho-Anatomical Adaptations in Limonium Species
4. Seed Germination under High Salinity, and Recovery of Germination
5. Plant Growth under Controlled Experimental Conditions
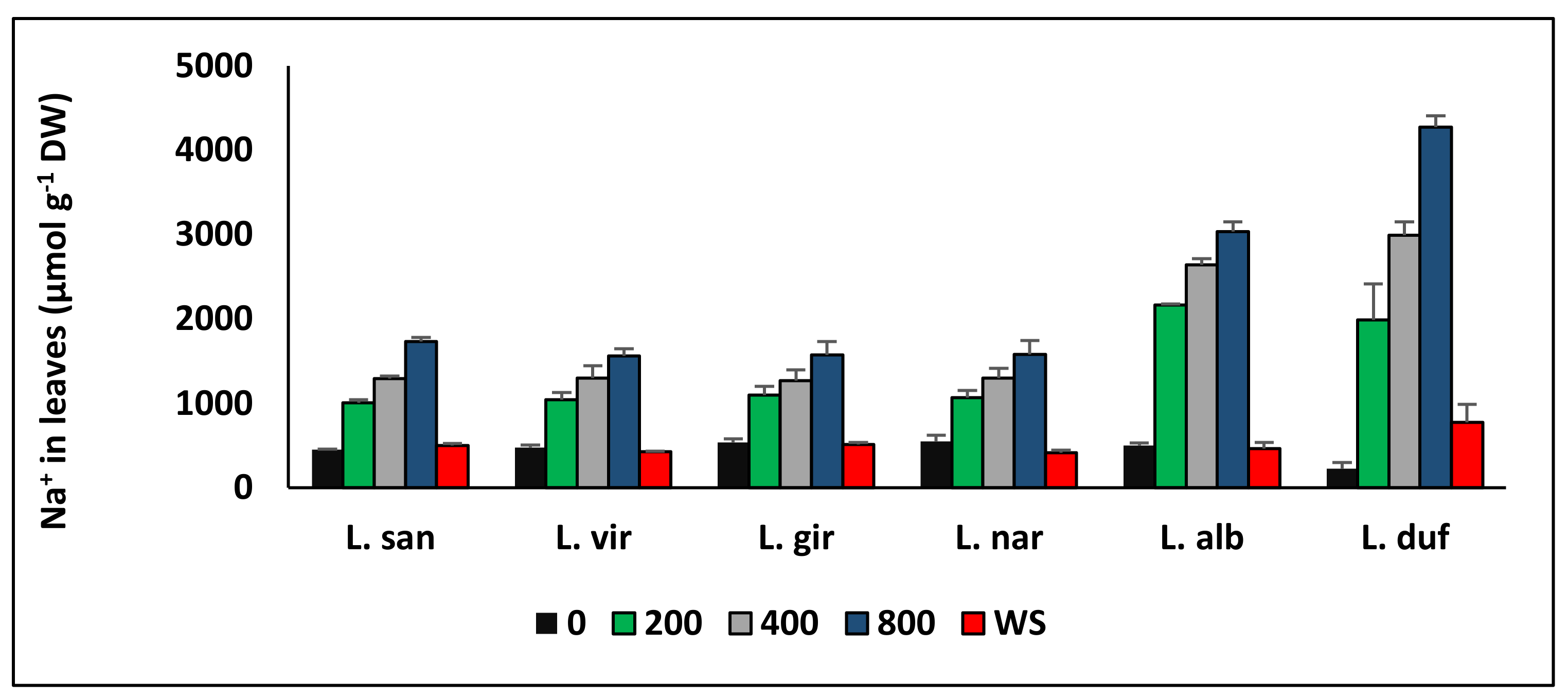
6. Ion Transport and Accumulation
7. Osmolyte Synthesis
8. Synthesis of Antioxidant Compounds and Activation of Antioxidant Enzymes
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Erben, M. Die Gattung Limonium im südwestmediterranen Raum. Mitt. Bot. Staatssamml. München. 1978, 14, 361–631. [Google Scholar]
- Koutroumpa, K.; Theodoridis, S.; Warren, B.H.; Jiménez, A.; Celep, F.; Doğan, M.; Romeiras, M.M.; Santos-Guerra, A.; Fernández-Palacios, J.M.; Caujapé-Castells, J.; et al. An expanded molecular phylogeny of Plumbaginaceae, with emphasis on Limonium (sea lavenders): Taxonomic implications and biogeographic considerations. Ecol. Evol. 2018, 1–8. [Google Scholar] [CrossRef]
- Buira, A.; Aedo, C.; Medina, L. Spatial patterns of the Iberian and Balearic endemic vascular flora. Biodivers. Conserv. 2017, 26, 479–508. [Google Scholar] [CrossRef]
- The IUCN National Red List Working Group (NRLWG). IUCN/NRLWG National Red List Database. Available online: https://www.nationalredlist.org/ (accessed on 20 April 2020).
- Laguna, E.; Fos, S.; Ferrando-Pardo, I.; Ferrer-Gallego, P.P. Endangered halophytes and their conservations: Lessons from Eastern Spain. In From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland; Heidelberg, Germany, 2020; pp. 1–64. [Google Scholar]
- European Commission. Council Directive 92/43 CEE on the Conservation of Natural Habitats and of Wild Fauna and Flora. OJL 1992, 206, 1–50. [Google Scholar]
- European Commission DG Environment. Interpretation Manual of European Union Habitats (Version EUR27); European Commission DG Environment: Brussels, Belgium, 2007. [Google Scholar]
- Ferrer-Gallego, P.P.; Navarro, A.; Pérez-Rovira, P.; Roselló, R.; Rosselló, J.A.; Rosato, M.; Laguna, E. A new polyploid species of Limonium (Plumbaginaceae) from the Western Mediterranean basin. Phytotaxa 2015, 234, 263–270. [Google Scholar] [CrossRef]
- Palacios, C.; Rosselló, J.A.; González-Candelas, F. Study of the evolutionary relationships among Limonium species (Plumbaginaceae) using nuclear and cytoplasmic molecular markers. Mol. Phylogen. Evol. 2000, 14, 232–249. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boissier, E. Plumbaginaceae. In Prodromus Systematis Naturalis Regni Vegetabilis; de Candolle, A.P., Ed.; Treuttel et Wurz: Paris, France, 1848; pp. 617–696. [Google Scholar]
- Boissier, E. Diagnoses Plantarum Orientalium Novarum; Baillière: Paris, France, 1859; pp. 61–71. [Google Scholar]
- Akhani, H.; Malekmohammadi, M.; Mahdavi, P.; Gharibiyan, A.; Chase, M.W. Phylogenetics of the Irano-Turanian taxa of Limonium (Plumbaginaceae) based on ITS nrDNA sequences and leaf anatomy provides evidence for species delimitation and relationships of lineages. Bot. J. Linn. Soc. 2013, 171, 519–550. [Google Scholar] [CrossRef]
- Lledó, M.D.; Crespo, M.B.; Fay, M.F.; Chase, M.W. Molecular phylogenetics of Limonium and related genera (Plumbaginaceae): Biogeographical and systematic implications. Am. J. Bot. 2005, 92, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Malekmohammadi, M.; Akhani, H.; Borsch, T. Phylogenetic relationships of Limonium (Plumbaginaceae) inferred from multiple chloroplast and nuclear loci. Taxon 2017, 66, 1128–1146. [Google Scholar] [CrossRef]
- Erben, M. Limonium Mill. In Flora Ibérica; Castroviejo, S., Aedo, C., Cirujano, S., Laínz, M., Montserrat, P., Morales, R., Muñoz Garmendia, F., Navarro, C., Paiva, J., Eds.; Editorial CSIC: Madrid, Spain, 1993; Volume 3, pp. 2–143. [Google Scholar]
- Al Hassan, M.; Estrelles, E.; Soriano, P.; López-Gresa, M.P.; Bellés, J.M.; Boscaiu, M.; Vicente, O. Unraveling salt tolerance mechanisms in halophytes: A comparative study on four Mediterranean Limonium species with different geographic distribution patterns. Front. Plant. Sci. 2017, 17, 1438. [Google Scholar] [CrossRef] [PubMed]
- González-Orenga, S.; Llinares, J.V.; Al Hassan, M.; Fita, A.; Collado, F.; Lisón, P.; Vicente, O.; Boscaiu, M. Physiological and morphological characterisation of Limonium species in their natural habitats: Insights into their abiotic stress responses. Plant Soil. 2020, 449, 267–284. [Google Scholar] [CrossRef]
- Yuan, F.; Leng, B.; Wang, B. Progress in studying salt secretion from the salt glands in recretohalophytes: How do plants secrete salt? Front. Plant Sci. 2016, 7, 977. [Google Scholar] [CrossRef]
- Shabala, S.; Bose, Y.; Hedrich, R. Salt bladders: Do they matter? Trends Plant Sci. 2014, 19, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Lyv, M.J.; Leng, B.Y.; Zheng, G.Y.; Feng, Z.T.; Li, P.H.; Wang, B.S. Comparative transcriptome analysis of developmental stages of the Limonium bicolor leaf generates insights into salt gland differentiation. Plant Cell. Environ. 2015, 38, 1637–1657. [Google Scholar] [CrossRef]
- Lipschitz, N.; Waisel, Y. Existence of salt glands in various genera of the Gramineae. New Phytol. 1974, 73, 507–513. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Yin, L.K.; Pan, B.R. A review on the study of salt glands of Tamarix. Acta Bot. Bor. Occid. Sin 2003, 23, 190–194. [Google Scholar]
- Grigore, M.N.; Toma, C. Anatomical Adaptations of Halophytes. A Review of Classic Literature and Recent Findings; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New. Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Türkan, I.; Demiral, T. Recent developments in understanding salinity tolerance. Environ. Exp. Bot. 2009, 67, 2–9. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Regulation of pH and generation of osmolarity in vascular plants: A cost-benefit analysis in relation to efficiency of use of energy, nitrogen and water. New Phytol. 1985, 101, 25–77. [Google Scholar] [CrossRef]
- Shabala, S.; Shabala, L. Ion transport and osmotic adjustment in plants and bacteria. Biomol. Concepts 2011, 2, 407–419. [Google Scholar] [CrossRef]
- Wyn Jones, R.; Storey, R.; Leigh, R.A.; Ahmad, N.; Pollard, A. A hypothesis on cytoplasmic osmoregulation. In Regulation of Cell membrane Activities in Plants; Marre, E., Ciferri, O., Eds.; Elsevier: Amsterdam, The Netherlands, 1977; pp. 121–136. [Google Scholar]
- Yeo, A.R. Salinity resistance: Physiologies and prices. Physiol. Plant. 1983, 58, 214–222. [Google Scholar] [CrossRef]
- Glenn, E.P.; Brown, J.J.; Blumwald, E. Salt tolerance and crop potential of halophytes. Crit. Rev. Plant Sci. 1999, 18, 227–255. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 217037. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Harikrishnan, M.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant. Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Vicente, O.; Boscaiu, M.; Naranjo, M.A.; Estrelles, E.; Bellés, J.M.; Soriano, P. Responses to salt stress in the halophyte Plantago crassifolia (Plantaginaceae). J. Arid Environ. 2004, 58, 463–481. [Google Scholar] [CrossRef]
- Ungar, I.A. Ecophysiology of Vascular Halophytes; CRC: Boca Raton, FL, USA, 1991. [Google Scholar]
- Chapman, V.J. Salt Marshes and Salt Deserts of the World. Ecol. Halophytes 1974, 79, 3–19. [Google Scholar]
- Sağlam, C.; Önder, S. The use of native halophytes in landscape design in The Central Anatolia, Turkey. Turk. J. Agric. Food Sci. Technol. 2018, 6, 1718–1726. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Llanderal, A.; Lao, M.T. Halophytes as an option for the restoration of degraded areas and landscaping. In Handbook of Halophytes, From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. Comptes Rendus Biol. 2008, 331, 865–873. [Google Scholar] [CrossRef]
- Yang, M.H.; Kim, N.H.; Heo, J.D.; Sung, S.H.; Jeong, E.J. Hepatoprotective effects of Limonium tetragonum, edible medicinal halophyte growing near seashores. Pharmacogn. Mag. 2014, 10, 563–568. [Google Scholar] [CrossRef]
- Cervelli, C.; Farina, E.; Dalla Guda, C.; Giovannini, A.; Liotta, A.; Paterniani, T.; Burchi, G.; Cacini, S.; Antonetti, M.; Zizzo, G.; et al. Development of new ornamental plants and germplasm selection in Mediterranean native species. Acta Hortic. 2012, 937, 45–50. [Google Scholar] [CrossRef]
- Burchi, G.; Mercuri, A. Results of a breeding activity on Limonium ssp. Acta Hort. 2006, 714, 43–50. [Google Scholar] [CrossRef]
- Kaninski, A.I.; Ivanova, I.; Bistrichanov, S.; Zapryanova, N.; Atanassova, B.; Iakimova, E.T. Ex situ conservation of endangered Limonium species in the Bulgarian flora. J. Fruit Ornam. Plant Res. 2012, 20, 115–129. [Google Scholar] [CrossRef]
- Lin, L.-C.; Chou, C.J. Flavonoids and phenolics from Limonium sinense. Planta Med. 2000, 66, 382–383. [Google Scholar] [CrossRef]
- Geng, D.; Chi, X.; Dong, Q.; Hu, F. Antioxidants screening in Limonium aureum by optimised online HPLC-DPPH assay. Ind. Crops Prod. 2015, 67, 492–497. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Di Gioia, F.; Ferreira, I.C.F.R.; Petropoulos, S.A. Halophytes for future horticulture. In Handbook of Halophytes. From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Medini, F.; Bourgou, S.; Lalancette, K.; Snoussi, M.; Mkadmini, K.; Coté, I.; Ksouri, R. Phytochemical analysis, antioxidant, anti-inflammatory, and anticancer activities of the halophyte Limonium densiflorum extracts on human cell lines and murine macrophages. S. Afr. J. Bot. 2015, 99, 158–164. [Google Scholar] [CrossRef]
- Blainski, A.; Gionco, B.; Oliveira, A.G.; Andrade, G.; Scarminio, I.S.; Silva, D.B.; Lopes, N.P.; Mello, J.C.P. Antibacterial activity of Limonium brasiliense (Baicuru) against multidrug-resistant bacteria using a statistical mixture design. J. Ethnopharmacol. 2017, 198, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Medini, F.; Legault, J.; Pichette, A.; Abdelly, C.; Ksouri, R. Antiviral efficacy of Limonium densiflorum against HSV-1 and influenza viruses. S. Afr. J. Bot. 2014, 92, 65–72. [Google Scholar] [CrossRef]
- Aniya, Y.; Miyagi, C.; Nakandakari, A.; Kamiya, S.; Imaizumi, N.; Ichiba, T. Free radical scavenging action of the medicinal herb Limonium wrightii from the Okinawa islands. Phytomedicine 2002, 9, 239–244. [Google Scholar] [CrossRef]
- Murray, A.P.; Rodriguez, S.; Frontera, M.A.; Tomas, M.A.; Mulet, M.C. Antioxidant metabolites from Limonium brasiliense (boiss.) Kuntze. Z. Naturforsch. C 2004, 59, 477–480. [Google Scholar] [CrossRef]
- Medini, F.; Ksouri, R.; Falleh, H.; Megdiche, W.; Trabelsi, N.; Abdelly, A.C. Effects of physiological stage and solvent on polyphenol composition, antioxidant and antimicrobial activities of Limonium densiflorum. J. Med. Plants Res. 2011, 5, 6719–6730. [Google Scholar]
- Rodrigues, M.J.; Soszynski, A.A.; Martins, A.; Rauter, A.P.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. Unravelling the antioxidant potential and the phenolic composition of different anatomical organs of the marine halophyte Limonium algarvense. Ind. Crops Prod. 2015, 77, 315–322. [Google Scholar] [CrossRef]
- Trabelsi, N.; Oueslati, S.; Falleh, H.; Waffo-Téguo, P.; Papastamoulis, Y.; Merillon, J.M.; Abdelly, C.; Riadh, K. Isolation of powerful antioxidants from the medicinal halophyte Limoniastrum guyonianum. Food Chem. 2012, 135, 1419–1424. [Google Scholar] [CrossRef]
- Ruiz-Riaguas, A.; Zengin, G.; Sinan, K.I.; Salazar-Mendías, C.; Llorent-Martínez, E.J. Phenolic profile, antioxidant activity, and enzyme inhibitory properties of Limonium delicatulum (Girard) Kuntze and Limonium quesadense Erben. J. Chem. 2020, 1016208. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Neves, V.; Martins, A.; Rauter, A.P.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. In vitro antioxidant and anti-inflammatory properties of Limonium algarvense flowers’ infusions and decoctions: A comparison with green tea (Camellia sinensis). Food Chem. 2016, 200, 322–329. [Google Scholar] [CrossRef]
- Kim, N.H.; Heo, J.D.; Rho, J.R.; Yang, M.H.; Jeong, E.J. Anti-obesity effect of halophyte crop, Limonium tetragonum in high-fat diet-induced obese mice and 3T3-L1 adipocytes. Biol. Pharm. Bull. 2017, 40, 1856–1865. [Google Scholar] [CrossRef]
- Souid, A.; Bellani, L.; Gabriele, M.; Pucci, L.; Smaoui, A.; Abdelly, C.; Hamed, K.B.; Longo, V. Phytochemical and biological activities in Limonium species collected in different biotopes of Tunisia. Chem. Biodivers. 2019, 16, e1900216. [Google Scholar] [CrossRef] [PubMed]
- Zia, S.; Khan, M.A. Effect of light, salinity, and temperature on seed germination of Limonium stocksii. Can. J. Bot. 2004, 82, 151–157. [Google Scholar] [CrossRef]
- El-Amier, Y.A.; Ejgholi, A.A. Fodder potentialities of three naturally growing in Egypt. J. Environ. Sci. 2014, 43, 647–662. [Google Scholar]
- Manousaki, E.; Kalogerakis, N. Halophytes—An Emerging Trend in Phytoremediation. Int. J. Phytoremed. 2011, 13, 959–969. [Google Scholar] [CrossRef]
- Manousaki, E.; Galanaki, K.; Papadimitriou, L.; Kalogerakis, N. Metal phytoremediation by the halophyte Limoniastrum monopetalum (L.) Boiss: Two contrasting ecotypes. Int. J. Phytoremed. 2014, 16, 755–769. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Bhowmik, P.C.; Hossain, M.A.; Rahman, M.M.; Prasad, M.N.; Ozturk, M.; Fujita, M. Potential use of halophytes to remediate saline soils. BioMed Res. Int. 2014, 589341. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Manuka, R.; Nikalje, G.C.; Penna, S. Halophytes as a potential resource for phytodesalination. In Handbook of Halophytes, From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Perrino, E.V.; Musarella, C.M.; Magazzini, P. Management of grazing Italian river buffalo to preserve habitats defined by Directive 92/43/EEC in a protected wetland area on the Mediterranean coast: Palude Frattarolo, Apulia, Italy. Euro-Mediterr. J. Environ. Integr. 2020. [Google Scholar] [CrossRef]
- Martínez Sánchez, M.J.; García Lorenzo, M.L.; Pérez Sirvent, C.; Bech, J. Trace element accumulation in plants from an aridic area affected by mining activities. J. Geochem. Explor. 2012, 123, 8–12. [Google Scholar] [CrossRef]
- Sheikh-Assadi, M.; Khandan-Mirkohi, A.; Alemardan, A.; Moreno-Jiménez, E. Mycorrhizal Limonium sinuatum (L.) Mill. Enhances accumulation of lead and cadmium. Int. J. Phytoremed. 2015, 17, 556–562. [Google Scholar] [CrossRef]
- Grigore, M.N.; Toma, C. Morphological and anatomical adaptations of halophytes: A review. In Handbook of Halophytes, From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Grigore, M.N.; Toma, C. Structure of salt glands of Plumbaginaceae. Rediscovering old findings from 19th century. ‘Mettenius’ or ‘Licopoli’ organs? J. Plant Dev. 2016, 23, 37–52. [Google Scholar]
- Grigore, M.N.; Toma, C. Integrative anatomy of halophytes from Mediterranean climate. In Handbook of Halophytes, From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Răvăruț, M. Plumbaginaceae. In Flora Republicii Populare Romîne; Săvulescu, T., Ed.; Editura Acdemiei Republicii Populare Romîne: Bucuresti, Romania, 1960; Volume 7, pp. 21–40. [Google Scholar]
- Linchevskii, L.A. Plumbaginaceae. In Flora of the U.S.S.R. (Series initiated by V. L. Komarov); Shishkin, B.K., Bobrov, E.G., Eds.; Translated from Russian; Israel Program for Scientific Translations: Jerusalem, Israel, 1967; pp. 216–348. [Google Scholar]
- Antonelli-Ushirobira, T.M.; Blainski, A.; Gancedo, N.C.; Gaburo, F.; Cardoso, K.A.K.; Leite-Melo, E.V.S.; Mello, J.C.P.; de Milaneze-Gutierre, M.A. Morpho-anatomical study of rhizome of Limonium brasiliense. Rev. Bras. Farmacogn. 2015, 25, 320–327. [Google Scholar] [CrossRef]
- Grigore, M.N.; Ivănescu, L.; Toma, C. Halophytes. An Integrative Anatomical Study; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Moțiu, T.; Toma, C.; Tiron, A.; Niță, M. Contribuții la cunoașterea structurii organelor vegetative de Limonium gmelini (Willd). Biol. Veget. 1987, 3, 11–14. [Google Scholar]
- Solereder, H. Systematic Anatomy of the Dicotyledons. A Handbook for Laboratories of Pure and Applied Botany; Clarendon: Oxford, UK, 1908; Volume 2. [Google Scholar]
- Colombo, P.; Trapani, S. Morpho-anatomical observations on three Limonium species endemic to the Pelagic Islands. Fl. Medit. 1992, 2, 77–90. [Google Scholar]
- Zoric, L.; Milic, D.; Karanovic, D.; Lukovic, J. Anatomical adaptations of halophytes within the Southern Pannonian Plain Region. In Handbook of Halophytes, From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Zoric, L.; Anackov, G.T.; Karanovic, D.; Lukovic, J. Leaf structural adaptations of two Limonium Miller (Plumbaginales, Plumbaginaceae) taxa. J. Nat. Sci. Matica Srpska Novi Sad 2013, 125, 43–54. [Google Scholar] [CrossRef]
- De Fraine, E. The morphology and anatomy of the genus Statice as represented at Blakeney Point. I. Statice binervosa G.E. Smith and Statice bellidifolia D.C. (=Statice reticulata). Ann. Bot. 1916, 30, 239–282. [Google Scholar] [CrossRef]
- Grigore, M.N.; Toma, C. Structuri Secretoare de Săruri la Halofite. O Abordare Integrativă; Academiei Române: Bucuresti, Romania, 2010. [Google Scholar]
- Yuan, F.; Wang, B. Adaptation of Recretohalophytes to Salinity. In Handbook of Halophytes, From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Caperta, A.D.; Róis, A.S.; Teixeira, G.; Garcia-Caparros, P.; Flowers, T.J. Secretory structures in plants: Lessons from the Plumbaginaceae on their origin, evolution and roles in stress tolerance. Plant. Cell. Environ. 2020, 43, 2912–2931. [Google Scholar] [CrossRef]
- Grigore, M.N.; Flowers, T.J. Evolution in angiosperm halophytes. In Handbook of Halophytes, From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Gul, B.; Ansari, R.; Flowers, T.; Khan, M.A. Germination strategies of halophyte seeds under salinity. Environ. Exp. Bot. 2013, 92, 4–18. [Google Scholar] [CrossRef]
- Kazachkova, Y.; Khan, A.; Acuña, T.; López-Díaz, I.; Carrera, E.; Khozin-Goldberg, I.; Fait, A.; Barak, S. Salt induces features of a dormancy like state in seeds of Eutrema (Thellungiella) salsugineum, a halophytic relative of Arabidopsis. Front. Plant Sci. 2016, 7, 1071. [Google Scholar] [CrossRef]
- Khan, M.A.; Gul, B. Halophyte seed germination. In Ecophysiology of High Salinity Tolerant Plants; Khan, M., Weber, D., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 11–30. [Google Scholar]
- Khan, M.A. Comparative influence of salinity and temperature on the germination of subtropical halophytes. In Halophyte Uses in Different Climates I: Ecological and Ecophysiological Studies. Progress in Biometeriology; Lieth, H., Moschenko, M., Lohman, M., Koyro, H.W., Hamdy, A., Eds.; Backhuys Publishers: Leiden, The Netherlands, 1999; pp. 77–88. [Google Scholar]
- Ungar, I.A. Seed germination and seed-bank ecology of halophytes. In Seed Development and Germination; Kigel, J., Galili, G., Eds.; Marcel and Dekker Inc.: New York, NY, USA, 1995. [Google Scholar]
- Giménez Luque, E.; Delgado-Fernández, I.; Gómez Mercado, F. Effect of salinity and temperature on seed germination in Limonium cossonianum. Botany 2013, 91, 12–16. [Google Scholar] [CrossRef]
- Delgado Fernández, I.C.; Giménez Luque, E.; Gómez Mercado, F.; Marrrero, J.M. Germination responses of Limonium insigne (Coss.) Kuntze to salinity and temperature. Pak. J. Bot. 2015, 47, 807–812. [Google Scholar]
- Delgado Fernández, I.C.; Giménez Luque, E.; Gómez Mercado, F.; Pedrosa, V. Influence of temperature and salinity on the germination of Limonium tabernense Erben from Tabernas Desert (Almería, SE Spain). Flora 2016, 218, 68–74. [Google Scholar] [CrossRef]
- Fos, M.; Alfonso, L.; Ferrer-Gallego, P.P.; Laguna, E. Effect of salinity, temperature and hypersaline conditions on the seed germination in Limonium mansanetianum an endemic and threatened Mediterranean species. Plant Biosyst. 2020, 155, 165–171. [Google Scholar] [CrossRef]
- Melendo, M.; Giménez, E. Seed germination responses to salinity and temperature in Limonium supinum (Plumbaginaceae), an endemic halophyte from Iberian Peninsula. Plant Biosyst. 2019, 153, 257–263. [Google Scholar] [CrossRef]
- González-Orenga, S.; Ferrer-Gallego, P.P.; Laguna, E.; López-Gresa, M.P.; Donat-Torres, M.P.; Verdeguer, M.; Vicente, O.; Boscaiu, M. Insights on salt tolerance of two endemic Limonium species from Spain. Metabolites 2019, 9, 294. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Mateos Naranjo, E.; Garzón, O.; Castillo, J.M.; Luque, T.; Figueroa, M.E. Effects of salinity on germination and seedling establishment of endangered Limonium emarginatum (Willd.) O. Kuntze. J. Coast. Res. 2008, 24, 201–205. [Google Scholar] [CrossRef]
- Monllor, M.; Soriano, P.; Llinares, J.V.; Boscaiu, M.; Estrelles, E. Assessing effects of temperature change on four Limonium species from threatened Mediterranean salt-affected habitats. Not. Bot. Horti Agrob. 2018, 46, 286–291. [Google Scholar] [CrossRef]
- Yildiz, M.; Cenkci, S.; Kargioglu, M. Effects of salinity, temperature, and light on seed germination in two Turkish endemic halophytes, Limonium iconicum and L. lilacinum (Plumbaginaceae). Seed Sci. Technol. 2008, 36, 646–656. [Google Scholar] [CrossRef]
- Kleemann, S.G.L.; Gill, G. Seed germination and seedling recruitment behavior of winged sea lavender (Limonium lobatum) in Southern Australia. Weed Sci. 2018, 66, 485–493. [Google Scholar] [CrossRef]
- Mahmoud, A.; El Sheikh, A.M.; Abdul Baset, S. Germination of two halophytes: Halopeplis perfoliata and Limonium axillare from Saudi Arabia. J. Arid Environ. 1983, 6, 87–98. [Google Scholar] [CrossRef]
- Woodell, S.R.J. Salinity and seed germination patterns in coastal plants. Vegetation 1985, 61, 223–229. [Google Scholar] [CrossRef]
- Ungar, I.A. Influence of salinity on seed germination in succulent halophytes. Ecology 1962, 3, 329–335. [Google Scholar] [CrossRef]
- Ungar, I.A. Influence of salinity and temperature on seed germination. Ohio J. Sci. 1967, 67, 120–123. [Google Scholar]
- Yuan, F.; Guo, J.; Shabala, S.; Wang, B. Reproductive physiology of halophytes: Current standing. Front. Plant Sci. 2019, 9, 1954. [Google Scholar] [CrossRef]
- Flowers, T.J.; Hajibagheri, M.A.; Clipson, N.J.W. Halophytes. Q. Rev. Biol. 1986, 61, 313–335. [Google Scholar] [CrossRef]
- Al Hassan, M.; Morosan, M.; López-Gresa, M.D.P.; Prohens, J.; Vicente, O.; Boscaiu, M. Salinity-induced aariation in biochemical markers provides insight into the mechanisms of salt tolerance in common (Phaseolus vulgaris) and runner (P. coccineus) beans. Int. J. Mol. Sci. 2016, 17, 1582. [Google Scholar] [CrossRef]
- González-Orenga, S.; Al Hassan, M.; Llinares, J.V.; Lisón, P.; López-Gresa, M.P.; Verdeguer, M.; Vicente, O.; Boscaiu, M. Qualitative and quantitative differences in osmolytes accumulation and antioxidant activities in response to water deficit in four Mediterranean Limonium species. Plants 2019, 8, 506. [Google Scholar] [CrossRef]
- Hameed, A.; Gulzar, S.; Aziz, I.; Hussain, T.; Gul, B.; Khan, M.A. Effects of salinity and ascorbic acid on growth, water status and antioxidant system in a perennial halophyte. AoB Plants 2015, 7, plv004. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Song, J.; Ruan, Y.; Wang, B.S. Comparison of the effects of NaCl and KCl at the roots on seedling growth, cell death and the size, frequency and secretion rate of salt glands in leaves of Limonium sinense. Acta Physiol. Plant. 2009, 31, 343–350. [Google Scholar] [CrossRef]
- Morales, M.A.; Olmos, E.; Torrecillas, A.; Sánchez-Blanco, M.J.; Alarcon, J.J. Differences in water relations, leaf ion accumulation and excretion rates between cultivated and wild species of Limonium sp. grown in conditions of saline stress. Flora 2001, 196, 345–352. [Google Scholar] [CrossRef]
- Souid, A.; Gabriele, M.; Longo, V.; Pucci, L.; Bellani, L.; Smaoui, A.; Abdelly, C.; Hamed, K. Salt tolerance of the halophyte Limonium delicatulum is more associated with antioxidant enzyme activities than phenolic compounds. Function. Plant Biol. 2016, 43, 607–619. [Google Scholar] [CrossRef]
- Grieve, C.M.; Poss, J.A.; Grattam, S.R.; Sheuse, P.J.; Lieth, J.H.; Zeng, L. Productivity and mineral nutrition of Limonium species irrigated with saline wastewaters. Hort. Sci. 2005, 40, 654–658. [Google Scholar] [CrossRef]
- Li, Y. Kinetics of the antioxidant response to salinity in the halophyte Limonium bicolor. Plant Soil Environ. 2008, 54, 493–497. [Google Scholar] [CrossRef]
- Xianzhao, L.; Chunzhi, W.; Qing, S. Screening for salt tolerance in eight halophyte species from Yellow River Delta at the two initial growth stages. ISRN Agronomy 2013. [Google Scholar] [CrossRef]
- Hamed, K.B.; Chibani, F.; Abdelly, C.; Magne, C. Growth, sodium uptake and antioxidant responses of coastal plants differing in their ecological status under increasing salinity. Biologia 2014, 69, 193–201. [Google Scholar] [CrossRef]
- Flowers, T.J. Salt tolerance in Suaeda maritima (L.) Dum: The effect of sodium chloride on growth, respiration, and soluble enzymes in a comparative study with Pisum sativum. J. Exp. Bot. 1972, 23, 310–321. [Google Scholar] [CrossRef]
- Khan, M.A.; Ungar, I.A.; Showalter, A.M. The effect of salinity on the growth, water status, and ion content of a leaf succulent perennial halophyte, Suaeda fruticosa (L.) Forssk. J. Arid Environ. 2000, 45, 73–84. [Google Scholar] [CrossRef]
- Hameed, A.; Hussain, T.; Gulzar, S.; Aziz, I.; Gul, B.; Khan, M. Salt tolerance of a cash crop halophyte Suaeda fruticosa: Biochemical responses to salt and exogenous chemical treatments. Acta Physiol. Plant. 2012, 34, 2331–2340. [Google Scholar] [CrossRef]
- Tabot, P.T.; Adams, J.B. Salt secretion, proline accumulation and increased branching confer tolerance to drought and salinity in the endemic halophyte Limonium linifolium. S. Afr. J. Bot. 2014, 94, 64–73. [Google Scholar] [CrossRef]
- Leng, B.Y.; Yuan, F.; Dong, X.X.; Wang, J.; Wang, B.S. Distribution pattern and salt excretion rate of salt glands in two recretohalophyte species of Limonium (Plumbaginaceae). S. Afr. J. Bot. 2018, 115, 74–80. [Google Scholar] [CrossRef]
- Ramadan, T. Ecophysiology of salt excretion in the xerohalophyte Reaumuria hirtella. New Phytol. 1998, 139, 273–281. [Google Scholar] [CrossRef]
- Hill, A.E. Ion and water transport in Limonium: I. Active transport by the leaf gland cells. Biochim. Biophys. Acta (BBA) Biomembr. 1967, 135, 454–460. [Google Scholar] [CrossRef]
- Zia, S.; Egan, T.; Khan, M. Growth and selective ion transport of Limonium stocksii (Plumbaginaceae) under saline conditions. Pak. J. Bot. 2008, 40, 697–709. [Google Scholar]
- Wang, S.; Changgui, W.; Yanrong, W.; Chen, H.; Zhou, Z.; Fu, H.; Sosebee, R.E. The characteristics of Na+, K+ and free proline distribution in several drought resistant plants of the Alxa Desert, China. J. Arid Environ. 2004, 56, 525–539. [Google Scholar] [CrossRef]
- Lv, S.; Jiang, P.; Chen, X.; Fan, P.; Wang, X.; Li, Y. Multiple compartmentalisation of sodium conferred salt tolerance in Salicornia europaea. Plant Physiol. Biochem. 2012, 51, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Borsai, O.; Hassan, M.A.; Negrușier, C.; Raigón, M.D.; Boscaiu, M.; Sestraș, R.E.; Vicente, O. Responses to salt stress in Portulaca: Insight into its tolerance mechanisms. Plants 2020, 9, 1660. [Google Scholar] [CrossRef] [PubMed]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef]
- Alarcon, J.J.; Morales, M.A.; Torrecillas, A.; Sanchez-Blango, M.J. Growth, water relations and accumulation of organic and inorganic solutes in the halophytes Limonium latifolium cv. avignon and its interspecific hybrid Limonium caspia X Limonium latifolium cv. bettlaard during salt stress. J. Plant Physiol. 1999, 154, 795–801. [Google Scholar] [CrossRef]
- Flowers, T.J.; Yeo, A.R. Ion relation of salt tolerance. In Solute Transport in Plant Cells and Tissues; Baker, D.A., Hall, J.L., Eds.; Longman Scientific and Technical: Harlow, UK, 1988; pp. 392–413. [Google Scholar]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.J.; Chen, H.Y.; Bai, W.P.; Yang, R.C.; Yang, P.Z.; Chen, R.J.; Hu, T.M.; Wang, S.M. Sodium-related adaptations to drought: New insights from the xerophyte plant Zygophyllum xanthoxylum. Front. Plant Sci. 2018, 9, 1678. [Google Scholar] [CrossRef]
- Carter, C.T.; Grieve, C.M.; Poss, J.A. Salinity effects on emergence, survival, and ion accumulation of Limonium perezii. J. Plant. Nutr. 2005, 28, 1243–1257. [Google Scholar] [CrossRef]
- Greenway, H.; Munns, R. Mechanisms of salt tolerance in non-halophytes. Annu. Rev. Plant Physiol. 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Gil, R.; Bautista, I.; Boscaiu, M.; Lidón, A.; Wankhade, S.; Sánchez, H.; Llinares, J.; Vicente, O. Responses of five Mediterranean halophytes to seasonal changes in environmental conditions. AoB Plants 2014, 6, plu049. [Google Scholar] [CrossRef] [PubMed]
- Percey, W.J.; Shabala, L.; Wu, Q.; Su, N.; Breadmore, M.C.; Guijt, R.M.; Bose, J.; Shabala, S. Potassium retention in leaf mesophyll as an element of salinity tissue tolerance in halophytes. Plant Physiol. Biochem. 2016, 109, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Assaha, D.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, S.; Fahad, S.; Anjum, M.A.; Nawaz, A.; Naz, S.; Hussain, S.; Ahmad, S. Role of osmolytes in the mechanisms of antioxidant defense of plants. Sustain. Agric. Rev. 2020, 39. [Google Scholar] [CrossRef]
- Tipirdamaz, R.; Gagneul, D.; Duhazé, C.; Aïnouche, A.; Monnier, C.; Özkum, D.; Larher, F. Clustering of halophytes from an inland salt marsh in Turkey according to their ability to accumulate sodium and nitrogenous osmolytes. Environ. Exp. Bot. 2006, 57, 139–153. [Google Scholar] [CrossRef]
- Gagneul, D.; Aïnouche, A.; Duhazé, C.; Lugan, R.; Larher, F.R.; Bouchereau, A. A reassessment of the function of the so-called compatible solutes in the halophytic Plumbaginaceae Limonium latifolium. Plant Physiol. 2007, 144, 1598–1611. [Google Scholar] [CrossRef]
- Al Hassan, M.; Pacurar, A.; López-Gresa, M.P.; Donat-Torres, M.P.; Llinares, J.V.; Boscaiu, M.; Vicente, O. Effects of salt stress on three ecologically distinct Plantago species. PLoS ONE 2016, 11, e0160236. [Google Scholar] [CrossRef]
- Al Hassan, M.; Chaura, J.; Donat-Torres, M.; Boscaiu, M.; Vicente, O. Antioxidant responses under salinity and drought in three closely related wild monocots with different ecological optima. AoB Plants 2017, 9, plx009. [Google Scholar] [CrossRef]
- Rhodes, D.; Nadolska-Orczyk, A.; Rich, P. Salinity, osmolytes and compatible solutes. In Salinity: Environment-Plants-Molecules; Läuchli, A., Lüttge, U., Eds.; Springer: Dordrecht, The Netherlands, 2002. [Google Scholar] [CrossRef]
- Youssef, A.M.; Hassanein, R.A.; Hassanein, A.A.; Morsy, A.A. Changes in quaternary ammonium compounds, proline and protein profiles of certain halophytic plants under different habitat conditions. Pak. J. Biol. Sci. 2003, 6, 867–882. [Google Scholar] [CrossRef][Green Version]
- Yasseen, B.T.; Abu-Al-Basal, M.A. Ecophysiology of Limonium axillare and Avicennia marina from the coastline of Arabian Gulf-Qatar. Coast Conserv. 2008, 12, 35–42. [Google Scholar] [CrossRef]
- Furtana, G.B.; Dumani, H.; Tipirdamaz, R. Seasonal changes of inorganic and organic osmolyte content in three endemic Limonium species of Lake Tuz (Turkey). Turk. J. Bot. 2013, 37, 455–463. [Google Scholar] [CrossRef]
- Hanson, A.D.; Rathinasabapathi, B.; Chamberlin, B.; Gage, D.A. Comparative physiological evidence that beta-alanine betaine and choline-O-sulfate act as compatible osmolytes in halophytic Limonium species. Plant Physiol. 1991, 97, 1199–1205. [Google Scholar] [CrossRef]
- Hanson, A.D.; Rathinasabapathi, B.; Rivoal, J.; Burnet, M.; Dillon, M.O.; Gage, D.A. Osmoprotective compounds in the Plumbaginaceae: A natural experiment in metabolic engineering of stress tolerance. Proc. Natl. Acad. Sci. USA 1994, 91, 306–310. [Google Scholar] [CrossRef]
- Liu, X.; Duan, D.; Li, W.; Tadano, T.; Khan, A.M. A comparative study on responses of growth and solute composition in halophytes Suaeda Salsa and Limonium Bicolor to salinity. In Ecophysiology of High Salinity Tolerant Plant. Tasks for Vegetation Science; Springer: Dordrecht, The Netherlands, 2006; Volume 40. [Google Scholar] [CrossRef]
- Bouchereau, A.; Duhazé, C.; Martin-Tanguy, J.; Guégan, J.P.; Larher, F. Improved analytical methods for determination of nitrogenous stress metabolites occurring in Limonium species. J. Chromatogr. 1999, 836, 209–221. [Google Scholar] [CrossRef]
- Murakeözy, É.P.; Smirnoff, N.; Nagy, Z.; Tuba, Z. Seasonal accumulation pattern of pinitol and other carbohydrates in Limonium gmelinii subsp. Hungarica. J. Plant Physiol. 2002, 159, 485–490. [Google Scholar]
- Murakeözy, É.P.; Nagy, Z.; Duhazé, C.; Bouchereau, A.; Tuba, Z. Seasonal changes in the levels of compatible osmolytes in three halophytic species of inland saline vegetation in Hungary. J. Plant Physiol. 2003, 160, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Grieve, C. Accumulation of chiro-inositol and other non-structural carbohydrates in Limonium species in response to saline irrigation waters. J. Am. Soc. Hortic. Sci. 2009, 134, 329–336. [Google Scholar] [CrossRef]
- Akat, Ö.; Çakar, H. Yield response of Limonium sinuatum cultivars under salinity stress. J. Environ. Biol. 2020, 41, 302–309. [Google Scholar] [CrossRef]
- Saibi, W.; Brini, F. Proline, a peculiar amino acid with astucious functions in development and salt tolerance process in plants. J. Food Nutr. Metab. 2020, 3, 1–8. [Google Scholar] [CrossRef]
- Rhodes, D.; Hanson, A.D. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 357–384. [Google Scholar] [CrossRef]
- Rivoal, J.; Hanson, A. Choline-O-sulfate biosynthesis in plants (Identification and partial characterisation of a salinity-inducible choline sulfotransferase from species of Limonium (Plumbaginaceae). Plant Physiol. 1994, 106, 1187–1193. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Černý, M.; Habánová, H.; Berka, M.; Luklová, M.; Brzobohatý, B. Hydrogen peroxide: Its role in plant biology and crosstalk with signalling networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef] [PubMed]
- Barsukova, M.E.; Veselova, I.A.; Shekhovtsova, T.N. Main methods and approaches to the determination of markers of oxidative stress—Organic peroxide compounds and hydrogen peroxide. J. Anal. Chem. 2019, 74, 425–436. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant responses of wheat plants under stress. Genet. Mol. Biol. 2016, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gunes, A.; Pilbeam, D.J.; Inal, A.; Bagci, E.G.; Coban, S. Influence of silicon on antioxidant mechanisms and lipid peroxidation in chickpea (Cicer arietinum L.) cultivars under drought stress. J. Plant Interact. 2007, 2, 105–113. [Google Scholar] [CrossRef]
- Rao, A.C.; Reddy, A.R. Glutathione reductase: A putative redox regulatory system in plant cells. In Sulfur Assimilation and Abiotic Stress in Plants; Khan, N.A., Singh, S., Umar, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 111–147. [Google Scholar]
- Fini, A.; Brunetti, C.; di Ferdinando, M.; Ferrini, F.; Tattini, M. Stress-induced flavonoid biosynthesis and th3 antioxidant machinery of plants. Plant Signal. Behav. 2011, 6, 709–711. [Google Scholar] [CrossRef]
- Davies, K.M.; Albert, N.W.; Zhou, Y.; Schwinn, K.E. Functions of flavonoid and betalain pigments in abiotic stress tolerance in plants. In Annual Plant Reviews Online; Roberts, J.A., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Jithesh, M.N.; Prashanth, S.R.; Sivaprakash, K.R.; Parida, A.K. Antioxidative response mechanisms in halophytes: Their role in stress defence. J. Gen. 2006, 85, 237–254. [Google Scholar] [CrossRef]
- Ozgur, R.; Uzilday, B.; Sekmen, A.H.; Turkan, I. Reactive oxygen species regulation and antioxidant defence in halophytes. Funct. Plant Biol. 2013, 40, 832–847. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Souid, A.; Bellani, L.; Magné, C.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Longo, V.; Hamed, B.K. Physiological and antioxidant responses of the sabkha biotope halophyte Limonium delicatulum to seasonal changes in environmental conditions. Plant Physiol. Biochem. 2018, 123, 180–191. [Google Scholar] [CrossRef] [PubMed]




| Species | Treatment | Compatible Solutes | Reference |
|---|---|---|---|
| L. albuferae | salt | Glu, Fru, Pro, GABA | [99] |
| L. axillare | field | βAB, Pro | [147] |
| field | Pro | [148] | |
| L. anatolicum | field | βAB, Cho, Glu, Fru; Pro | [149] |
| L. aureum | salt | βAB; COS | [150] |
| L. diffusum | field | PB | [151] |
| L. bicolor | salt | Pro | [152] |
| L. dufourii | salt | Pro, GABA | [99] |
| L. dumosum | βAB; COS; Pro; Tryamine | [153] | |
| L. ferulaceum | PB 146 | [151] | |
| L. girardianum | salt | Pro, Fru, Suc | [16] |
| drought | Pro, GB, Glc | [111] | |
| field | Pro, Fru | [17] | |
| L. globuliferum | field | Pro, COS, βAB | [142,151] |
| L. iconicum | field | GB, COS, βAB | [142] |
| field | GB, βAB, Cho, Glu, Fru | [149] | |
| L. latifolium | field | βAB; COS | [151] |
| Hoagland | βAB; COS; Pro | [153] | |
| salt | Gln, Suc, Fru, Glc, cInos, mInos | [143] | |
| L. lilacinum | field | βAB, Cho, Glu, Fru, Pro | [149] |
| L. gmelinii | Hoagland | βAB; COS; Pro, Tryamine; Glutamate; Methionine | [153] |
| field | Pin | [154] | |
| field | Pin, βAB, COS | [155] | |
| L. liniifolium | drought | Pro, oxalic acid | [123] |
| L. guyonianum | field | PB, Hydroxyproline betaine | [151] |
| L. macrophyllum | field | Pro, COS | [151] |
| L. mucrontaum | field | Pro, COS | [151] |
| L. monopetalum | Hydroxyproline betaine; PB | [151] | |
| L. narbononense | salt | Pro, GB, Fru | [16] |
| drought | R, GB | [111] | |
| field | Pro, Gb, Fru | [17] | |
| L. pectinatum | field | Pro, COS | [151] |
| L. perezii | salt | GB, COS | [151] |
| Glutamate, Tyrosine, Methionine, Ornithine, GB; COS | [153] | ||
| salt | cInos, mInos, Fru, Glc, Suc | [156] | |
| L. plumosum | field | Pro, COS | [151] |
| L. puberulum | field | Pro, COS | [151] |
| L. salicorniaceum | field | Hydroxyproline betaine | [151] |
| L. santapolense | salt | GB, Fru, Suc | [16] |
| drought | Pro, Suc, Fru, GB | [111] | |
| field | Suc, Fru | [18] | |
| L. sinuatum | salt | GB, COS | [150] |
| Hoagland | GB, COS, Glutamate | [153] | |
| salt | cInos, mInos Fru, Glc, Suc | [156] | |
| salt | Pro | [157] | |
| L. tataricum | Hoagland | βAB; COS; Pro | [153] |
| L. virgatum | salt | Pro, GB, Suc | [16] |
| drought | GB | [111] | |
| field | Fru, GB, Pro | [17] | |
| L. vulgare | salt | βAB; COS | [150] |
| Hoagland | Tryamine, Pro; βAB; COS | [153] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Orenga, S.; Grigore, M.-N.; Boscaiu, M.; Vicente, O. Constitutive and Induced Salt Tolerance Mechanisms and Potential Uses of Limonium Mill. Species. Agronomy 2021, 11, 413. https://doi.org/10.3390/agronomy11030413
González-Orenga S, Grigore M-N, Boscaiu M, Vicente O. Constitutive and Induced Salt Tolerance Mechanisms and Potential Uses of Limonium Mill. Species. Agronomy. 2021; 11(3):413. https://doi.org/10.3390/agronomy11030413
Chicago/Turabian StyleGonzález-Orenga, Sara, Marius-Nicusor Grigore, Monica Boscaiu, and Oscar Vicente. 2021. "Constitutive and Induced Salt Tolerance Mechanisms and Potential Uses of Limonium Mill. Species" Agronomy 11, no. 3: 413. https://doi.org/10.3390/agronomy11030413
APA StyleGonzález-Orenga, S., Grigore, M.-N., Boscaiu, M., & Vicente, O. (2021). Constitutive and Induced Salt Tolerance Mechanisms and Potential Uses of Limonium Mill. Species. Agronomy, 11(3), 413. https://doi.org/10.3390/agronomy11030413









