Biochar Promotes Nitrogen Transformation and Tomato Yield by Regulating Nitrogen-Related Microorganisms in Tomato Cultivation Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Materials
2.2. Experimental Setup
2.3. Measurements
2.3.1. Soil Water Content and Water Use Efficiency
2.3.2. Tomato Yield and Total N Uptake (TN) of Tomato Plants
2.3.3. NO3− and NH4+ of the Soil
2.3.4. Soil Enzyme Activity and the Quantification of Nitrifying and Denitrifying Bacteria
2.3.5. Microbial Biomass Analysis
2.3.6. Partial-Factor Productivity of N and Cost–Benefit Analysis
2.4. Statistical Analysis
3. Results
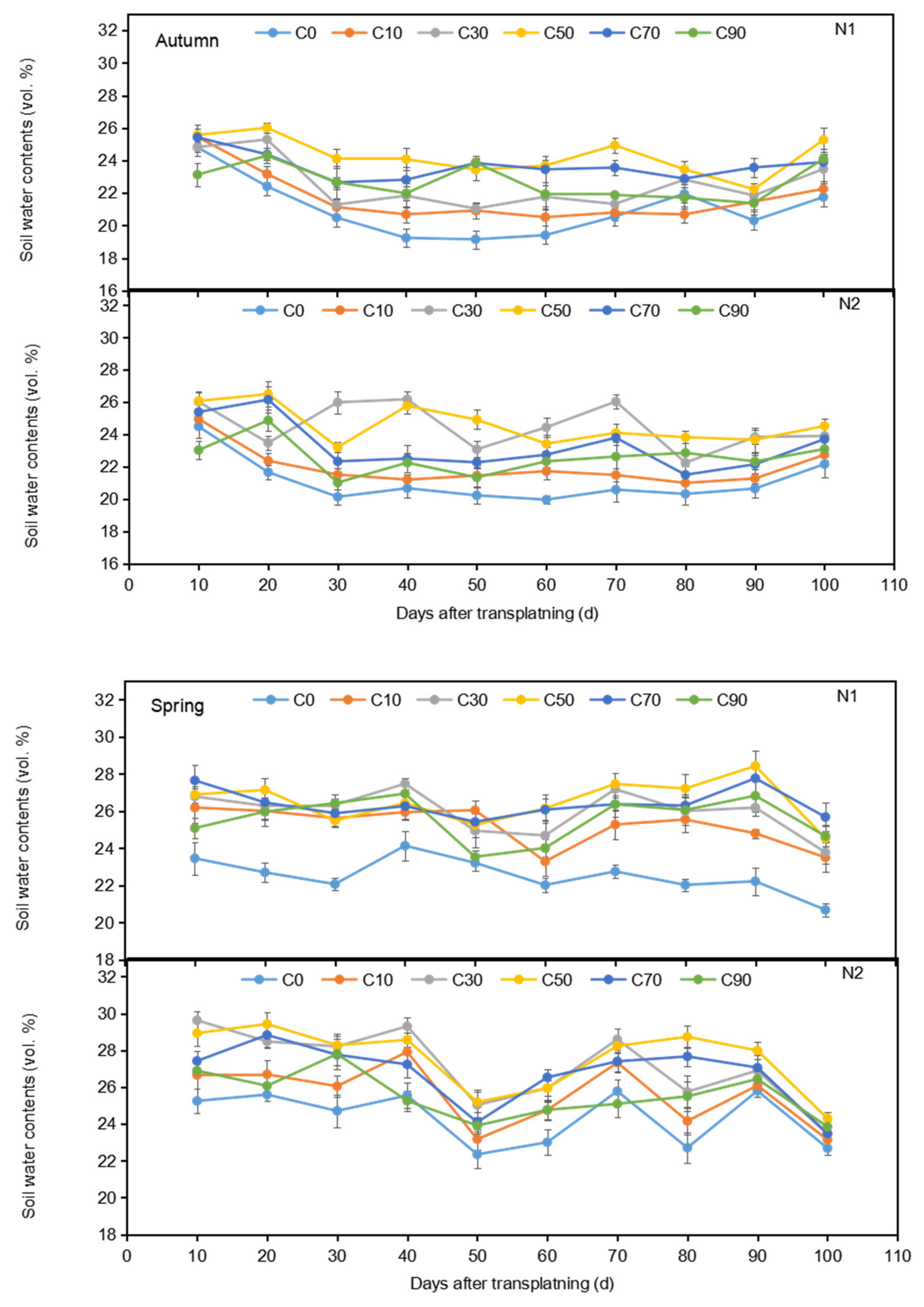
3.1. Soil Water Content
3.2. Soil Enzyme Activity and Abundance of Nitrifying and Denitrifying Bacteria
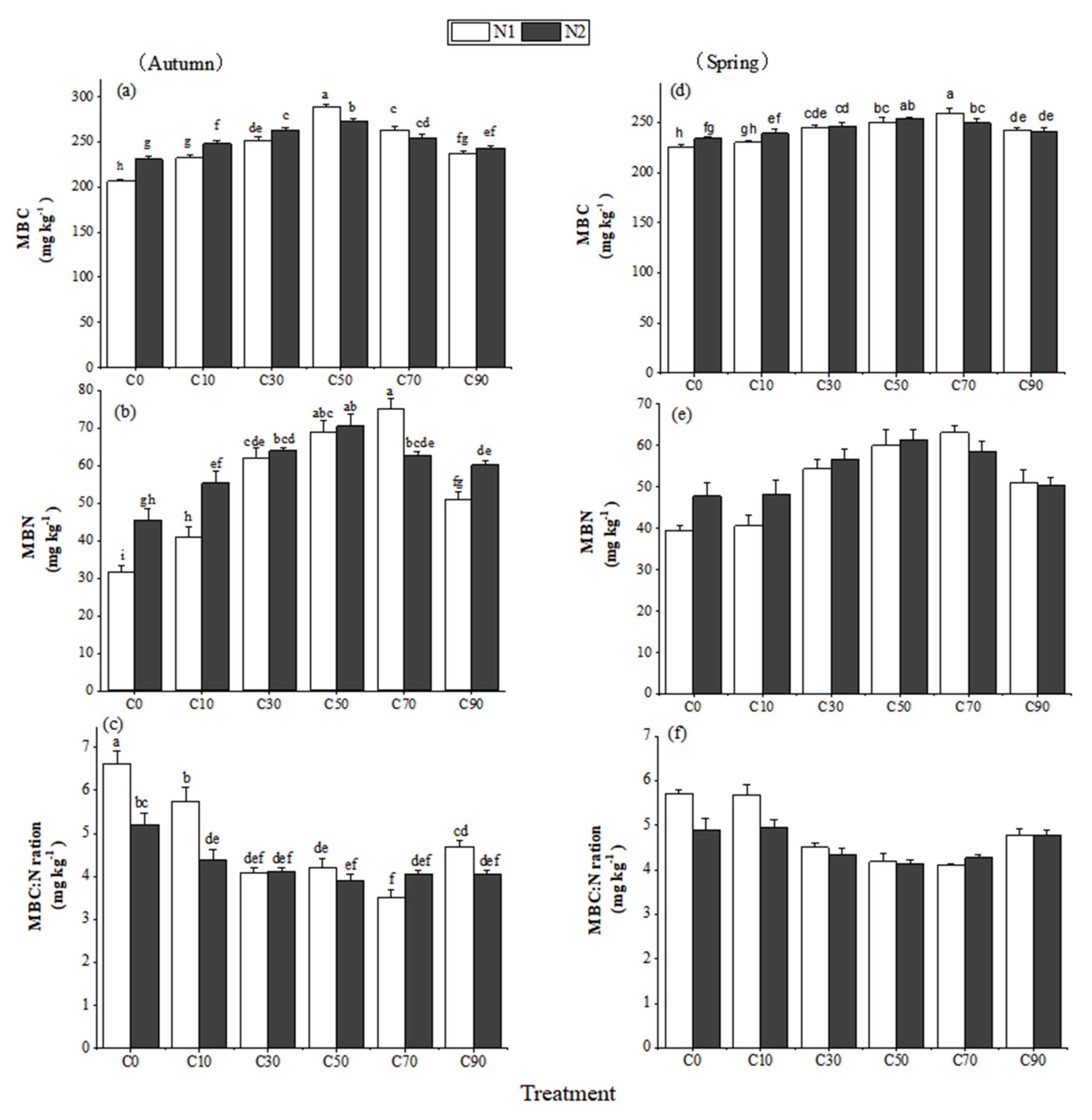
3.3. Soil Microbial Biomass
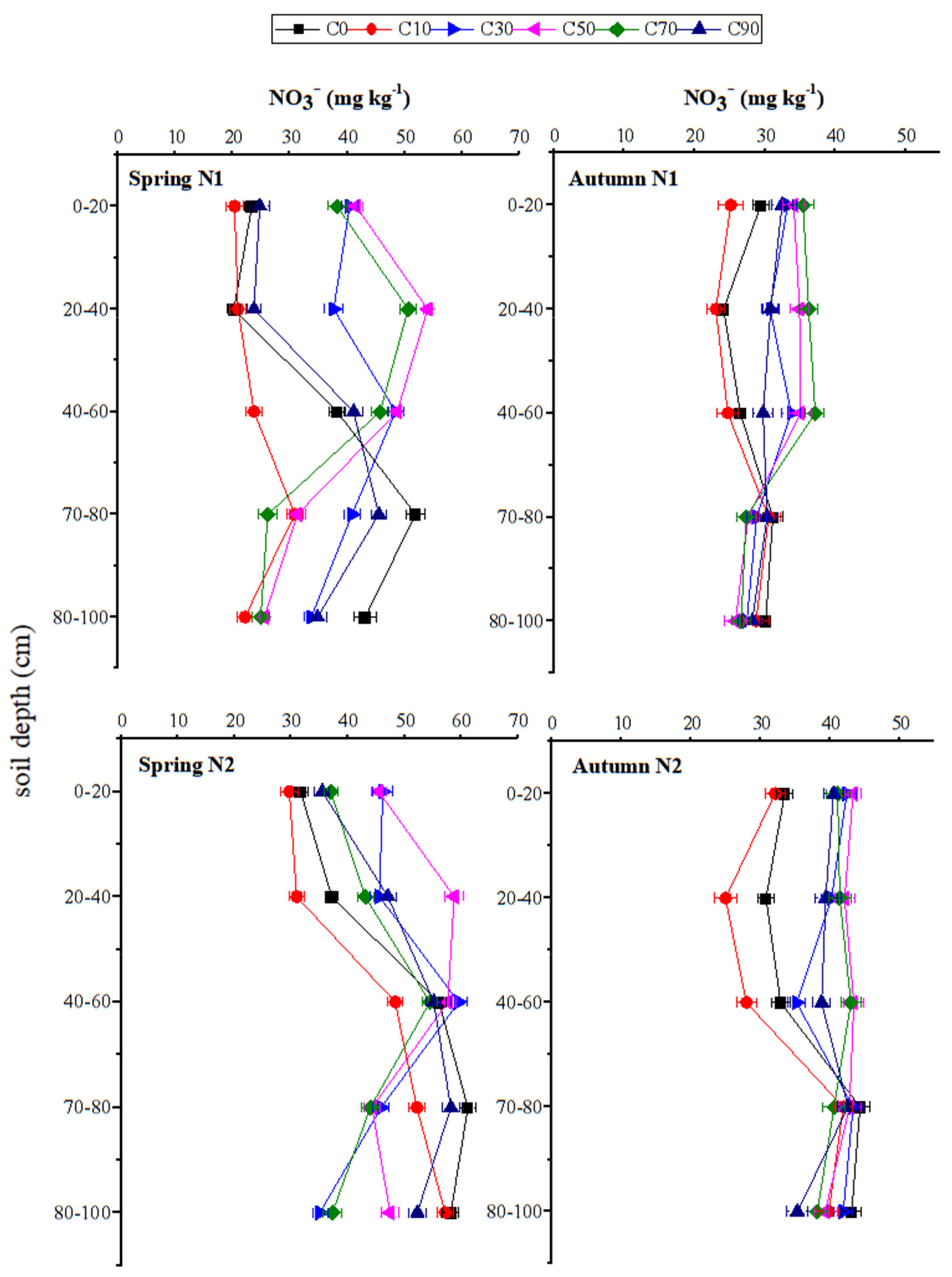
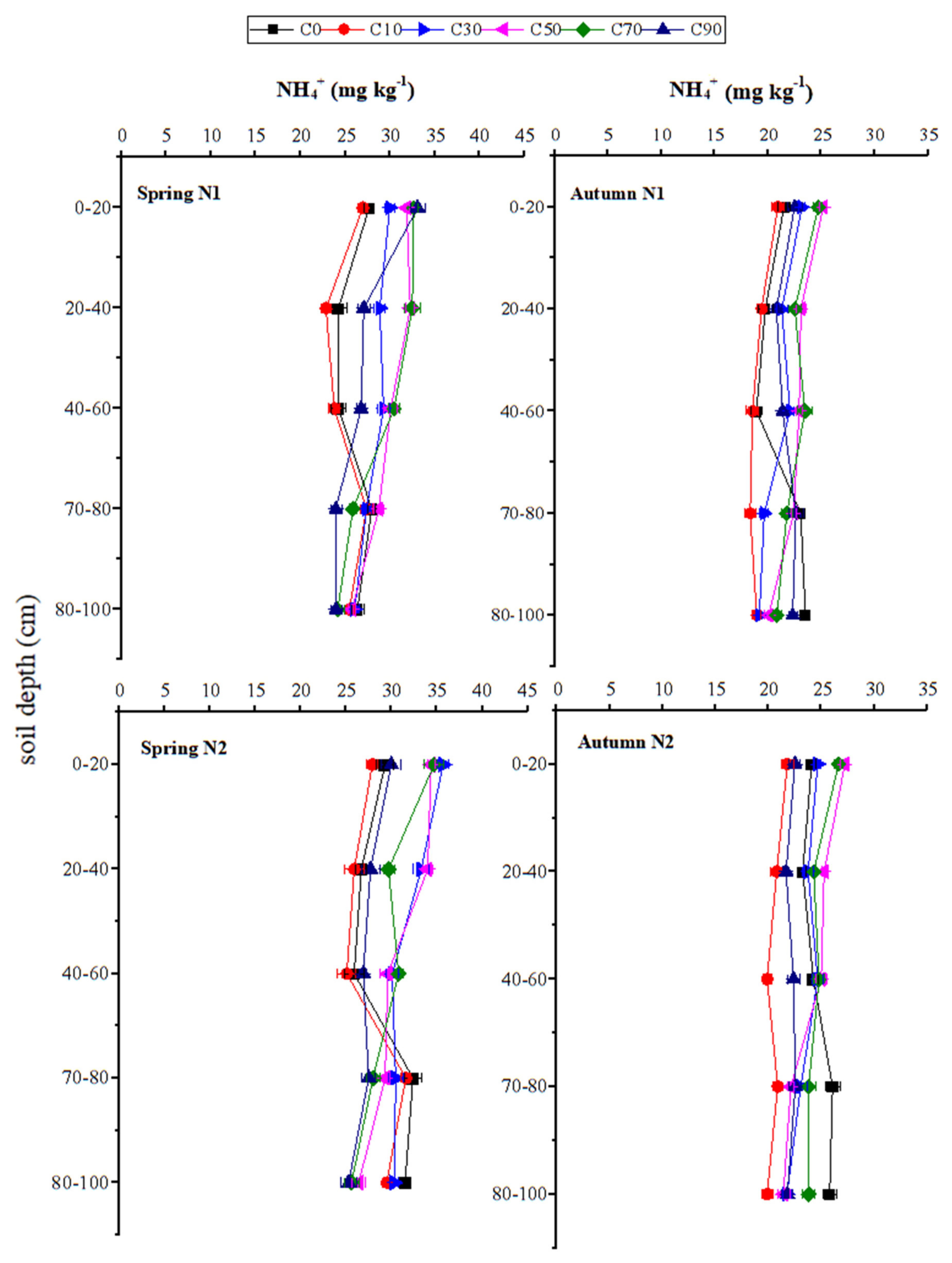
3.4. Distributions of NO3– and NH4+ at Different Soil Depths
3.5. Total N Uptake of Tomato Plants
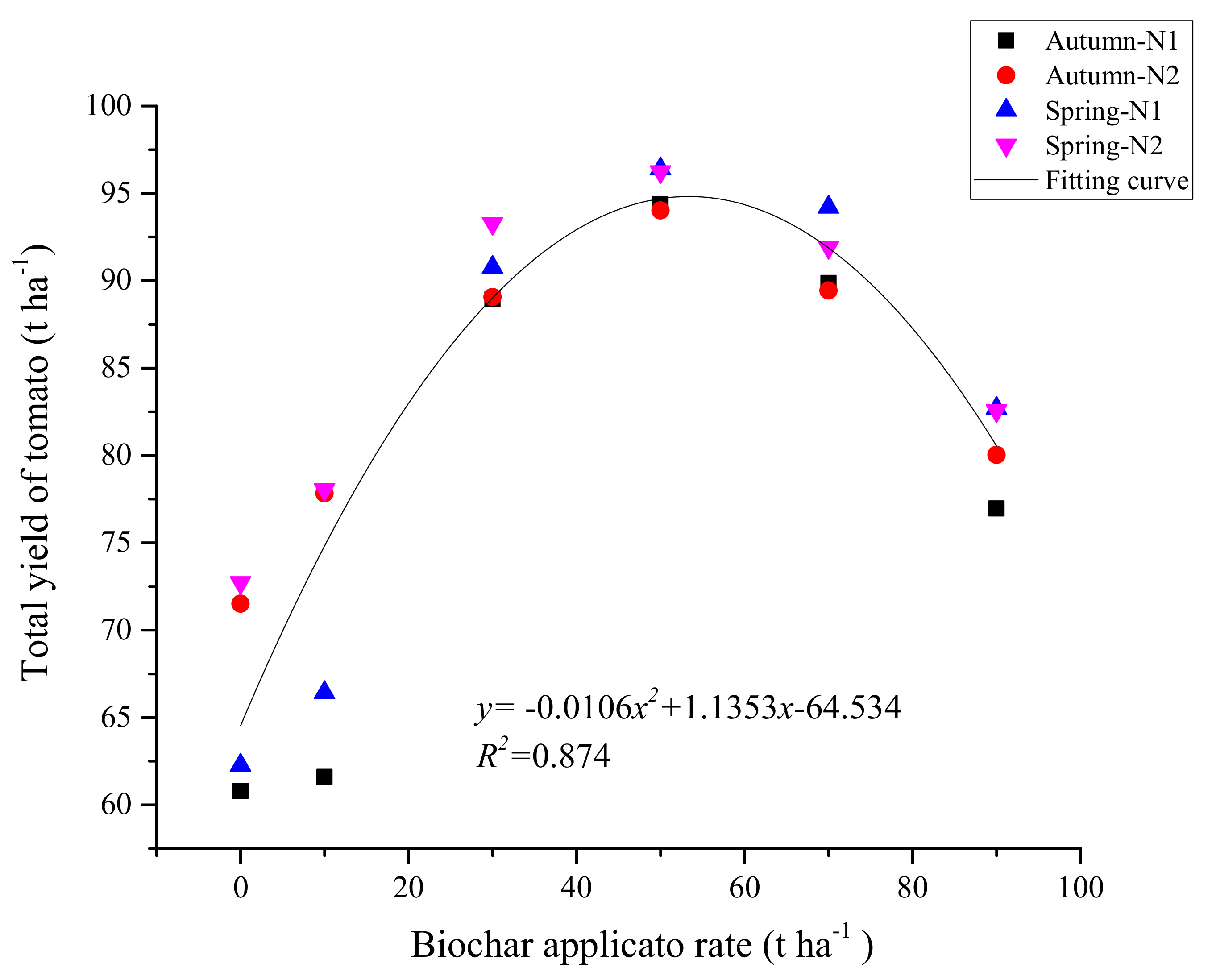
3.6. Tomato Yield and Utilisation of Water and N Fertiliser
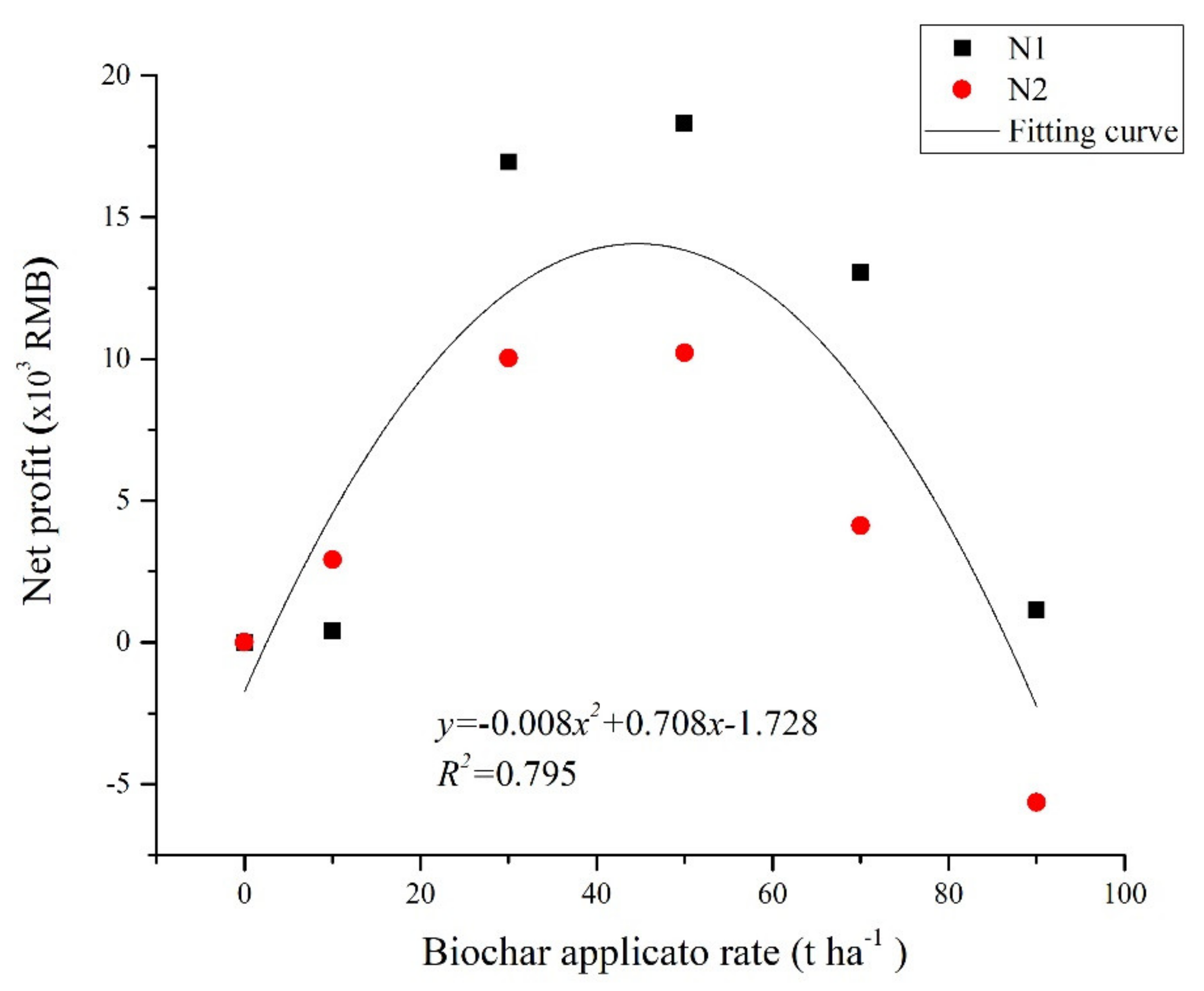
3.7. Relatively Suitable Combination of N Fertiliser and Biochar
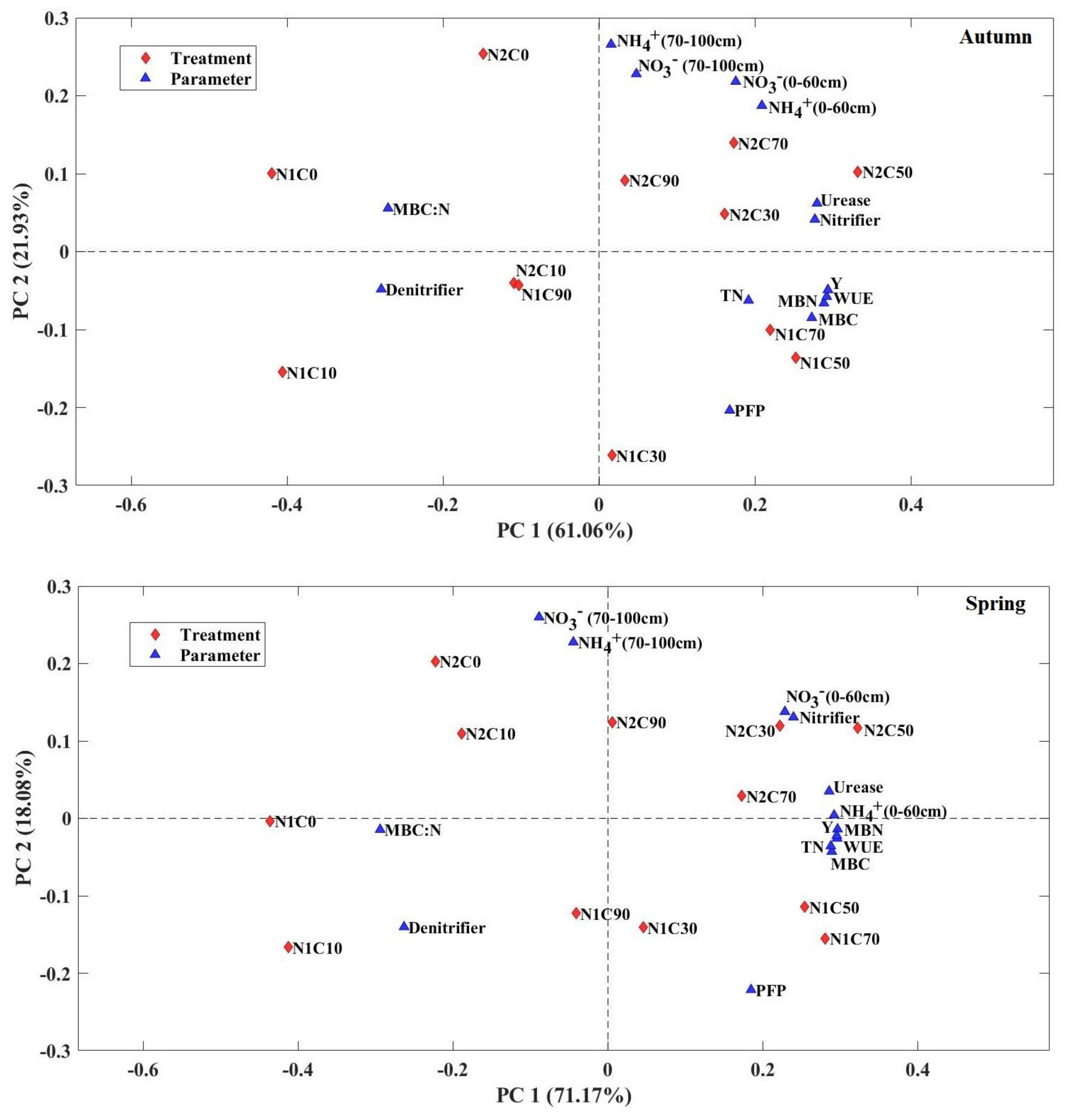
3.8. PCA of the Soil–Plant Parameters as Affected by the Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef]
- Yu, H.; Zou, W.; Chen, J.; Chen, H.; Yu, Z.; Huang, J.; Tang, H.; Wei, X.; Gao, B. Biochar amendment improves crop production in problem soils: A review. J. Environ. Manag. 2019, 232, 8–21. [Google Scholar] [CrossRef]
- Tan, Z.; Ye, Z.; Zhang, L.; Huang, Q. Application of the (15)N tracer method to study the effect of pyrolysis temperature and atmosphere on the distribution of biochar nitrogen in the biomass-biochar-plant system. Sci. Total Environ. 2018, 622, 79–87. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Xu, C.Y.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of biochar on soil available inorganic nitrogen: A review and meta-analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- Jeffery, S.; Memelink, I.; Hodgson, E.; Jones, S.; van de Voorde, T.F.J.; Martijn Bezemer, T.; Mommer, L.; van Groenigen, J.W. Initial biochar effects on plant productivity derive from N fertilization. Plant. Soil 2017, 415, 435–448. [Google Scholar] [CrossRef]
- Mandal, S.; Thangarajan, R.; Bolan, N.S.; Sarkar, B.; Khan, N.; Ok, Y.S.; Naidu, R. Biochar-induced concomitant decrease in ammonia volatilization and increase in nitrogen use efficiency by wheat. Chemosphere 2016, 142, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Nelissen, V.; Rütting, T.; Huygens, D.; Ruysschaert, G.; Boeckx, P. Temporal evolution of biochar’s impact on soil nitrogen processes—a15N tracing study. GCB Bioenergy 2015, 7, 635–645. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Turner, S.; Meyer-Stüve, S.; Schippers, A.; Guggenberger, G.; Schaarschmidt, F.; Wild, B.; Richter, A.; Dohrmann, R.; Mikutta, R. Microbial utilization of mineral-associated nitrogen in soils. Soil Biol. Biochem. 2017, 104, 185–196. [Google Scholar] [CrossRef]
- Yang, H.; Sheng, R.; Zhang, Z.; Wang, L.; Wang, Q.; Wei, W. Responses of nitrifying and denitrifying bacteria to flooding-drying cycles in flooded rice soil. Appl. Soil Ecol. 2016, 103, 101–109. [Google Scholar] [CrossRef]
- Li, Y.; Chang, S.X.; Tian, L.; Zhang, Q. Conservation agriculture practices increase soil microbial biomass carbon and nitrogen in agricultural soils: A global meta-analysis. Soil Biol. Biochem. 2018, 121, 50–58. [Google Scholar] [CrossRef]
- Song, Y.; Song, C.; Hou, A.; Ren, J.; Wang, X.; Cui, Q.; Wang, M. Effects of temperature and root additions on soil carbon and nitrogen mineralization in a predominantly permafrost peatland. Catena 2018, 165, 381–389. [Google Scholar] [CrossRef]
- Du, Z.; Wang, Y.; Huang, J.; Lu, N.; Liu, X.; Lou, Y.; Zhang, Q. Consecutive Biochar Application Alters Soil Enzyme Activities in the Winter Wheat–Growing Season. Soil Sci. 2014, 179, 75–83. [Google Scholar] [CrossRef]
- Du, Y.-D.; Zhang, Q.; Cui, B.-J.; Sun, J.; Wang, Z.; Ma, L.-H.; Niu, W.-Q. Aerated irrigation improves tomato yield and nitrogen use efficiency while reducing nitrogen application rate. Agric. Water Manag. 2020, 235, 106152. [Google Scholar] [CrossRef]
- Li, C.; Xiong, Y.; Qu, Z.; Xu, X.; Huang, Q.; Huang, G. Impact of biochar addition on soil properties and water-fertilizer productivity of tomato in semi-arid region of Inner Mongolia, China. Geoderma 2018, 331, 100–108. [Google Scholar] [CrossRef]
- Du, Y.; Niu, W.; Zhang, Q.; Cui, B.; Gu, X.; Guo, L.; Liang, B. Effects of Nitrogen on Soil Microbial Abundance, Enzyme Activity, and Nitrogen Use Efficiency in Greenhouse Celery under Aerated Irrigation. Soil Sci. Soc. Am. J. 2018, 82, 606. [Google Scholar] [CrossRef]
- Kandeler, E.G. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, Y.; Xie, S.; Li, J. Implication of Nitrifying and Denitrifying Bacteria for Nitrogen Removal in a Shallow Lake. Clean Soil Air Water 2017, 45, 1500319. [Google Scholar] [CrossRef]
- Horita, M.; Sakai, Y.K. Specific detection and quantification of Ralstonia pseudosolanacearum race 4 strains from Zingiberaceae plant cultivation soil by MPN-PCR. J. Gen. Plant. Pathol. 2020, 86, 393–400. [Google Scholar] [CrossRef]
- Nakaho, Y.I.K. Sensitive quantitative detection of Ralstonia solanacearum in soil by the most probable number-polymerase chain reaction (MPN-PCR) method. Appl. Microbiol. Biotechnol. 2014, 98, 4169–4177. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Awad, Y.M.; Ok, Y.S.; Abrigata, J.; Beiyuan, J.; Beckers, F.; Tsang, D.C.W.; Rinklebe, J. Pine sawdust biomass and biochars at different pyrolysis temperatures change soil redox processes. Sci. Total Environ. 2018, 625, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Joseph, S.D.; Ji, M.; Nielsen, S.; Mitchell, D.R.G.; Donne, S.; Horvat, J.; Wang, J.; Munroe, P.; Thomas, T. Chemolithotrophic processes in the bacterial communities on the surface of mineral-enriched biochars. ISME J. 2017, 11, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, B.A.; Ellis, N.; Kim, C.S.; Bi, X.; Emam, A.E. Engineered biochar from microwave-assisted catalytic pyrolysis of switchgrass for increasing water-holding capacity and fertility of sandy soil. Sci. Total Environ. 2016, 566, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Yoo, G.; Kim, H.; Chen, J.; Kim, Y. Effects of Biochar Addition on Nitrogen Leaching and Soil Structure following Fertilizer Application to Rice Paddy Soil. Soil Sci. Soc. Am. J. 2014, 78, 852–860. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Li, G.; Andersen, M.N.; Liu, F. Biochar enhances yield and quality of tomato under reduced irrigation. Agric. Water Manag. 2014, 138, 37–44. [Google Scholar] [CrossRef]
- Brockhoff, S.R.; Christians, N.E.; Killorn, R.J.; Horton, R.; Davis, D.D. Physical and Mineral-Nutrition Properties of Sand-Based Turfgrass Root Zones Amended with Biochar. Agron. J. 2010, 102, 1627–1631. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; McGrouther, K.; Huang, H.; Lu, K.; Guo, X.; He, L.; Lin, X.; Che, L.; Ye, Z.; et al. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. Res. Int. 2016, 23, 974–984. [Google Scholar] [CrossRef]
- Dempster, D.N.; Murphy, D.L.J.D.V. Decreased soil microbial biomass and nitrogen mineralisation with Eucalyptus biochar addition to a coarse textured soil. Plant Soil 2012. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, L.; Li, Y.; Brookes, P.C.; Xu, J.; Luo, Y. The effects of combinations of biochar, lime, and organic fertilizer on nitrification and nitrifiers. Biol. Fertil. Soils 2016, 53, 77–87. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Wang, H.; Zhao, X.; Cui, H.; Wei, Z. Reducing nitrogen loss and phytotoxicity during beer vinasse composting with biochar addition. Waste Manag. 2017, 61, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Ducey, T.F.; Ippolito, J.A.; Cantrell, K.B.; Novak, J.M.; Lentz, R.D. Addition of activated switchgrass biochar to an aridic subsoil increases microbial nitrogen cycling gene abundances. Appl. Soil Ecol. 2013, 65, 65–72. [Google Scholar] [CrossRef]
- DeLuca, T.; MacKenzie, M.; Gundale, M.; Holben, W. Wildfire-produced charcoal directly influences nitrogen cycling in ponderosa pine forests. Soil Sci. Soc. Am. J. 2006, 70, 448–453. [Google Scholar] [CrossRef]
- Van Zwieten, L. Influence of biochars on flux of N2O and CO2 from Ferrosol. Aust. J. Soil Res. 2010, 48, 555–568. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, P.; Shi, G.; Cheng, K.; Bian, R.; Liu, X.; Zhang, X.; Zheng, J.; Crowley, D.E. Short-term biochar manipulation of microbial nitrogen transformation in wheat rhizosphere of a metal contaminated Inceptisol from North China plain. Sci. Total Environ. 2018, 640, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Ning, L.; Xun, M.; Feng, F.; Li, P.; Yue, S.; Song, J.; Zhang, W.; Yang, H. Biochar can increase nitrogen use efficiency of Malus hupehensis by modulating nitrate reduction of soil and root. Appl. Soil Ecol. 2019, 135, 25–32. [Google Scholar] [CrossRef]
- Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar]
- Chen, J.; Sun, X.; Li, L.; Liu, X.; Zhang, B.; Zheng, J.; Pan, G. Change in active microbial community structure, abundance and carbon cycling in an acid rice paddy soil with the addition of biochar. Eur. J. Soil Sci. 2016, 67, 857–867. [Google Scholar] [CrossRef]
- Kushwaha, C.P.; Tripathi, S.K.; Singh, K.P. Variations in soil microbial biomass and N availability due to residue and tillage management in a dryland rice agroecosystem. Soil Tillage Res. 2000, 56, 153–166. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Dijkstra, F.A.; Liu, X.R.; Wang, Y.D.; Huang, J.; Lu, N. Effects of biochar on soil microbial biomass after four years of consecutive application in the north China Plain. PLoS ONE 2014, 9, e102062. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.J.; Watts, D.W.; Amonette, J.E.; Ippolito, J.A.; Lima, I.M.; Gaskin, J.; Das, K.C.; Steiner, C.; Ahmedna, M.; et al. Biochars Impact on Soil-Moisture Storage in an Ultisol and Two Aridisols. Soil Sci. 2012, 177, 310–320. [Google Scholar] [CrossRef]
- Rajkovich, S. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Biol. Fertil. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36. [Google Scholar] [CrossRef]

| Factor | Soil | Biochar |
|---|---|---|
| Nominal peak temperature (°C) | – | 450 |
| Surface area (m2 g−1) | – | 87.1 |
| Total ash (%) | – | 19.8 |
| Bulk density (g cm−1) | 1.35 | – |
| pH | 7.35 | 10.51 |
| organic matter (g kg−1) | 16.48 | – |
| C (%) | – | 72.38 |
| H (%) | – | 2.62 |
| O (%) | – | 23.81 |
| Mineral N (mg kg−1) | – | <3 |
| Total N (g kg−1) | 0.96 | 0.98 |
| NH4+–N (mg kg−1) | 10.1 | 1.67 |
| NO3−–N (mg kg−1) | 15.3 | 0.59 |
| Total K (g kg−1) | 10.4 | – |
| Total P (g kg−1) | 0.87 | 0.76 |
| Treatment | Nitrogen Fertiliser (kg/ha) | Biochar (t/ha) |
|---|---|---|
| N1C0 | 190 | 0 |
| N1C10 | 190 | 10 |
| N1C30 | 190 | 30 |
| N1C50 | 190 | 50 |
| N1C70 | 190 | 70 |
| N1C90 | 190 | 90 |
| N2C0 | 250 | 0 |
| N2C10 | 250 | 10 |
| N2C30 | 250 | 30 |
| N2C50 | 250 | 50 |
| N2C70 | 250 | 70 |
| N2C90 | 250 | 90 |
| Treatment | Yield (t) | Input Cost (×103 RMB) | Income (×103 RMB) | Benefit (×103 RMB) | Net Profit Relative to No Biochar (×103 RMB) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biochar | Fertiliser and Pesticide | Pipe System | Water and Electricity | Seedlings | Other | |||||
| N1C0 | 8.21 | 0.00 | 1.16 | 2.02 | 0.10 | 2.00 | 0.50 | 45.95 | 40.17 | 0.00 |
| N1C10 | 8.53 | 1.33 | 1.16 | 2.02 | 0.10 | 2.00 | 0.60 | 47.79 | 40.58 | 0.41 |
| N1C30 | 11.98 | 4.00 | 1.16 | 2.02 | 0.10 | 2.00 | 0.70 | 67.10 | 57.12 | 16.95 |
| N1C50 | 12.72 | 6.67 | 1.16 | 2.02 | 0.10 | 2.00 | 0.80 | 71.23 | 58.48 | 18.31 |
| N1C70 | 12.27 | 9.33 | 1.16 | 2.02 | 0.10 | 2.00 | 0.90 | 68.72 | 53.21 | 13.04 |
| N1C90 | 10.64 | 12.00 | 1.16 | 2.02 | 0.10 | 2.00 | 1.00 | 59.61 | 41.33 | 1.16 |
| N2C0 | 9.62 | 0.00 | 1.26 | 2.02 | 0.10 | 2.00 | 0.50 | 53.85 | 47.97 | 0.00 |
| N2C10 | 10.39 | 1.33 | 1.26 | 2.02 | 0.10 | 2.00 | 0.60 | 58.19 | 50.88 | 2.91 |
| N2C30 | 12.16 | 4.00 | 1.26 | 2.02 | 0.10 | 2.00 | 0.70 | 68.07 | 57.99 | 10.03 |
| N2C50 | 12.68 | 6.67 | 1.26 | 2.02 | 0.10 | 2.00 | 0.80 | 71.03 | 58.18 | 10.22 |
| N2C70 | 12.09 | 9.33 | 1.26 | 2.02 | 0.10 | 2.00 | 0.90 | 67.70 | 52.09 | 4.12 |
| N2C90 | 10.84 | 12.00 | 1.26 | 2.02 | 0.10 | 2.00 | 1.00 | 60.70 | 42.32 | -5.65 |
| Treatment | Autumn | Spring | ||||
|---|---|---|---|---|---|---|
| Urease (mg g−1 24 h−1) | amoA-Type Nitrifier (103 g−1) | nirS-Type Denitrifier (103 g−1) | Urease (mg g−1 24 h−1) | amoA-Type Nitrifier (103 g−1) | nirS-Type Denitrifier (103 g−1) | |
| N1C0 | 1.86 ± 0.04 i | 1.89 ± 0.11 e | 4.08 ± 0.07 b | 1.78 ± 0.02 fg | 1.59 ± 0.3 e | 4.86 ± 0.35 ab |
| N1C10 | 1.68 ± 0.07 j | 1.46 ± 0.31 e | 4.43 ± 0.13 a | 1.68 ± 0.03 g | 1.53 ± 0.17 e | 5.95 ± 0.6 a |
| N1C30 | 2.15 ± 0.05 efg | 2.53 ± 0.18 d | 3.66 ± 0.15 c | 2.09 ± 0.03 d | 2.44 ± 0.38 cde | 3.43 ± 0.46 cd |
| N1C50 | 2.31 ± 0.04 cd | 3.96 ± 0.17 b | 2.05 ± 0.1 g | 2.33 ± 0.04 b | 3.11 ± 0.24 bcd | 2.48 ± 0.64 cdef |
| N1C70 | 2.21 ± 0.02 def | 4.28 ± 0.18 ab | 2.04 ± 0.07 g | 2.27 ± 0.03 bc | 3.55 ± 0.34 bc | 2.37 ± 0.42 def |
| N1C90 | 2.02 ± 0.07 gh | 2.88 ± 0.05 cd | 2.87 ± 0.08 e | 1.94 ± 0.06 e | 2.17 ± 0.54 de | 3.92 ± 0.15 bc |
| N2C0 | 1.95 ± 0.07 hi | 2.65 ± 0.14 d | 3.24 ± 0.12 d | 1.81 ± 0.04 f | 2.48 ± 0.54 cde | 3.14 ± 0.63 cde |
| N2C10 | 2.06 ± 0.06 fgh | 2.56 ± 0.18 d | 3.50 ± 0.13 cd | 1.84 ± 0.02 ef | 2.1 ± 0.46 de | 3.56 ± 0.61 bcd |
| N2C30 | 2.41 ± 0.08 bc | 3.32 ± 0.1 c | 2.47 ± 0.14 f | 2.46 ± 0.02 a | 5.45 ± 0.31 a | 1.6 ± 0.31 f |
| N2C50 | 2.59 ± 0.02 a | 4.49 ± 0.09 a | 1.83 ± 0.06 g | 2.38 ± 0.03 ab | 6.28 ± 0.58 a | 1.22 ± 0.09 f |
| N2C70 | 2.48 ± 0.03 ab | 3.27 ± 0.12 c | 2.72 ± 0.05 ef | 2.30 ± 0.04 bc | 4.06 ± 0.56 b | 1.93 ± 0.48 ef |
| N2C90 | 2.27 ± 0.03 cde | 3.87 ± 0.12 b | 2.82 ± 0.16 e | 2.20 ± 0.06 c | 3.99 ± 0.38 b | 1.97 ± 0.06 ef |
| ANOVA | ||||||
| N | *** | *** | *** | *** | *** | *** |
| C | *** | *** | *** | *** | *** | *** |
| N×C | ns | ** | ns | *** | *** | *** |
| Factors | Autumn | Spring | ||||||
|---|---|---|---|---|---|---|---|---|
| TN | MBC | MBN | MBC:N | TN | MBC | MBN | MBC:N | |
| N | *** | ** | ** | *** | *** | ns | ns | ns |
| C | *** | *** | *** | *** | *** | *** | *** | *** |
| N×C | * | *** | *** | *** | ** | ** | ns | ns |
| Treatment | Autumn | Spring | ||||
|---|---|---|---|---|---|---|
| Y(t/ha) | WUE/(kg m−3) | PFP | Y(t/ha) | WUE/(kg m−3) | PFP | |
| N1C0 | 60.80 ± 0.33 i | 38.48 ± 0.36 h | 319.99 ± 1.75 c | 62.28 ± 0.18 j | 30.17 ± 0.34 j | 327.79 ± 0.92 c |
| N1C10 | 61.60 ± 0.36 h | 39.02 ± 0.53 h | 324.19 ± 1.89 a | 66.42 ± 0.19 i | 32.49 ± 0.52 i | 349.57 ± 1.04 a |
| N1C30 | 88.95 ± 0.13 c | 57.17 ± 0.30 c | 468.14 ± 0.68 d | 90.78 ± 0.15 e | 44.23 ± 0.54 e | 477.78 ± 0.79 e |
| N1C50 | 94.38 ± 0.23 a | 60.91 ± 0.21 a | 496.77 ± 1.23 b | 96.40 ± 0.16 a | 47.26 ± 0.31 a | 507.39 ± 0.82 b |
| N1C70 | 89.87 ± 0.26 b | 57.43 ± 0.29 c | 473.02 ± 1.37 i | 94.21 ± 0.24 b | 46.05 ± 0.14 bc | 495.85 ± 1.29 i |
| N1C90 | 76.96 ± 0.18 f | 49.76 ± 0.36 f | 405.05 ± 0.92 e | 82.71 ± 0.09 f | 40.68 ± 0.61 f | 435.31 ± 0.49 e |
| N2C0 | 71.51 ± 0.36 g | 45.03 ± 0.82 g | 286.03 ± 1.44 k | 72.72 ± 0.09 h | 35.10 ± 0.71 h | 290.89 ± 0.36 k |
| N2C10 | 77.83 ± 0.28 e | 49.44 ± 0.57 f | 311.31 ± 1.09 f | 78.04 ± 0.13 g | 38.10 ± 0.48 g | 312.17 ± 0.53 f |
| N2C30 | 89.07 ± 0.24 c | 56.02 ± 0.25 d | 356.27 ± 0.97 j | 93.27 ± 0.31 c | 45.30 ± 0.23 cd | 373.07 ± 1.24 j |
| N2C50 | 94.03 ± 0.33 a | 59.97 ± 0.38 b | 376.11 ± 1.31 g | 96.23 ± 0.30 a | 46.76 ± 0.68 ab | 384.92 ± 1.21 g |
| N2C70 | 89.43 ± 0.26 bc | 56.72 ± 0.63 cd | 357.74 ± 1.01 h | 91.90 ± 0.16 d | 44.66 ± 0.34 de | 367.61 ± 0.62 h |
| N2C90 | 80.03 ± 0.40 d | 51.67 ± 0.36 e | 320.12 ± 1.61 c | 82.56 ± 0.18 f | 40.57 ± 0.44 f | 330.23 ± 0.69 c |
| ANOVA | ||||||
| B | *** | *** | *** | *** | *** | *** |
| N | *** | *** | *** | *** | *** | *** |
| B×N | *** | *** | *** | *** | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Yu, H.; Niu, W.; Kharbach, M. Biochar Promotes Nitrogen Transformation and Tomato Yield by Regulating Nitrogen-Related Microorganisms in Tomato Cultivation Soil. Agronomy 2021, 11, 381. https://doi.org/10.3390/agronomy11020381
Guo L, Yu H, Niu W, Kharbach M. Biochar Promotes Nitrogen Transformation and Tomato Yield by Regulating Nitrogen-Related Microorganisms in Tomato Cultivation Soil. Agronomy. 2021; 11(2):381. https://doi.org/10.3390/agronomy11020381
Chicago/Turabian StyleGuo, Lili, Huiwen Yu, Wenquan Niu, and Mourad Kharbach. 2021. "Biochar Promotes Nitrogen Transformation and Tomato Yield by Regulating Nitrogen-Related Microorganisms in Tomato Cultivation Soil" Agronomy 11, no. 2: 381. https://doi.org/10.3390/agronomy11020381
APA StyleGuo, L., Yu, H., Niu, W., & Kharbach, M. (2021). Biochar Promotes Nitrogen Transformation and Tomato Yield by Regulating Nitrogen-Related Microorganisms in Tomato Cultivation Soil. Agronomy, 11(2), 381. https://doi.org/10.3390/agronomy11020381







