Effect of H2O-Based Low-Pressure Plasma (LPP) Treatment on the Germination of Bambara Groundnut Seeds
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
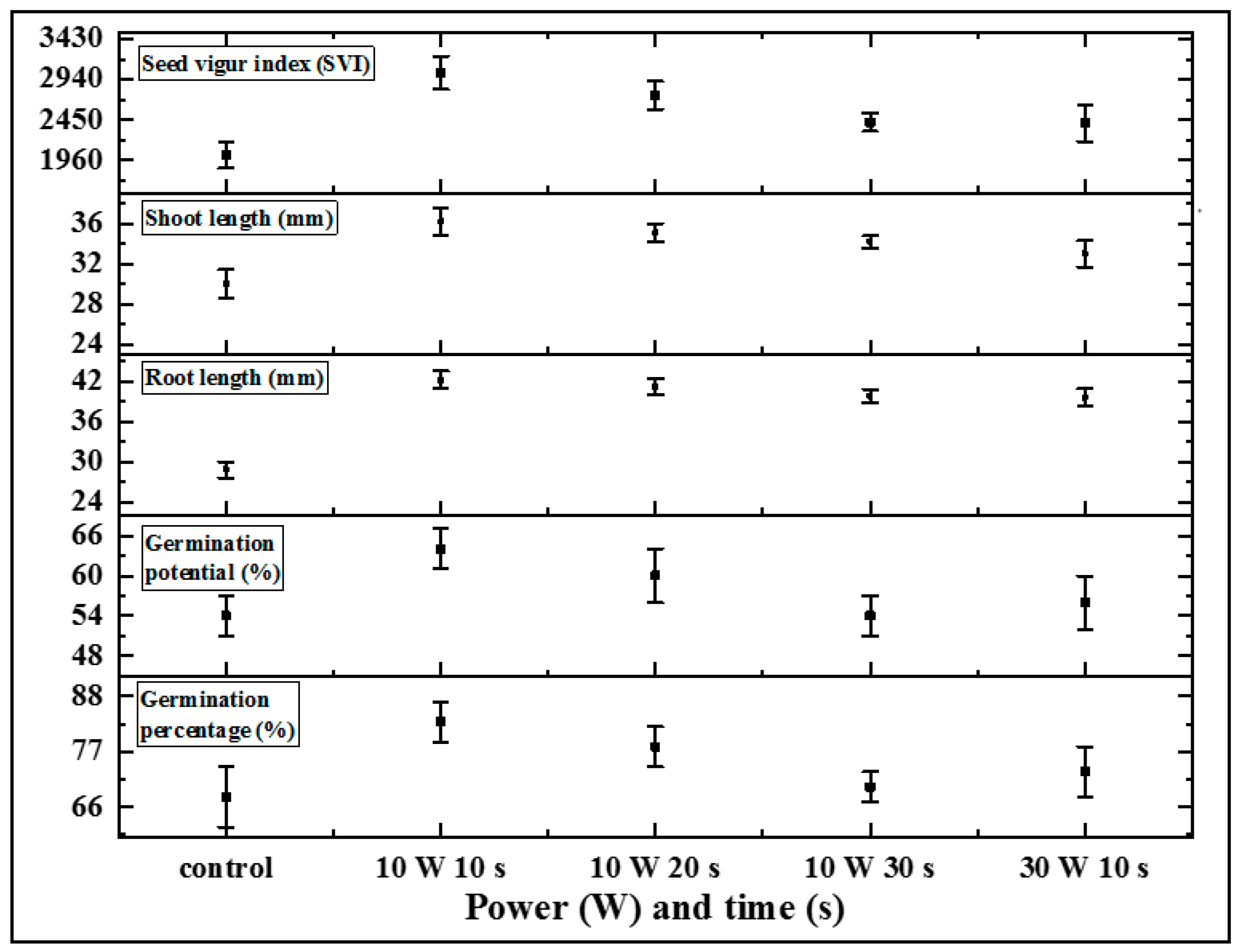
3.1. Seed Germination
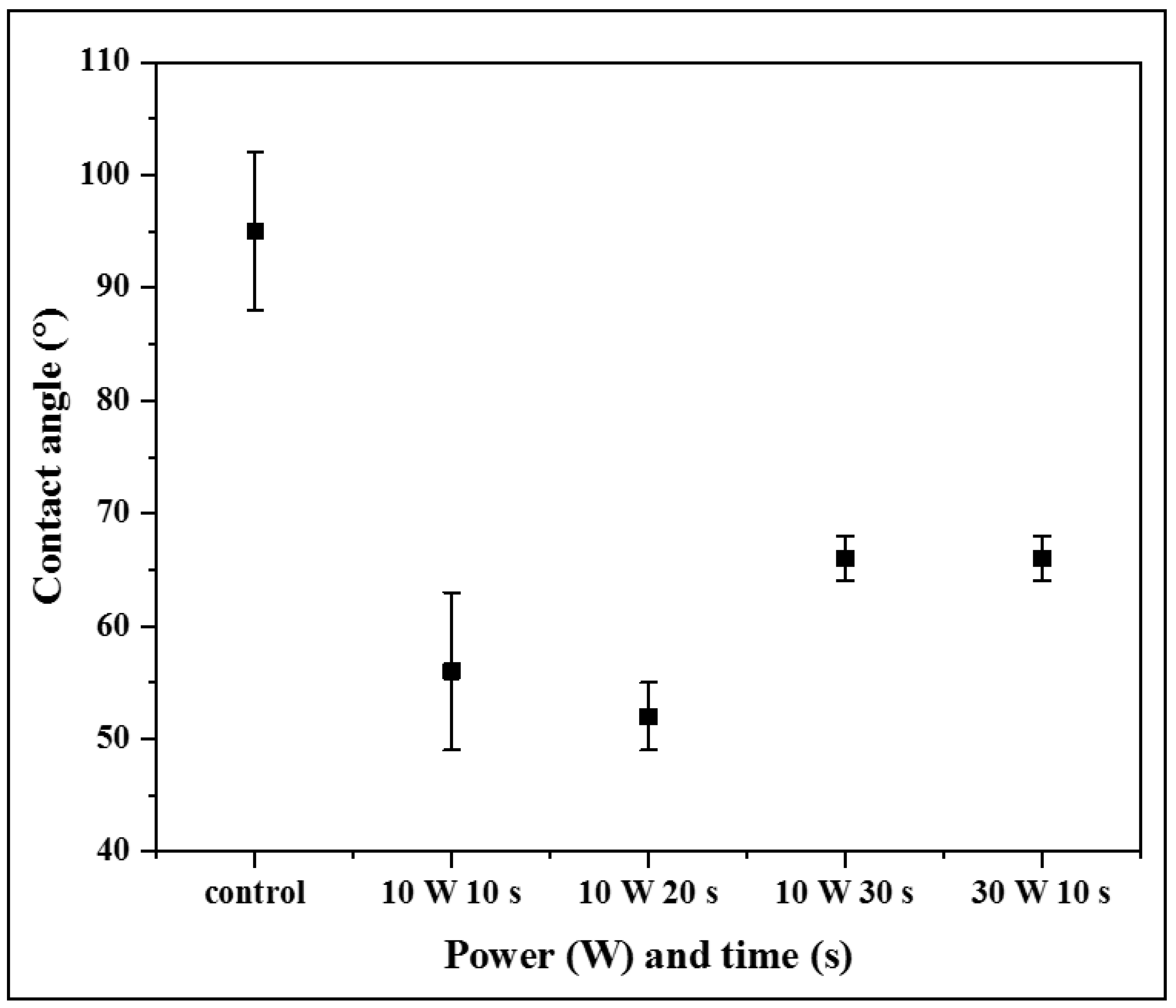
3.2. Water Contact Angle (WCA)
3.3. Effect of UV Radiation
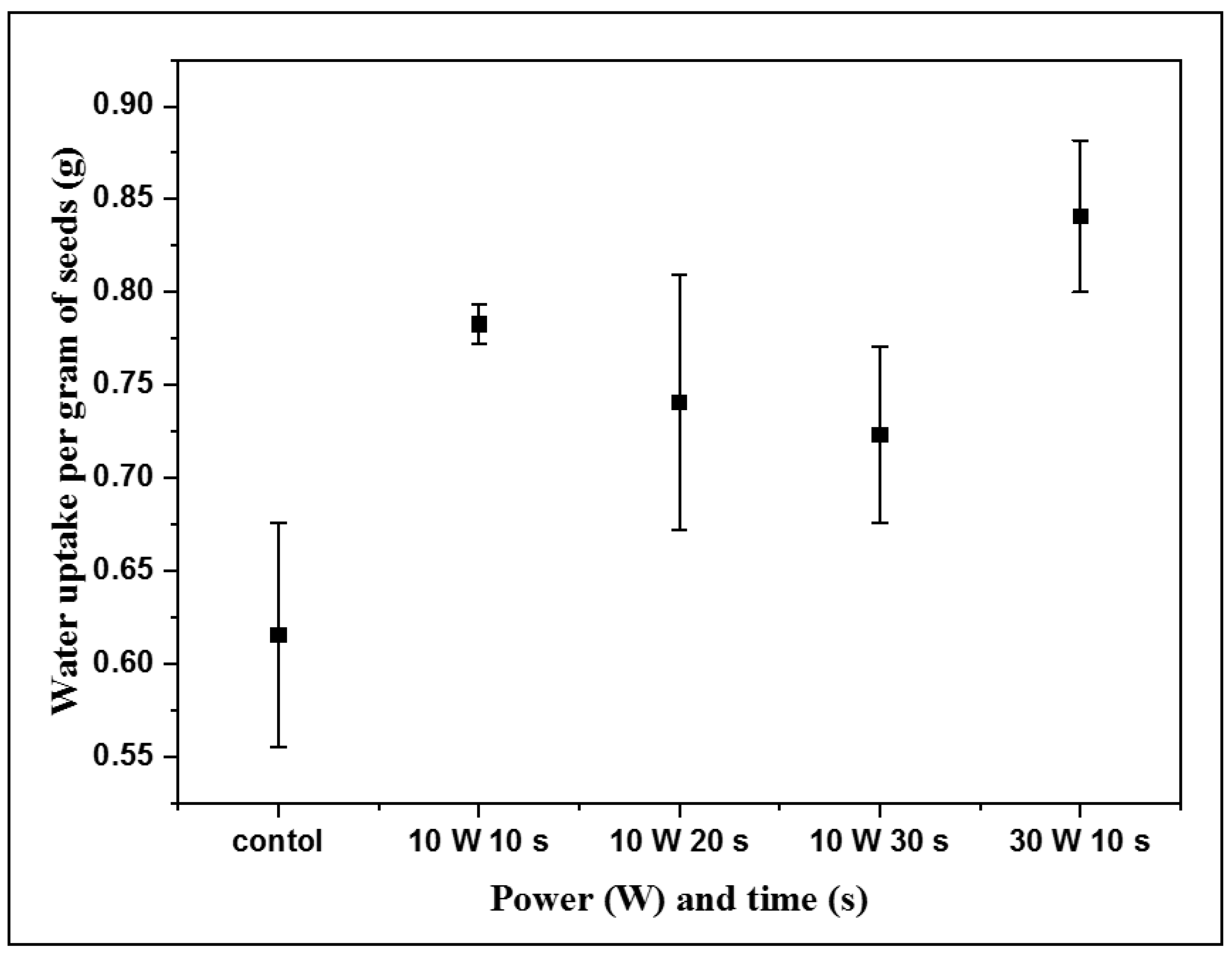
3.4. Water Uptake Analysis
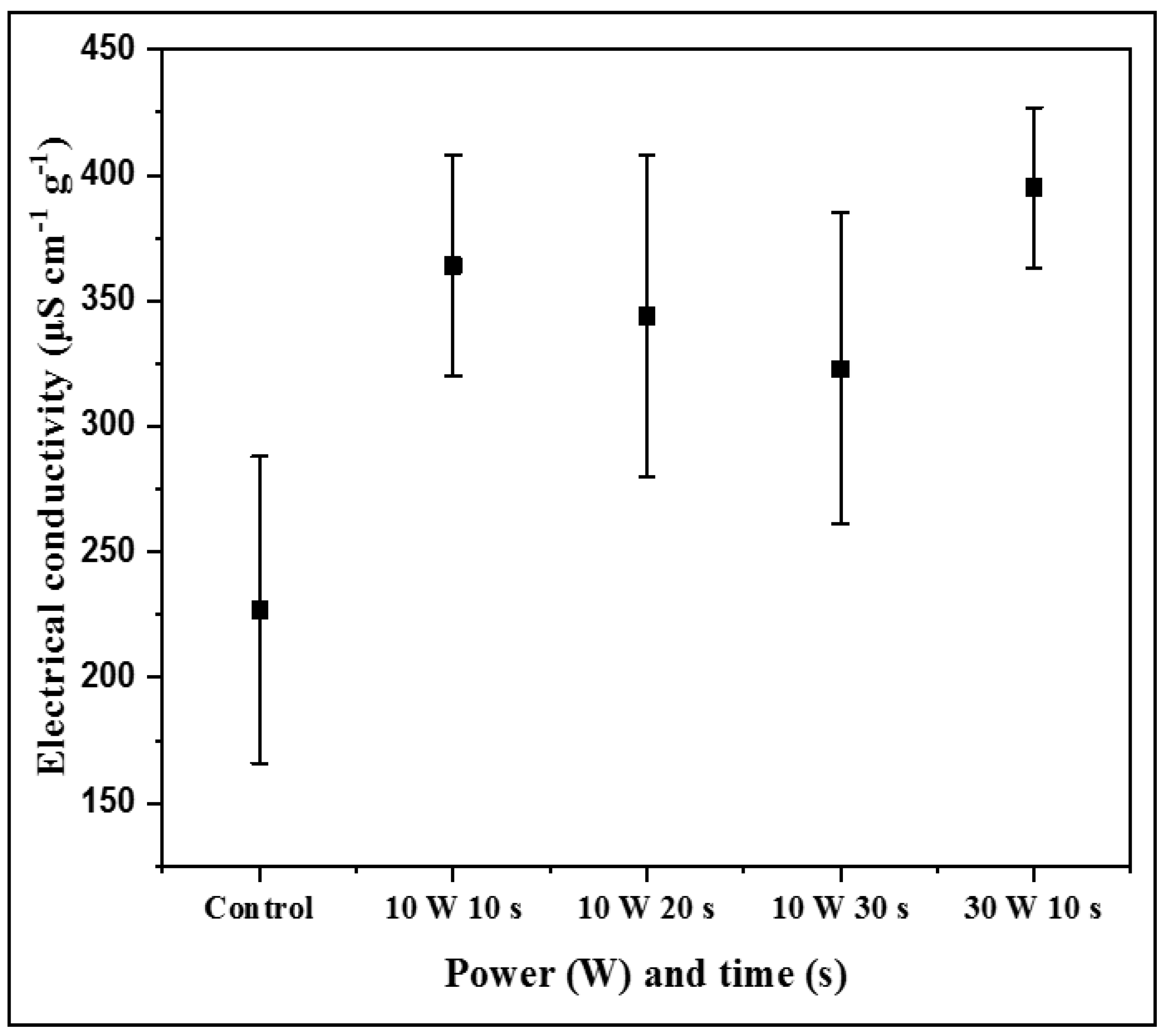
3.5. Electrical Conductivity
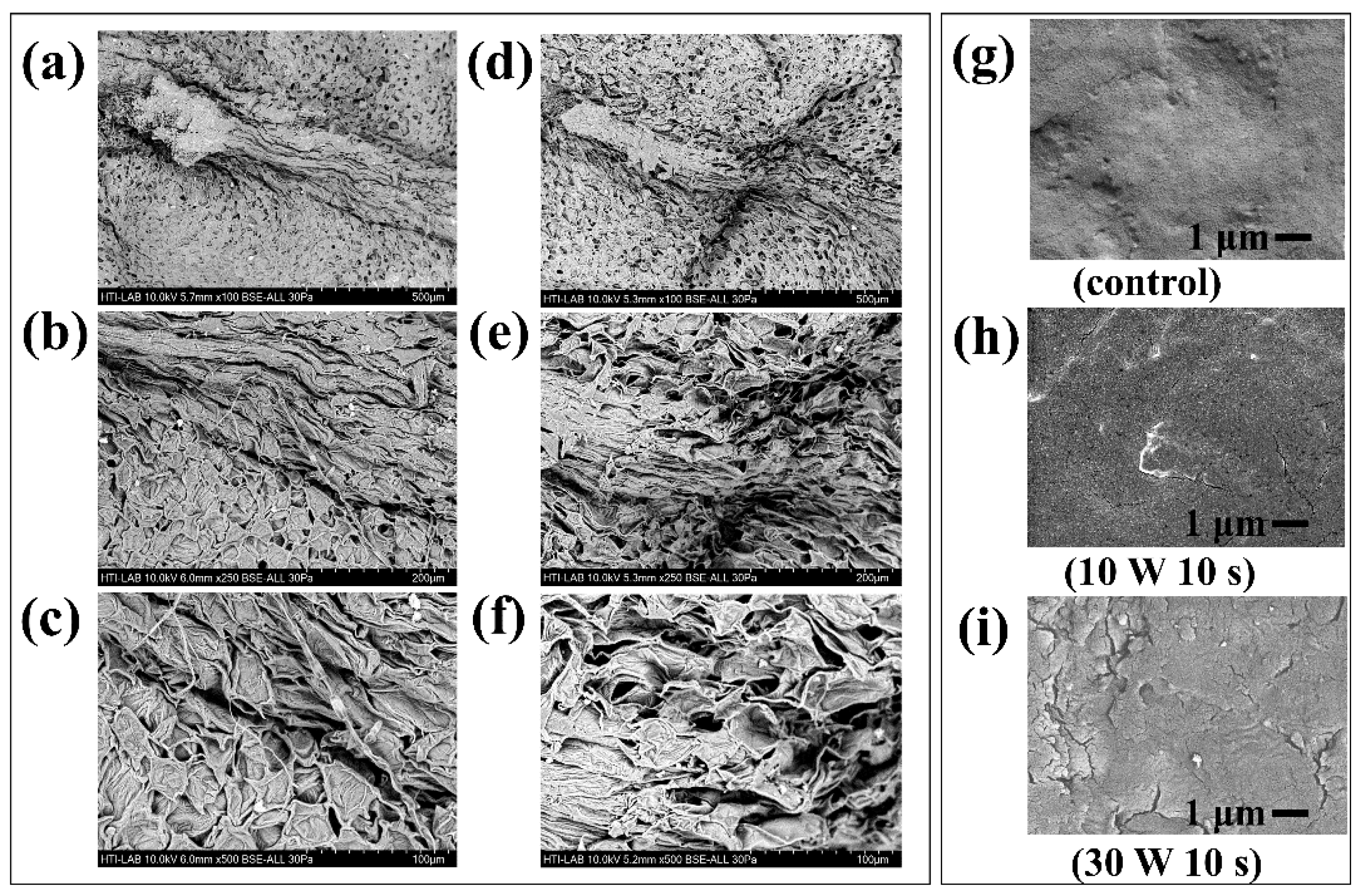
3.6. Surface Morphology
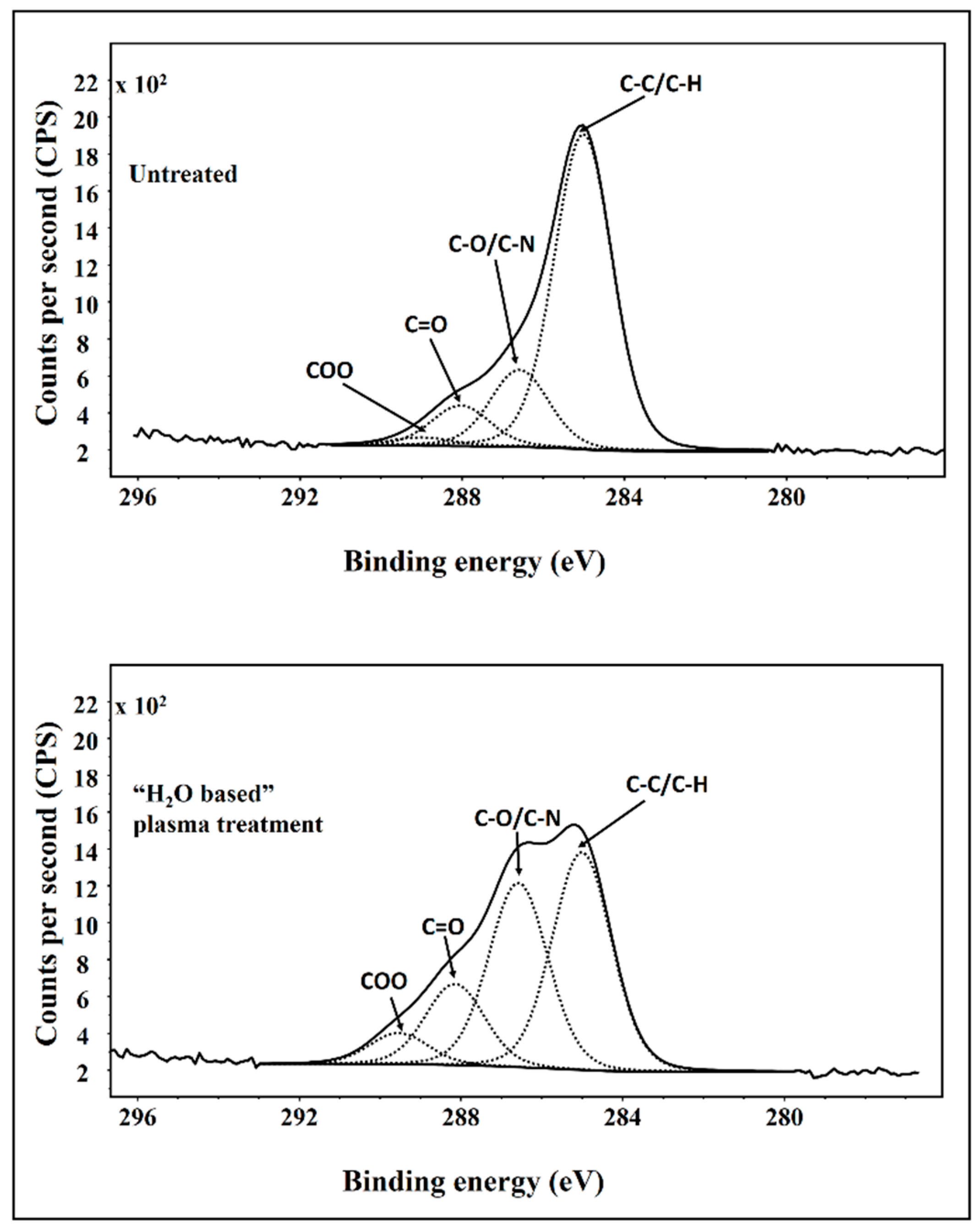
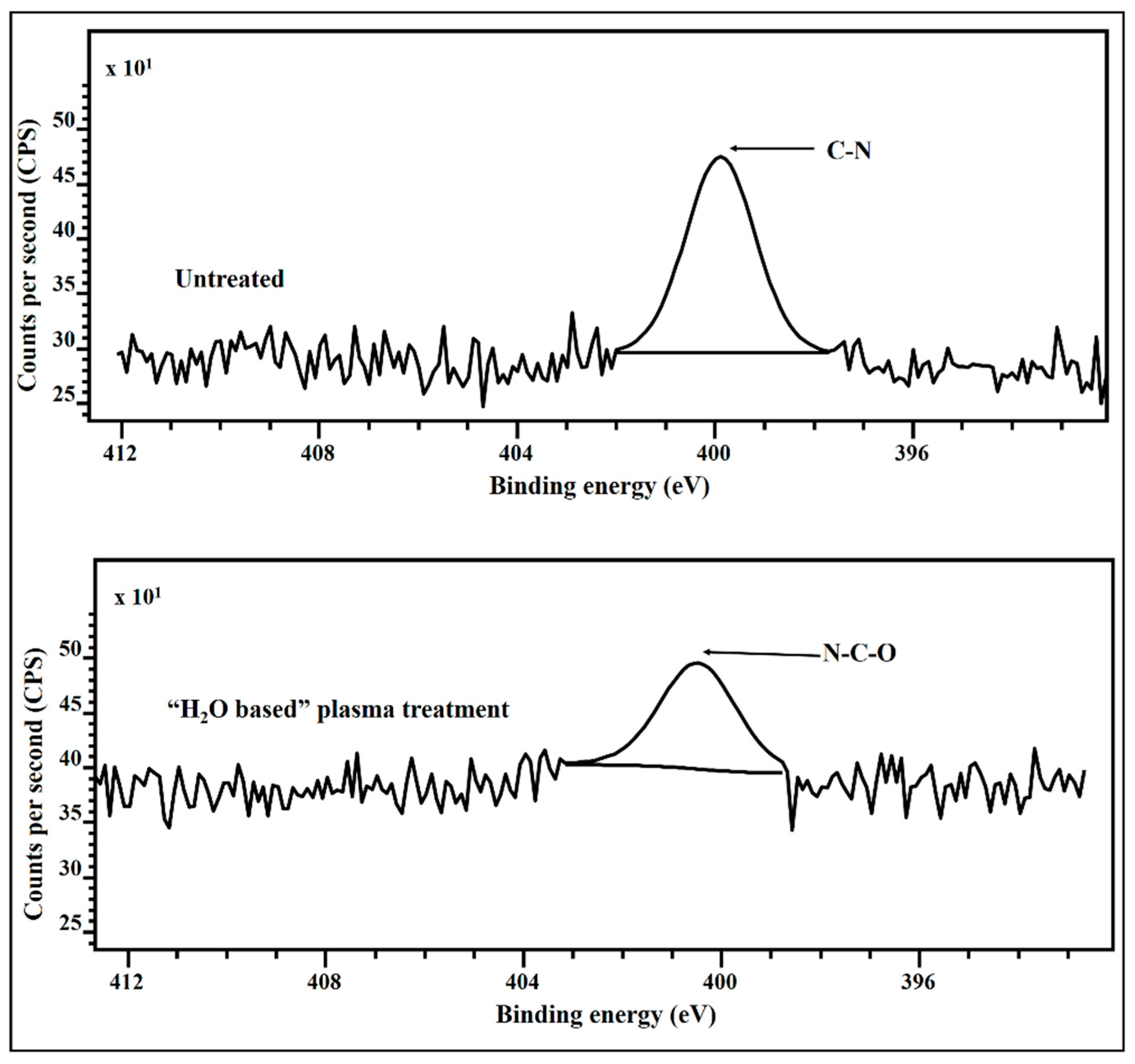
3.7. X-ray Photoelectron Spectroscopy (XPS)
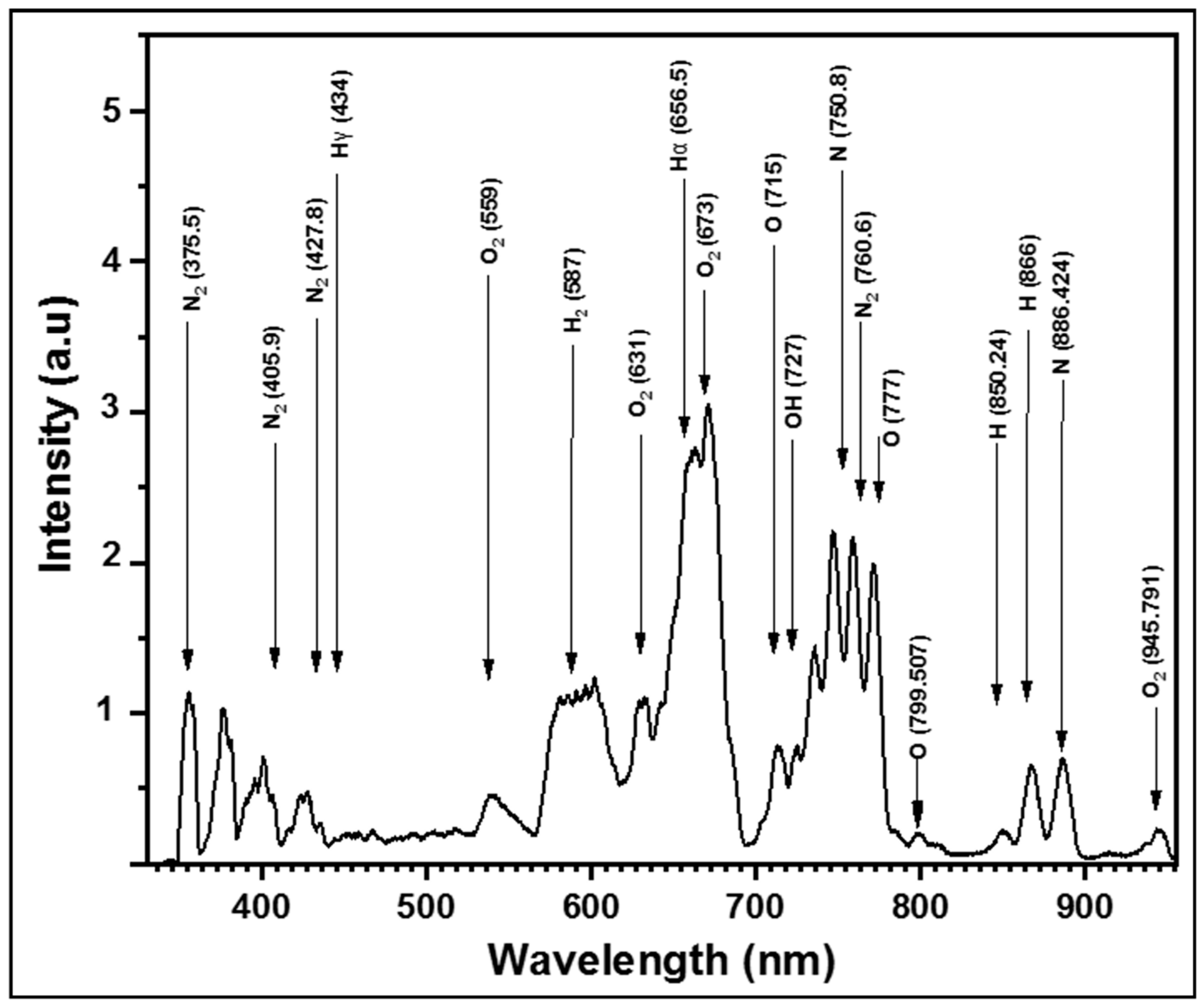
3.8. Optical Emission Spectroscopy (OES)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cassidy, E.S.; West, P.C.; Gerber, J.S.; Foley, J.A. Redefining agricultural yields: From tonnes to people nourished per hectare. Environ. Res. Lett 2013, 8, 034015. [Google Scholar] [CrossRef]
- Ho, W.K.; Chai, H.H.; Kendabie, P.; Ahmad, N.S.; Jani, J.; Massawe, F.; Kilian, A.; Mayes, S. Integrating genetic maps in bambara groundnut [Vigna subterranea (L) Verdc.] and their syntenic relationships among closely related legumes. BMC Genom. 2017, 18, 192. [Google Scholar] [CrossRef]
- Ahmad, N.S.; Redjeki, E.S.; Ho, W.K.; Aliyu, S.; Mayes, K.; Massawe, F.; Kilian, A.; Mayes, S. Construction of a genetic linkage map and QTL analysis in bambara groundnut. Genome 2016, 59, 459–472. [Google Scholar] [CrossRef]
- Chai, H.H.; Ho, W.K.; Graham, N.; May, S.; Massawe, F.; Mayes, S. A Cross-Species Gene Expression Marker-Based Genetic Map and QTL Analysis in Bambara Groundnut. Genes (Basel) 2017, 8, 84. [Google Scholar] [CrossRef]
- Mayes, S.; Ho, W.K.; Chai, H.H.; Gao, X.; Kundy, A.C.; Mateva, K.I.; Zahrulakmal, M.; Hahiree, M.; Kendabie, P.; Licea, L.C.S.; et al. Bambara groundnut: An exemplar underutilised legume for resilience under climate change. Planta 2019, 250, 803–820. [Google Scholar] [CrossRef]
- Mubaiwa, J.; Fogliano, V.; Chidewe, C.; Bakker, E.J.; Linnemann, A.R. Utilization of bambara groundnut (Vigna subterranea (L.) Verdc.) for sustainable food and nutrition security in semi-arid regions of Zimbabwe. PLoS ONE 2018, 13, e0204817. [Google Scholar] [CrossRef]
- Murevanhema, Y.Y.; Jideani, V.A. Potential of Bambara groundnut (Vigna subterranea (L.) Verdc) milk as a probiotic beverage-a review. Crit. Rev. Food Sci. Nutr. 2013, 53, 954–967. [Google Scholar] [CrossRef]
- Oyeyinka, S.A.; Singh, S.; Adebola, P.O.; Gerrano, A.S.; Amonsou, E.O. Physicochemical properties of starches with variable amylose contents extracted from bambara groundnut genotypes. Carbohydr. Polym. 2015, 133, 171–178. [Google Scholar] [CrossRef]
- Draweel, M.; Waluyo, B.; Saptadi, D.; Rahmi Ardiarini, N.; Kuswanto, K. Seed size and water imbibition to germination rate in bambara groundnut (Vigna subterranean (L.) Verdc.). TPGM 2018, 8, 95–99. [Google Scholar]
- Kishinevsky, B.; Zur, M.; Friedman, Y.; Meromi, G.; Ben-Moshe, E.; Nemas, C. Variation in nitrogen fixation and yield in landraces of Bambara groundnut (Vigna subterranea L.). Field Crops Res. 1996, 48, 57–64. [Google Scholar] [CrossRef]
- Lale, N.E.S.; Ajayi, F.A. Suppression of development of Callosobruchus maculatus (F.) (Col.: Bruchidae) in bambara groundnut seeds exposed to solar heat in the Nigerian savanna. J. Pest Sci. 2001, 74, 133–137. [Google Scholar] [CrossRef]
- Singh, H.; Jassal, R.K.; Kang, J.S.; Sandhu, S.S.; Kang, H.; Grewal, K. Seed priming techniques in field crops—A review. Agric. Rev. 2015, 36, 251–264. [Google Scholar] [CrossRef]
- Dhayal, M.; Lee, S.-Y.; Park, S.-U. Using low-pressure plasma for Carthamus tinctorium L. seed surface modification. Vacuum 2006, 80, 499–506. [Google Scholar] [CrossRef]
- Chen, H.H.; Chang, H.C.; Chen, Y.K.; Hung, C.L.; Lin, S.Y.; Chen, Y.S. An improved process for high nutrition of germinated brown rice production: Low-pressure plasma. Food Chem. 2016, 191, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Štěpánová, V.; Slavíček, P.; Kelar, J.; Prášil, J.; Smékal, M.; Stupavská, M.; Jurmanová, J.; Černák, M. Atmospheric pressure plasma treatment of agricultural seeds of cucumber (Cucumis sativus L.) and pepper (Capsicum annuum L.) with effect on reduction of diseases and germination improvement. Plasma Process. Polym. 2018, 15, 1700076. [Google Scholar] [CrossRef]
- Filatova, I.; Lyushkevich, V.; Goncharik, S.; Zhukovsky, A.; Krupenko, N.; Kalatskaja, J. The effect of low-pressure plasma treatment of seeds on the plant resistance to pathogens and crop yields. J. Phys. D Appl. Phys. 2020, 53, 244001. [Google Scholar] [CrossRef]
- Meiqiang, Y.; Mingjing, H.; Buzhou, M.; Tengcai, M. Stimulating effects of seed treatment by magnetized plasma on tomato growth and yield. Plasma Sci. Technol. 2005, 7, 3143. [Google Scholar] [CrossRef]
- Park, Y.; Oh, K.S.; Oh, J.; Seok, D.C.; Kim, S.B.; Yoo, S.J.; Lee, M.-J. The biological effects of surface dielectric barrier discharge on seed germination and plant growth with barley. Plasma Process. Polym. 2018, 15, 1600056. [Google Scholar] [CrossRef]
- Bafoil, M.; Le Ru, A.; Merbahi, N.; Eichwald, O.; Dunand, C.; Yousfi, M. New insights of low-temperature plasma effects on germination of three genotypes of Arabidopsis thaliana seeds under osmotic and saline stresses. Sci. Rep. 2019, 9, 8649. [Google Scholar] [CrossRef] [PubMed]
- de Groot, G.; Hundt, A.; Murphy, A.B.; Bange, M.P.; Mai-Prochnow, A. Cold plasma treatment for cotton seed germination improvement. Sci. Rep. 2018, 8, 14372. [Google Scholar] [CrossRef] [PubMed]
- Los, A.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Bourke, P. Investigation of mechanisms involved in germination enhancement of wheat ( Triticum aestivum ) by cold plasma: Effects on seed surface chemistry and characteristics. Plasma Process. Polym. 2019, 16, 1800148. [Google Scholar] [CrossRef]
- Phan, K.T.K.; Phan, H.T.; Brennan, C.S.; Regenstein, J.M.; Jantanasakulwong, K.; Boonyawan, D.; Phimolsiripol, Y. Gliding arc discharge non-thermal plasma for retardation of mango anthracnose. LWT Food Sci. Technol 2019, 105, 142–148. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Zhang, X.; Zhuang, J.; Yang, S.; Bazaka, K.; Ken Ostrikov, K. Effects of Atmospheric-Pressure N2, He, Air, and O2 Microplasmas on Mung Bean Seed Germination and Seedling Growth. Sci. Rep. 2016, 6, 32603. [Google Scholar] [CrossRef]
- Ximing, X.; Gengenbach, T.R.; Griesser, H.J. Changes in wettability with time of plasma-modified perfluorinated polymers. J. Adhes. Sci. Technol. 1992, 6, 1411–1431. [Google Scholar] [CrossRef]
- Goldblatt, R.; Ferreiro, L.; Nunes, S.; Thomas, R.; Chou, N.; Buchwalter, L.; Heidenreich, J.; Chao, T. Characterization of water vapor plasma-modified polyimide. J. Appl. Polym. Sci. 1992, 46, 2189–2202. [Google Scholar] [CrossRef]
- Ling, L.; Jiafeng, J.; Jiangang, L.; Minchong, S.; Xin, H.; Hanliang, S.; Yuanhua, D. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 2014, 4, 5859. [Google Scholar] [CrossRef]
- Ahn, C.; Gill, J.; Ruzic, D.N. Growth of Plasma-Treated Corn Seeds under Realistic Conditions. Sci. Rep. 2019, 9, 4355. [Google Scholar] [CrossRef]
- Mildažienė, V.; Aleknavičiūtė, V.; Žūkienė, R.; Paužaitė, G.; Naučienė, Z.; Filatova, I.; Lyushkevich, V.; Haimi, P.; Tamošiūnė, I.; Baniulis, D. Treatment of common sunflower (Helianthus annus L.) seeds with radio-frequency electromagnetic field and cold plasma induces changes in seed phytohormone balance, seedling development and leaf protein expression. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.F.; Siow, K.S.; Ng, P.Y.; Majlis, B.Y. Enhancing the biocompatibility of the polyurethane methacrylate and off-stoichiometry thiol-ene polymers by argon and nitrogen plasma treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 613–621. [Google Scholar] [CrossRef]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Deposition and XPS and FTIR Analysis of Plasma Polymer Coatings Containing Phosphorus. Plasma Process. Polym. 2014, 11, 133–141. [Google Scholar] [CrossRef]
- Kone, M.; Kone, T.; Silue, N.; Soumahoro, A.B.; Kouakou, T.H. In Vitro Seeds Germination and Seedling Growth of Bambara Groundnut (Vigna subterranea (L.) Verdc. (Fabaceae)). Sci. World J. 2015, 2015, 595073. [Google Scholar] [CrossRef] [PubMed]
- Manmathan, H.; Shaner, D.; Snelling, J.; Tisserat, N.; Lapitan, N. Virus-induced gene silencing of Arabidopsis thaliana gene homologues in wheat identifies genes conferring improved drought tolerance. J. Exp. Bot. 2013, 64, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Dezfuli, P.M.; Sharif-Zadeh, F.; Janmohammadi, M. Influence of priming techniques on seed germination behavior of maize inbred lines (Zea mays L.). ARPN J. Agric. Biol. Sci. 2008, 3, 22–25. [Google Scholar]
- Rasband, W. Image v. 1.53g, Image processing and analysis in Java. Available online: https://imagej.nih.gov/ij/download.html (accessed on 29 January 2021).
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Drori, E. Cold radiofrequency plasma treatment modifies wettability and germination speed of plant seeds. Sci. Rep. 2012, 2, 741. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.F.; Siow, K.S.; Ng, P.Y.; Nai, M.H.; Lim, C.T.; Yeop Majlis, B. Ageing properties of polyurethane methacrylate and off-stoichiometry thiol-ene polymers after nitrogen and argon plasma treatment. J. Appl. Polym. Sci. 2016, 133, 44107. [Google Scholar] [CrossRef]
- Siow, K.S.; Kumar, S.; Griesser, H.J. Low-Pressure Plasma Methods for Generating Non-Reactive Hydrophilic and Hydrogel-Like Bio-Interface Coatings—A Review. Plasma Process. Polym. 2015, 12, 8–24. [Google Scholar] [CrossRef]
- Siow, K.S. Low pressure plasma modifications for the generation of hydrophobic coatings for biomaterials applications. Plasma Process. Polym. 2018, 15, 1800059. [Google Scholar] [CrossRef]
- Kessler, F.; Kühn, S.; Radtke, C.; Weibel, D.E. Controlling the surface wettability of poly(sulfone) films by UV-assisted treatment: Benefits in relation to plasma treatment. Polym. Int. 2013, 62, 310–318. [Google Scholar] [CrossRef]
- Stoffels, E.; Sakiyama, Y.; Graves, D.B. Cold atmospheric plasma: Charged species and their interactions with cells and tissues. IEEE Plasma Sci. 2008, 36, 1441–1457. [Google Scholar] [CrossRef]
- Pawlat, J.; Starek, A.; Sujak, A.; Kwiatkowski, M.; Terebun, P.; Budzeń, M. Effects of atmospheric pressure plasma generated in GlidArc reactor on Lavatera thuringiaca L. seeds’ germination. Plasma Process. Polym. 2018, 15, 1700064. [Google Scholar] [CrossRef]
- Tong, J.; He, R.; Zhang, X.; Zhan, R.; Chen, W.; Yang, S. Effects of Atmospheric Pressure Air Plasma Pretreatment on the Seed Germination and Early Growth ofAndrographis paniculata. Plasma Sci. Technol. 2014, 16, 260–266. [Google Scholar] [CrossRef]
- Vieira, R.D.; Scappa Neto, A.; Bittencourt, S.R.M.d.; Panobianco, M. Electrical conductivity of the seed soaking solution and soybean seedling emergence. Sci. Agric. 2004, 61, 164–168. [Google Scholar] [CrossRef]
- Mirdad, Z.; Powell, A.A.; Matthews, S. Prediction of germination in artificially aged seeds of Brassica spp. using the bulk conductivity test. Seed Sci. Technol. 2006, 34, 273–286. [Google Scholar] [CrossRef]
- Demir, I.; Cebeci, C.; Guloksuz, T. Electrical conductivity measurement to predict germination of commercially available radish seed lots. Seed Sci. Technol. 2012, 40, 229–237. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Zheng, G.H.; Wang, X.F.; Liu, Y.; Yan, Y.T.; Lin, J. Possible involvement of K+/Na+ in assessing the seed vigor index. J. Integr. Plant Biol. 2005, 47, 935–941. [Google Scholar] [CrossRef]
- Sera, B.; Spatenka, P.; Serý, M.; Vrchotova, N.; Hruskova, I. Influence of plasma treatment on wheat and oat germination and early growth. IEEE Plasma Sci. 2010, 38, 2963–2968. [Google Scholar] [CrossRef]
- Koga, K.; Attri, P.; Kamataki, K.; Itagaki, N.; Shiratani, M.; Mildaziene, V. Impact of radish sprouts seeds coat color on the electron paramagnetic resonance signals after plasma treatment. Jpn. J. Appl. Phys. 2020, 59, SHHF01. [Google Scholar] [CrossRef]
- Sahu, B.B.; Jin, S.B.; Han, J.G. Development and characterization of a multi-electrode cold atmospheric pressure DBD plasma jet aiming plasma application. J. Anal. At. Spectrom. 2017, 32, 782–795. [Google Scholar] [CrossRef]
- Hosseini, S.I.; Mohsenimehr, S.; Hadian, J.; Ghorbanpour, M.; Shokri, B. Physico-chemical induced modification of seed germination and early development in artichoke (Cynara scolymus L.) using low energy plasma technology. Phys. Plasmas 2018, 25, 013525. [Google Scholar] [CrossRef]
- Holc, M.; Junkar, I.; Primc, G.; Iskra, J.; Titan, P.; Mlakar, S.G.; Kovac, J.; Mozetic, M. Improved sprout emergence of garlic cloves by plasma treatment. Plasma Med. 2016, 6. [Google Scholar] [CrossRef]


| Seed Type | Water Uptake Per Gram (g) | Water Contact Angle (°) |
|---|---|---|
| Untreated seeds | 0.6158 ± 0.0604 | 95 ± 7 |
| UV-treated | 0.6102 ± 0.0721 | 94 ± 6 |
| Element | C | N | O | N/C | O/C |
|---|---|---|---|---|---|
| Control | 81.0 | 4.0 | 15.0 | 0.05 | 0.18 |
| Treated | 67.8 | 5.0 | 27.2 | 0.07 | 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, N.; Masood, A.; Siow, K.S.; Wee, M.F.M.R.; Auliya, R.Z.; Ho, W.K. Effect of H2O-Based Low-Pressure Plasma (LPP) Treatment on the Germination of Bambara Groundnut Seeds. Agronomy 2021, 11, 338. https://doi.org/10.3390/agronomy11020338
Ahmed N, Masood A, Siow KS, Wee MFMR, Auliya RZ, Ho WK. Effect of H2O-Based Low-Pressure Plasma (LPP) Treatment on the Germination of Bambara Groundnut Seeds. Agronomy. 2021; 11(2):338. https://doi.org/10.3390/agronomy11020338
Chicago/Turabian StyleAhmed, Naeem, Asad Masood, Kim S. Siow, M. F. Mohd Razip Wee, Rahmat Zaki Auliya, and Wai Kuan Ho. 2021. "Effect of H2O-Based Low-Pressure Plasma (LPP) Treatment on the Germination of Bambara Groundnut Seeds" Agronomy 11, no. 2: 338. https://doi.org/10.3390/agronomy11020338
APA StyleAhmed, N., Masood, A., Siow, K. S., Wee, M. F. M. R., Auliya, R. Z., & Ho, W. K. (2021). Effect of H2O-Based Low-Pressure Plasma (LPP) Treatment on the Germination of Bambara Groundnut Seeds. Agronomy, 11(2), 338. https://doi.org/10.3390/agronomy11020338








