Abstract
The water–development nexus is essential for the advancement and progress of cities in the face of problems such as climate change, water security and increasing environmental stress in the agricultural sector. Aiming for a circular economy and, at the same time, improving the resilience of water supply alternatives and achieving a goal of zero waste, this work presents a technical–economic study of a novel continuous ultrafiltration (c-UF) system with self-cleaning capacity coupled to an ozonation process, for the treatment of urban WWTP effluent. The removal efficiencies achieved were analysed both through macroscopic parameters (suspended solids, turbidity) and for the most frequently occurring contaminants of emerging concern (CECs). Consequently, an effluent suitable for irrigation was obtained, with a total recovery factor of 97.92%, a concentration of suspended solids (SS) below 1 mg L−1, 0.06 NTU turbidity and toxicity free, complying with the new European Regulation on Water Reuse (EU 2020/741). A comparative analysis of the proposed process with regard to conventional tertiary treatment revealed that the proposed process was 39.1% more economic, with a cost of 0.0325 € m−3. This alternative treatment will be of great interest because of its favourable technical–economic characteristics, being postulated as a basic process for implementation in modern water reuse plants.
1. Introduction
Urbanization is one of the most significant trends of the 21st century. By 2050, it is expected that more than 68% of the world’s population will reside in large cities, which will mean a movement of more than 4000 million people migrating from rural areas to large population centres [1]. Currently, many of these cities are experiencing rapid and uncontrolled growth, largely by people with low per capita income to which the government is not able to provide infrastructures consistent with those of a developed city. Authors such as Luthy et al. [2] concluded in their study of cities in California that most people living in newly developed residential areas were living in areas with limited water availability, and more than 68% of these new settlements in large cities lacked an adequate sanitation system. Many of these untreated or conventional primary treatment wastewaters end up in ditches or urban rivers with consequent risks to public health [3].
Water pollution is one of the largest threats to health, human development and the environment. The composition of these wastewaters generally varies depending on the different polluting sources [4]. Domestic wastewater is characterized by a high concentration of nutrients (N, P, K, Ca and Mg) and by high bacterial loads, such as pathogenic bacteria, viruses, common protozoa (Giardia and Cryptosporidium) and helminths, which can pose a direct threat to human health. Therefore, given the epidemiological health hazard, eliminating pathogens is one of the objectives of wastewater treatment systems [5]. In addition, these urban wastewaters can also contain contaminants of emerging concern (CECs). This category includes any synthetic or naturally occurring chemical or any microorganism that is not commonly controlled and that can cause adverse effects on both human health and the environment [6]. Among the main categories of CECs are Pharmaceuticals and Personal Care Products (PPCPs) and endocrine disruptors (EDCs). In 2013, the European Parliament expanded the classification by Directive 2013/39/EU to include steroid hormones, surfactants, perfluorinated compounds, flame retardants, industrial additives, drugs and UV filters. Many of these contaminants, due to their low concentration of approximately 3 ng L−1 [7], are only regulated to a certain extent in the European Union (EU) and USA. In the EU, the implementation of this regulation is collected through a list of priority substances listed as CECs in Decision 2015/495/EU and Decision 2018/840/EU. The European Commission, as it became aware of the adverse effects of other substances not included in this list, has expanded the number of substances to be monitored through the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH). In the USA, the CECs are regulated from the Contaminants Candidate List of unregulated contaminants [8]. The long-term impacts of CECs are unknown since they can bioaccumulate in aquatic species. Regulatory authorities, such as the European Environmental Agency (EEA)—integrated by 32 Member States and seven cooperating countries—the International Chemical Safety Program (IPCS), the World Health Organization (WHO) and the U.S. Environmental Protection Agency (EPA) [9], are responsible for developing regulations and directives that improve the protection and sanitary safety of these freshwater resources.
The effects of water pollution also directly or indirectly influence the economic activity of large cities, such as industrial production, fishing, aquaculture and tourism [10]. In short, poor water quality hinders economic development. For this reason, large cities must adapt to this new reality and improve wastewater management by properly treating it and its subsequent reuse to minimize the negative impact of discharge and at the same time maximize the economic benefits it can bring without harming the environment and human health. This will change the way in which wastewater is viewed, becoming a point on which to obtain an economic benefit instead of a burden to deal with [1]. One of the potential sectors that could benefit from this new concept is horticulture. Reusing urban wastewater in peri-urban horticultural facilities in addition to securing continuous water resources would provide ecological services [11]. The produce obtained through this approach would reduce the carbon footprint of the food that people eat, in addition to modifying favourably the surrounding landscape. This solution would be most effective in arid or semiarid areas, whose population are notably increasing. In addition, in many countries where certain political and social conflicts makes it difficult to transport water from one region to another, the reuse of properly treated urban wastewater would provide a solution to this problem. Furthermore, having to construct expensive transfer pipelines, dams, etc., would be avoided. In short, proper water management and subsequent reuse in peri-urban agriculture would protect and optimize the use of natural resources [4,12].
For example, in Spain, one of the countries in Europe with the highest water consumption per capita (800.9 m3 inhabitant−1) and with an agricultural consumption of 25.47 × 109 m3 year−1 (68.19% of total use) is the first European country with reuse capacity and one of the few that has its own legislation that regulates it through Royal Decree 1620/2007 [13]. In addition, the European Union, aware of a regulatory gap, has released a new regulation on the minimum requirements that reused water must meet (EU 2020/741) [14], with mandatory compliance within a period of 3 years, from 26 June 2023, for all countries of the European Union that will use this unconventional water source. This new regulation aims to ensure that the quality of reclaimed water for agricultural irrigation reaches the same levels of quality and control in all countries of the European Union. This community regulation defines the types of crops where reclaimed waters can be used, the minimum water quality requirements and stipulates the quality control that these waters must comply with. The reuse of urban waters in peri-urban agriculture would considerably reduce the environmental impact of this important economic activity and its dependence on weather effects, freeing up resources that could be used for domestic consumption. In turn, this approach would directly connect with the new paradigm of a circular economy in the new cities of the 21st century [15].
Today’s purification technology generates effluents of reclaimed water of varying quality, even up to drinking water standards. To apply them for any agricultural use, advanced tertiary treatments are required in waste water treatment plants (WWTPs) [16,17]. However, reuse is not without risks, both in terms of its possible lack of agronomic suitability for irrigation as well as its sanitary suitability, due to the possible presence of pathogenic microorganisms in inadequately reclaimed waters [18]. The microbiological health risks associated with irrigating with purified water depend on several factors, which can be grouped into (i) factors associated with the characteristics of the water and the treatment received, among which include the microbial load and the purification system used; and (ii) the factors derived from the manipulation of water in irrigation. To avoid any risk due to misuse and to ensure adequate water quality for reuse, from a health and environmental point of view, it is necessary to implement robust purification processes together with an analytical control or self-control program.
For compliance and obtaining an effluent that meets the legal requirements for subsequent reuse in agriculture [5], different conventional and advanced physical and biological treatment options have been investigated for eliminating pathogenic microorganisms and CECs [19].
Table 1 shows the treatments that have performed best for eliminating CECs as well as other pathogens based on the use of physical, biological or chemical treatments. Biological processes have been one of the options that have most attracted researchers due to their low cost of implementation, ease of scaling and sustainability with the environment [20]. Among the most researched options are the active sludge process [21]. However, these biological treatment processes are inefficient, especially with regard to pharmaceutical products and are not sufficient for effectively eliminating several nonbiodegradable CECs [22].

Table 1.
Technologies for eliminating a wide range of contaminants of emerging concern (CECs) and mitigation of pathogenic organisms of effluents from secondary treatment.
For physical processes, these alternatives involve the use of adsorption processes [23], membranes, such as microfiltration [24], or ultrafiltration (UF) [4], despite the problem of surface fouling [25]. It is noteworthy that the removal of CECs by conventional physical treatments is unfeasible due to the low octanol/water partition coefficients [26]. According to Table 1, UF-based processes have greater advantages in terms of longevity and sustainability of the process [27], unlike processes such as adsorption, which presents difficulties once the adsorbent is saturated and involves high reclamation costs, with the only alternative being landfill management or energy recovery [28]. In the case of UF, separation is achieved mainly by the adsorption of contaminants on the surface of the membrane and electrostatic repulsion. In any case, the retention capacity depends on the specific matrix of the water to be treated and the presence of organic matter. Therefore, UF has application potential for obtaining high-quality effluents since it reduces parameters such as turbidity, suspended solids, bacteria (such as E. coli), coliforms and a wide range of CECs; it also has a low capital investment, versatility, no generation of sludge, no odours and ease of scaling that guarantees consistent quality of the treated water, regardless of the changes in the influent [29]. One of the drawbacks highlighted by authors such as Kim et al. [25] is the appearance of fouling, which significantly influences the membrane properties, such as porosity and hydrophobicity, in addition to operating conditions, such as pressure. Therefore, a system that counteracts the negative effects of fouling and allows normal operation is something that would facilitate the large-scale implementation of this technology in urban WWTPs.
Another issue that the application of UF in the WWTP must resolve is the treatment of the concentrates from the reject stream. This stream is composed of organic contaminants, refractory products (such as PPCPs) and pathogenic microorganisms. Although the discharge to the water medium in many cases is not sufficiently regulated, the uncontrolled discharge of these streams into the environment is not acceptable. Here, AOPs can play a fundamental role in treating this stream. According to Table 1, ozonation processes demonstrate a high CEC removal yield between 90 and 100% with a high oxidation rate due to the high reactivity of ozone and hydroxyl radicals towards many of the organic compounds present, such as carbamazepine, diclofenac or bisphenol A, among others. However, this requires specific reaction conditions, such as pH. In this case, a pH = 9, which is usual for ozonation of this kind of effluent, was used [45,46]. In addition, it is a powerful disinfecting agent capable of inactivating a wide range of pathogens, such as bacteria, viruses, protozoa and prion proteins, without leaving a toxic residue [47]. Added to the outstanding advantages is that it does not generate sludge and it makes it possible to generate ozone using renewable energy sources.
In short, this new problem of this century constitutes one of the great priority challenges society must solve. Such is the importance that the United Nations in 2015 included it in the Sustainable Development Goals (SDGs) adopted by most countries, through SDG 6 for Clean Water and Sanitation, which addresses the problem of water scarcity, sanitation and hygiene, treatment and reuse of wastewater. Linked to this problem, SDG 3 for good health and well-being, SDG 11 for sustainable cities and communities and SDG 12 for responsible production and consumption promote the economic development of new cities together with sustainable agriculture and proximity to population centres. In this context, the nexus between the development of sustainable cities contributes through the appropriate reuse of their wastewater for local, sustainable and safe agriculture. Therefore, based on the background described, the use of a continuous ultrafiltration (c-UF) process that mitigates the effects of dreaded fouling through a self-cleaning system together with the treatment of the reject stream through an ozonation process is a real and low-cost alternative to conventional tertiary treatment based on coagulation–flocculation followed by a disinfection process using UV light or sodium hypochlorite for the efficient treatment of CECs and pathogenic microorganisms present in the urban waters of a WWTP.
This study aims to study the reuse of effluent from a secondary treatment of a real urban WWTP for use in agricultural irrigation through a c-UF pilot plant with a self-cleaning system coupled to an ozonation system for treating the reject stream. For this, the main parameters related to the quality of the treated water obtained (suspended solids, turbidity, total organic carbon and microbiology) will be analysed for both the UF and ozonation processes in addition to the removal yields of the most frequent CECs. Additionally, a study will be carried out in which the operational cost of the proposed technology and configuration will be evaluated and compared with the cost of tertiary treatment in a conventional WWTP.
2. Materials and Methods
2.1. Reagents and Chemical Used
Acetaminophen (C8H9NO2), atrazine (C8H14CIN5), diuron (C9H10Cl2N2O), ciprofloxacin hydrochloride (C17H19ClFN3O3), norfloxacin hydrochloride (C16H19ClFN3O3), isoproturon (C12H18N2O), simazine (C7H12ClN5), sulfamethoxazole (C10H11N3O3S) and trimethoprim (C14H18N4O3) were acquired from Fluka (Buchs, Switzerland). Amitriptyline hydrochloride (C20H23N), butylparaben (C11H14O3), 2-hydroxybenzothiazole (C7H5NOS), caffeine (C8H10N4O2), clomipramine hydrochloride (C19H24Cl2N2), carbamazepine (C15H12N2O), potassium perfluoro-1-octanesulfonate (C8F17KO3S), diclofenac sodium salt (C14H10Cl2NNaO2), methylparaben (C8H8O3), imipramine hydrochloride (C19H24N2), nortriptyline hydrochloride (C19H21N), perfluoro-n-octanoic acid (C8HF15O2), phenytoin (C15H12N2O2), potassium perfluoro-1-butanesulfonate (C4F9KO3S), progesterone (C21H30O2), sulfadiazine (C10H10N4O2S) and testosterone (C19H28O2) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Clofibric acid (C10H11ClO3), ketoprofen (C16H14O3), bezafibrate (C19H20ClNO4) and propranolol hydrochloride (C16H21NO2) were acquired from MP Biomedicals (Illkirch-Graffenstaden, France). Genistein (C15H10O5), genistin (C21H20O10) and glycitin (C22H22O10) were purchased from Extrasynthese (Lyon, France), perfluorooctane sulfonamide (C8H2F17NO2S) from Dr. Ehrenstorfer (Augsburg, Germany), losartan potassium (C22H22ClKN6O) from Merck (Darmstadt, Germany), valsartan (C24H29N5O3) and telmisartan (C33H30N4O2) from Boehringer (Ingelheim am Rhein, Germany), irbesartan (C25H28N6O) from Sanofi (Paris, France) and eprosartan mesylate (C24H28N2O7S2) from Solvay Pharmaceuticals (Brussels, Belgium). Acesulfame potassium (C4H4KNO4S) and sucralose (C12H19Cl3O8) were supplied by Supelco (Bellefonte, PA, USA). The purity of all the target analytes was >95%. Methanol (CH3OH, HPLC grade, 99.9%), ethyl acetate (C4H8O2, 99.8%) and n-hexane (C6H14, HPLC grade, 95%) were supplied by Labscan (Dublin, Ireland); ethylenediaminetetraacetic sodium salt (Na2EDTA, 99.0–101.1%) and ammonia solution (25% as NH3) by Panreac (Barcelona, Spain); formic acid (HCOOH ≥ 98%) by Scharlau (Barcelona, Spain); and sodium chloride (NaCl, >99.8%), sodium sulfite (Na2SO3, >100%) and acetic acid (CH3COOH, 100%) by Merck (Darmstadt, Germany).
2.2. Equipment Used
2.2.1. Galindo WWTP
The experiments were carried out in the Galindo WWTP, located in the municipality of Sestao, in the historical territory of Bizkaia, Basque Country (43°18′35.38″ N and 3°0′25.92″ W, north of Spain). The WWTP of Galindo serves the entire area of influence of Greater Bilbao, with a population equivalent to 1,200,000 inhabitants and is capable of treating in secondary treatment an average daily flow of 6.0 L s−1 with a maximum allowable in the primary treatment flow of 12 L s−1. The treatment line of this WWTP consists of a primary, secondary and tertiary treatment, according to the following units:
- The primary treatment consists of a fine grinding unit followed by a degreasing and grit removal system. The water from the sandblasting passes through a distribution channel subjected to agitation where the installed rectangular decanters are sent. In this operation, approximately 75% of the suspended solids (SS) and 50% of the organic matter are removed.
- The secondary treatment consists of extended aeration in a biological reactor and secondary decantation. Approximately 10 t day−1 of sludge accumulates in the decanters, which is treated and energetically recovered to supply part of the needs of the WWTP facilities.
- The tertiary treatment consists of a coagulation process with Al2(SO4)3 and sodium hypochlorite (NaClO) followed by a flocculation system and subsequent lamellar decantation. The sludge produced is sent to the water line. The clarified water is directed to sand filters with an average filtration rate of 7.0 m3 m−2 h−1, adopting a filtration surface of 21.0 m2. Subsequently, the water is disinfected in a chamber consisting of 80 lamps with a nominal consumption of 250 W lamp−1 and a maximum power consumption of 12 kW.
The effluent obtained from the Galindo plant complies with Directive 91/271/EEC for urban wastewater treatment. The objective of this study is to propose an alternative treatment technology that is cheaper than the existing conventional tertiary treatment and that complies with the new regulation of the European Commission [14] in terms of water reuse for agricultural use so that the treated effluent currently discharged into the Ballonti River can be adequately treated with the technology described in the following section and reused for irrigation in peri-urban agricultural facilities of Greater Bilbao.
2.2.2. Membrane Technology Coupled to Ozonisation Treatment
A part of the effluent from the secondary treatment is diverted to the c-UF pilot plant at a flow rate of 3.3 m3 h−1. The system consists of two independent frames. In the first frame, there are 4 UF modules (c-UF4XS, FLUYTEC, Erandio, Spain), containing a bundle of ultrafiltration membranes inside. In this same frame is the set of valves, pipes and instrumentation for the supply, drainage and permeate outlet. The second frame is composed of a main water tank intended for washing, a feed and washing pump, a compressed air system, roughing filtration (prefilter) and the corresponding valves, pipes and instrumentation.
In this ultrafiltration unit, hollow fibre membranes made from polyethersulfone (PES) are used, which are best suited for the operation and operate with an inside–outside configuration. The properties of this membrane are shown in Table 2.

Table 2.
Characteristics of the ultrafiltration (UF) membranes.
The c-UF plant has a self-cleaning device that extends the filtration operating time, decreasing the number of cleaning cycles that must be performed to maintain the membrane properties over time. This system consists of a filter that is placed prior to the c-UF equipment, which has a self-cleaning system through a rotating purge arm, as shown in Figure 1.
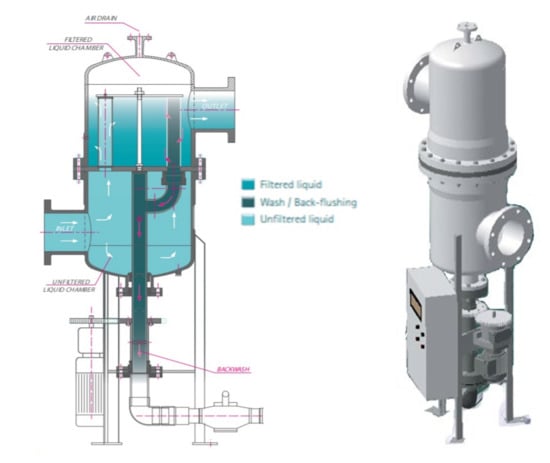
Figure 1.
Diagram of the self-cleaning device of the c-UF pilot plant [48].
According to Figure 1, when the pressure difference between the inlet, untreated water and outlet of the drainage pipe reaches a predetermined value, this arm goes into operation and starts the backwashing process automatically. The arm is connected to the filter element to be cleaned, opening a solenoid valve, which produces vacuum pressure inside this element. Thus, part of the filtered water is forced to pass in the opposite direction, dragging the solids and encrustations that block the filter. This process is repeated with each of the filter elements until the washing cycle is completed.
The reject stream and the wash waters were treated by an ozonation process in a pilot-scale gas-liquid contactor connected to the c-UF equipment. The ozonisation pilot system consisted of a 33.03 L stainless steel cylindrical contactor (with dimensions 795 mm × 230 mm), where the wastewater to be treated was introduced. Ozone was produced from extra pure oxygen in the ozone generator (TRIOGEN LAB2B, Triogen, De Goor, The Netherlands) and introduced into the reactor through a gas diffuser installed at the bottom of contactor, thus ensuring a perfect homogenization of the system. To improve the contact between the descending liquid and ascending gas, the column is provided on the top by a spray that atomizes the liquid. Part of the dissolution that the centrifugal pump (TMP04.08 S GF VN2BEN3, Argal Pumps, Brescia, Italy) recirculates is thrown up and returns to the reactor at the top where it is sprayed, which can also destroy the foams that are often formed due to the strong agitation of the system. The recirculation system provides enough stirring to get a good mixture in the system.
2.3. Experimental Setup and Procedures
2.3.1. Operation of Pilot Plant
The method of operating the c-UF equipment was differentiated into two modes: filtration mode and washing mode. During the filtration mode, dead-end filtration was performed with an inside–outside configuration. In this way, all the water was forced to cross the membrane from the inside out, retaining the suspended solids and solutes (CECs, pathogenic microorganisms) on the inner surface of the fibres.
The c-UF equipment was fed through a feeding branch connected to the secondary treatment at a rate of 3.3 m3 h−1 with a centrifugal pump (ZMR02.30 S GF V BS8 B EN3, Argal Pumps, Brescia, Italy), installed at the bottom of the equipment. The permeate obtained with each module, with a flow rate of 41.5 L m−2 h−1, was collected in the same branch of the filtered product. This parameter was continuously recorded through flow metres (FLS F3.80, Aliaxis, Okondo-Araba, Spain) (see Figure 2) to control membrane fouling and keep the production of treated water constant. Each filter operating cycle lasted 47 min. At the end of each cycle, the transmembrane pressure increased from the initial 0.6 bar to 2.3 bar.
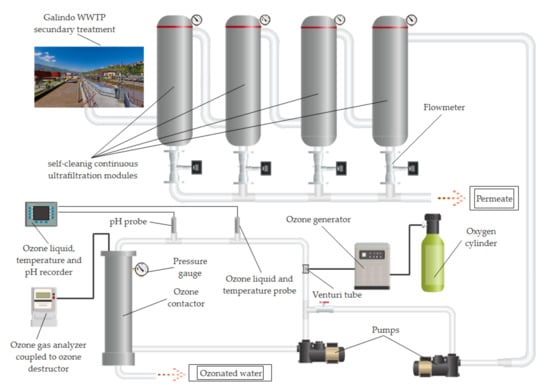
Figure 2.
Diagram of the continuous ultrafiltration process coupled to the ozonation system for the treatment of the reject stream.
The second mode corresponded to washing. This mode consisted of a sequence of washes (flushing, backwashing and rinsing composed of Chemical Enhanced Flushing (CEF) and Cleaning In Place (CIP)), whose main objective was to recover the initial state of the membrane. This mode of operation effectively removed the solids and encrustations deposited on the inner surface of the fibres. This sequence of washes was performed module by module or in pairs, with the intention of keeping the permeate flow constant. This process lasted 9 min, at which time the transmembrane pressure reached a pressure of 0.6 bar. The ozonation experiments of the reject stream consisted of a batch treatment. For this, the ozonation contactor was filled with 30.0 L of water from the reject stream. Subsequently, ozone was introduced into the contactor through a venturi tube placed in the recirculation loop followed by a static mixer. In addition, the contactor was provided with a liquid atomizer at the top to improve the gas–liquid (G-L) contact (see Figure 2). The experiments were carried out at a temperature of 15.0 °C, an initial pH of 9.0, a pressure of 1.5 bar, under constant flow of ozone (4 L min−1) and constant ozone concentration at the inlet of 6.4 mg L−1 during 180 min. The system pressure was controlled with a manometer (111.10, Wika Instruments, Barcelona, Spain) and the ozone concentration in the gas phase was monitored with an ozone analyser (BMT 964C, BMT MESSTECHNIK GMBH, Stahnsdorf, Germany). The dissolved ozone concentration in the liquid phase and temperature were measured with a probe (Rosemount 499AOZ-54, Emerson, Alcobendas, Spain). The pH was controlled with a probe (Rosemount 399-09-62, Emerson, Alcobendas, Spain), integrated with an ozone probe in a recorder (Rosemount Solu Comp II, Emerson, Alcobendas, Spain). Gas-phase residual ozone was removed with a thermocatalytic ozone destructor (KVM 20-2, ZonoSistem, El Puerto de Santa María, Spain). All experiments were conducted in duplicate.
2.3.2. Sampling Method
The analysed samples of the influent and effluent of the c-UF process and the ozonation of the reject stream were collected according to the procedure established by the EPA [49] for composite samples. Samples composed of 2.0 L were collected in triplicate over four selected days in May 2019. On each day, the samples were collected at 4 different hours that were sufficiently representative, taking into account the variable wastewater stream and contaminant concentration (8:00, 12:00, 15:00 and 20:00 h). Each 2.0 L sample in turn consisted of 4 subsamples taken every 5 min around the sampling time. For the feed influent of the c-UF plant, the hydraulic retention times of the secondary treatment were considered, which fluctuated between 28 and 38 h, depending on the organic load of the raw wastewater.
The samples were collected from authorized sampling points. Once collected they were transported to the laboratory in a portable cooler (4 °C). The collected samples were filtered with a 0.45 µm MF-Millipore membrane (Merck KGaA, Darmstadt, Germany) for the subsequent identification and quantification of CECs. In the case of the samples taken from the ozonator, sodium bisulphite was used as a reducing agent to stop the ozone reaction at a rate of 2.2 g Na2SO3 g of O3−1 [50].
2.4. Analytical Techniques
The identification and determination of the concentration of the CECs present in the water samples were analysed in triplicate according to the analysis method described by Mijangos et al. [51]. The method consisted of taking 100.0 mL of each sample to which 4.25 mL of Na2EDTA (0.2 M) and 0.8 mL of an HCOOH solution were added to acidify the sample to pH = 2.0. The CECs were subjected to solid-phase extraction (SPE) (OASIS-HLB, hydrophilic–lipophilic-balanced, 200 mg, Waters, Milford, USA). The cartridge was previously conditioned with 5.0 mL of methanol (CH3OH), 5.0 mL of Milli-Q water (MQ) and 5 mL of acidified Milli-Q water at pH = 2.0. Subsequently, the cartridges were rinsed with 6 mL of Milli-Q water to remove any impurities that could interfere with the analysis. Then, the SPE cartridges were vacuum-dried for 1 h. The sample was loaded into the cartridge at a constant flow of 5.0 mL min−1. Methanol (6.0 mL) was used to elute the analytes to subsequently evaporate the extract at 35.0 °C under a nitrogen stream. Finally, before introducing the samples into the liquid chromatography with tandem mass spectrometry (LC-MS-MS) equipment, they were reconstituted in 200.0 µL of a CH3OH:H2O phase (30:70, v:v) and filtered with a 0.22 µm polypropylene filter (Phenomenex, Torrance, CA, USA).
The LC–MS-MS analysis was performed using an Agilent 1260 series HPLC chromatograph coupled to an Agilent 6430 triple quadrupole (QqQ) mass spectrometer equipped with an electrospray ionization source (ESI) (Agilent Technologies, Palo Alto, CA, USA). The separation of the target analytes was carried out using a Kinetex F5 100 Å core-shell 2.1 mm × 100 mm, 2.6 µm column coupled to a Kinetex F5 pre-column 2.1 mm × 4.6 mm, 2.6 µm (Phenomenex, Torrance, CA, USA). Then, 10 µL of sample was injected into the system and the column was maintained at 35 °C during the chromatographic run. The separation was performed at a constant flow of 0.3 mL min−1 under gradient elution with a binary mixture of: H2O:CH3OH (95:5, v:v) (mobile phase A) and CH3OH:H2O (95:5, v:v) (mobile phase B), both containing 0.1% formic acid. The gradient profile started with 30% B, which was increased to 50% after 4 min and maintained for 12 min. Then, it was increased to 90% B, where it was maintained for 10 min. Initial gradient conditions (30% B) were then achieved in 6 min, where it was finally held for another 10 min (post-run step). Electrospray ionization was carried out using a N2 flow rate of 12 L min−1, a capillary voltage of 3500 V, a nebulizer pressure of 3.0 bar and a source temperature of 350 °C. Quantification was performed recording the three most intense transitions for each analyte. Both voltages, according to the target analytes, were simultaneously applied in a single injection.
The determination of suspended solids (SS) was carried out according to the procedure described in ISO 11923:1997, “Water quality—Determination of suspended solids by filtration through glass-fibre filters”. Turbidity was analysed using the turbidimeter Eutech TN-100 (Thermo Scientific, Singapore). The degree of mineralization was quantified by dissolved organic carbon (DOC) analysis on a Shimadzu TOC-VSCH analyser with ASI-V autosampler (Izasa Scientific, Alcobendas, Spain). Toxicity was evaluated in duplicate using the Microtox® bioassay in a Microtox® toxicity analyser, Azur 500 model (Microbics Corp., New Castle, Delaware, USA). The measurements were carried out according to ISO 11348-3 (1998), “Water Quality—Determination of the inhibitory effect of water samples on the light emission of Aliivibrio fischeri (Luminescent bacteria test)—Part 3: Method using freeze-dried bacteria” [52]. The results of this assay are usually expressed as EC50, which represents the percentage of sample dilution (%, v:v) that causes a 50% reduction in bacteria luminescence after 15 min of exposure. The Toxicity (TU) parameter used was defined according to Equation (1) [46]:
where C0 corresponds to the concentration of the compound used to obtain its EC50 value. Total coliforms and Escherichia coli (E. coli) measurements were analysed according with the Standard Methods for the Examination of Water and Wastewater, 9222 B and 9221 B, in a laboratory incubator Culture Model 153 (Hach Lange GmbH, Düsseldorf, Germany) [52].
3. Results and Discussion
3.1. Application of c-UF for the Removal of CECs from a Secondary Treatment
3.1.1. Characterization of the Influent and Effluent at Galindo WWTP
The influent and effluent of the Galindo WWTP during May 2019 were characterized. Table 3 shows the maximum, minimum and mean values of the different parameters analysed, as well as the standard deviation. The values of the different parameters observed indicate that the WWTP treats medium pollution loads [53].

Table 3.
Physicochemical characterization of the treated wastewater at Galindo WWTP (May 2019).
The variability observed between the maximum and minimum values is due to the different lifestyle habits of the population served by the Galindo WWTP. Regarding CECs in these waters, concentrations similar or lower than those detected in other WWTPs around the world were identified. For example, in Greece, they detected ciprofloxacin concentrations of 460 ng L−1 [54], and in Rome, caffeine concentrations of 20 µg L−1 [55].
The removal of parameters such as dissolved organic carbon (85.2%) and toxicity (<1 TU) shows that the WWTP of Galindo achieves a quality effluent in accordance with Spanish regulations (Royal Decree Law 11/1995). However, certain CECs, such as 2-hydroxybenzothiazole or carbamazepine, yielded 64.6% and 14.1%, respectively. According to Machado et al. [56] or Egea-Corbacho et al. [55], not completely eliminating CECs could create problems in human health for reuse applications in agricultural activities and the environment due to their low biodegradability.
3.1.2. Removal Yields of CECs in the c-UF Pilot Plant
In Table 4, the removal yields of the CECs detected in the Galindo WWTP were analysed, both for the biological treatment and for the c-UF plant following this process.

Table 4.
Removal yields of the different CECs in the secondary treatment and through the c-UF pilot plant with a self-cleaning system.
The variability of the observed yields for the different CECs is due to the strong dependence of the water stream treated in the WWTP with the precipitations [57]. It was observed that CECs such as genistein or progesterone were completely removed in the secondary treatment, in line with what was observed by Gros et al. [58], due to removal mechanisms based on adsorption and biodegradation. According to the study by Yu et al. [59], of the two possible pathways, biodegradation under aerobic conditions would be the main pathway removing both compounds.
However, drugs such as phenytoin (24.4%), irbesartan (7.2%) or carbamazepine (8.7%) were observed to be completely refractory to biological treatment [60]. Guedes-Alonso et al. [61] obtained carbamazepine removal yields of 5.7%, which are similar to those obtained in the Galindo WWTP. This low removal yield of carbamazepine is due to its molecular structure and hydrophilicity. For irbesartan or phenytoin, removal yields were similar to those observed by Das et al. [62] (with 21.1% and 6.5%, respectively) because these antibiotics induce an inhibition process of biocenosis [63].
Regarding the removal of CECs by ultrafiltration, it was observed that compounds such as amitriptyline (63.9%) and perfluorooctanesulfonamide (48.4%) performed better than others such as ciprofloxacin (16.7%) or sulfamethoxazole (10.2%). The difference in performance is due to the changing adsorption capacity of the filter membrane according to the CEC. Ferreiro et al. [9] studied the adsorption mechanism that occurred in an ultrafiltration process at certain concentrations of CECs, such as amitriptyline or sulfamethoxazole. According to the adsorption equilibria of such compounds [9], the high concentration of both CECs at the c-UF inlet favours retention during filtration.
To evaluate the efficiency of this c-UF unit, it is necessary to evaluate and determine a recovery factor, Y (%), that takes into account the overall performance of this operation, as defined in Equation (2):
where QP is the permeate stream, QA is the feed stream and QR is the reject stream in m3 h−1. According to Equation (2), the recovery rate associated with the c-UF unit was 97.9% for 425.67 min, in which a complete cycle of ultrafiltration followed by washing occurred. The recovery obtained from this c-UF system is slightly higher than that obtained by other ultrafiltration systems studied in the literature. Fan et al. [64] studied the influence that materials had on the operation of ultrafiltration modules on a pilot scale. According to this study, they obtained recoveries of 93.5%, 95.3% and 95.9% for the modules built in PES, polyvinylidene fluoride (PVDF) and polyacrylonitrile (PAN), respectively. The slight increase in the recovery factor of 4.4% is largely due to the c-UF self-cleaning system, which is explained in the following subsection.
3.1.3. Effect of the Self-Cleaning System on the Water Quality
To evaluate the effect of self-cleaning, the suspended solids (SS), turbidity and dissolved organic carbon (DOC) were followed both in the permeate and in the flushing and backwashing operations (see Figure 3). A significant reduction of 87.5% in SS was observed in the permeate stream. In contrast, the streams corresponding to washing the membranes presented a much higher SS concentration compared to the feed stream. It should be noted that within the washing cycle, flushing had the highest SS removal (71 mg L−1), while for backwashing, only 30 mg L−1 was removed. This indicates that the washing cycles are not nearly effective enough to partially recover the yield at the beginning of the filtration operation. According to Bourgeous et al. [65], this inefficiency is due to progressive fouling over time, either by solid material encrusting on the membrane surface or deposition and accumulation of particles in the form of gel inside the pores. The differences observed in the degree of SS removal between the four selected days explain the variability of the characteristics of the effluent from the secondary treatment.
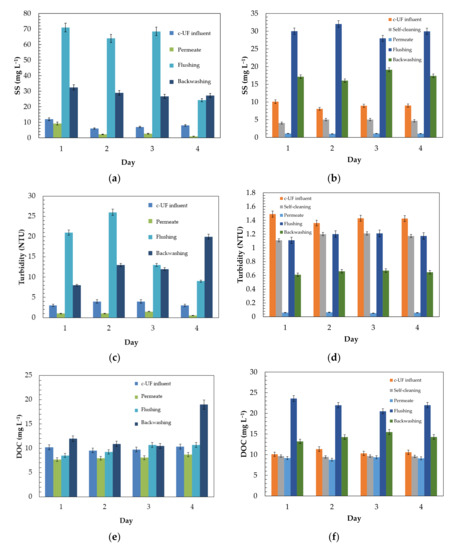
Figure 3.
Comparison of the traditional c-UF system and self-cleaning c-UF on an effluent from the secondary treatment. Evaluation of macroscopic parameters of water quality: (a) suspended solids (SS); (c) turbidity; and (e) dissolved organic carbon (DOC); (b,d,f) with self-cleaning system.
Regarding the experiments performed with the self-cleaning system, a reduction in SS of 90.1–100% in the permeate stream was observed (see Figure 3b). This is because this novel system prevents the formation of a cake and the accumulation of a number of particles that block the membrane surface. Installing a self-cleaning system reduces the concentration of solids between 38 and 60%. This improvement is also noticeable in the wash streams, in which there was a lower concentration of solids in the flushing streams of 32.0 mg L−1.
With respect to turbidity, Figure 3c illustrates that, without using the self-cleaning system, the reduction in turbidity was between 63 and 83%. On the other hand, with respect to the washing streams, a very high turbidity of 26.2 NTU was observed in flushing. These yields are in line with those obtained by authors such as Chen et al. [66] with a PVDF membrane, in which they achieved a reduction of 60% with an initial turbidity of 20 NTU. This decrease in yield may be because a large number of particles distributed along the membrane surface were able to form a layer in the form of a cake so thick that it contributed to the fouling of the membrane [67].
Regarding the use of the self-cleaning system with the c-UF unit, Figure 3d shows a greater reduction in turbidity than that achieved without it, obtaining a reduction of between 95 and 96% in the permeate. On the other hand, according to Figure 3d, with the self-cleaning system alone, it was possible to reduce de facto by 12–25% the turbidity of the effluent from the secondary treatment. In addition, the wash stream corresponding to flushing showed a very high turbidity, somewhat indicative of a more efficient operation of the system, unlike a conventional UF system. From the SS and turbidity analyses, it was observed that similar trends followed in terms of greater production efficiency in the presence of the self-cleaning system. Likewise, there is a relationship between SS and turbidity, as studied by Oliveira et al. [68], in which they observed that a decrease in solids necessarily implied a decrease in turbidity in the water.
In addition to SS and turbidity, the removal of dissolved organic carbon (DOC) was studied, as shown in Figure 3e,f. It was observed that the yields achieved in the c-UF system without the self-cleaning system did not exceed 15–25%. This is mainly because ultrafiltration processes are not the most effective for removing dissolved organic material [37,69]. When the self-cleaning system is included, it did not bring with it a significant reduction since the small observed improvement of 2.6% was within the range of experimental error. For the effective removal of dissolved organic matter, a nanofiltration membrane would be required, which would be totally inadvisable because of the fouling problem, causing a rapid blockage of the membrane pores that would prevent maintaining an adequate permeate stream for the process [70]. Another aspect that would contraindicate its use would be the high energy cost involved since an operating pressure of at least 5–20 bar is necessary [71].
In addition to the presence of CECs, another risk involved in the reuse of these resources of water is the presence of harmful microorganisms, which frequently produce toxic compounds once they come into contact with humans [72]. Therefore, the presence of E. coli bacteria, total coliforms and toxicity were studied as macroparameters indicative of the quality of the water intended for reuse, as shown in Table 5.

Table 5.
Evaluation of the removal of toxicity and microbial density using c-UF technology with and without a self-cleaning system.
According to Table 5, regardless of whether the self-cleaning system was used in the c-UF module, both E. coli and total coliforms were completely removed in the permeate stream. The pathogen removal yield of this system is in line with that observed by other researchers in which they inactivated E. coli in ranges ranging between 80 and 99% and between 95 and 100% [73,74]. The complete removal of pathogenic microorganisms together with the low levels of turbidity observed in the permeate stream below 1 NTU for both situations indicates that when the large particles are completely removed, this tends to eradicate the pathogens since they are not able to adhere to or remain embedded in the particles where they can reproduce more effectively [73]. In addition, this is because the pore size of the membrane (20 nm) is small enough to retain and block the passage of coliform species [75]. The results observed in the wash streams, both flushing and backwashing, indicate that the functioning of the c-UF wash cycles is adequate to notably increase the presence of coliforms and E. coli. However, when the self-cleaning system is included, it was observed that this would increase the operating times of water production by eliminating a lower volume of pathogens that would have been retained on the surface of the membrane. The same was observed by Slavik et al. [76] in a study that evaluated the impact of washing procedures on filtration productivity. According to this study, the backwashing mode had no impact on the efficiency of water production but did on the length of each cycle, which can be prolonged by optimizing the washing operations or introducing some type of additional system cleansing that avoids the irreversible adhesion of the retained compounds and particles.
Although UF technology is highly efficient at removing SS and pathogenic microorganisms, it is important to demonstrate that it is also effective in removing other compounds that could not be detected, either due to their low frequency of appearance or the low concentrations presented, below the limits of detection of the analytical techniques [9]. According to Table 5, it was observed that, in both systems, the toxicity decreased sharply until reaching a value less than 1. This indicates that the effluent produced was not toxic and that the UF operation was efficient enough to also retain the compounds identified as unknown.
3.2. Reject Stream Treatment via Ozonation Technologies
3.2.1. Analysis of Ozonation Removal Yields
To reduce the environmental impact of the UF operation and increase water production, the reject streams were treated in an ozonation process. In Table 6, the removal yields obtained from the identified CECs and the overall yield are shown. For this, the part removed during secondary treatment and the c-UF operation itself were taken into account.

Table 6.
Removal yields obtained after ozonation of the CECs identified in the reject stream from the self-cleaning c-UF system. Experimental conditions: FG = 1.6 mg min−1, P = 1.5 bar, = 6.4 mg L-1, pH0 = 9.0, T = 15.0 °C and V = 30.0 L.
In general, the high oxidation selectivity of ozone was observed due to its particular electronic structure. Molecular ozone was more effective with CECs that contained functional groups such as amines, phenols and double bonds [45,77,78]. This is because these CECs can be more easily degraded into degradation byproducts and acids by redox reactions and electrophilic substitution reactions. According to Table 6, in general, all identified CECs were completely removed with the exception of 2-hydroxybenzothiazole and caffeine, with 39.7% and 38.1%, respectively. This is because they are two of the CECs most refractory to ozonation. In the case of 2-hydroxybenzothiazole, the low yield is due to the low selectivity and reactivity of the hydroxyl radicals towards this compound. This was observed by Valdés et al. [79] in a study where they determined that the direct reaction with ozone was more selective and reactive (k = 2.3 M−1 s−1) than the indirect reaction at an alkaline pH (k = 5.6 × 10−9 M−1 s−1). Regarding the yield of caffeine, at pH = 7.02, the degradation kinetic constant (k = 0.92 M−1 s−1) was significantly lower than that corresponding to 2-hydroxybenzothiazole. Rosal et al. [80] observed that the presence of an intermediate reaction during the ozonation of caffeine could promote a stronger ozone decomposition. For other compounds, such as trimethoprim, with a higher removal percentage (97.8%), authors such as Kuang et al. [81] observed that the major contributors to the oxidation reaction were hydroxyl radicals, while the molecular ozone pathway played a minor role. Regarding the overall removal yield, those CECs removed via biological treatment, such as valsartan (96.4%) or acetaminophen (99.7%), had better removal yields than the less biodegradable and more recalcitrant compounds, such as carbamazepine (45.2%) or irbesartan (35.3%). This would partly explain the importance of secondary treatment in the removal of CECs, apart from a significant improvement of the c-UF system itself through the incorporation of a self-cleaning system. The improvement resulting from secondary treatment implies a mitigation of problematic fouling on the membrane surface and a greater rejection of hydrophobic drugs [82].
As in the permeate stream, in the monitoring of the c-UF-treated reject stream, the microbiological parameters were evaluated. Figure 4 summarizes the toxicity and microbial density measured, such as E. coli and total coliforms, during the ozonation process of the reject stream.
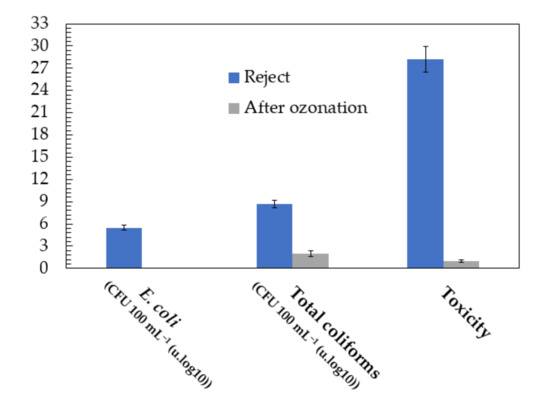
Figure 4.
Reduction in toxicity and pathogenic microorganisms averaged over the 4 days analysed by applying an ozonation treatment. Experimental conditions: FG = 1.6 mg min−1, P = 1.5 bar, = 6.4 mg L−1, pH0 = 9.0, T = 15.0 °C and V = 30.0 L.
According to Figure 4, the waters from the reject stream of the c-UF operation contained 2.9 × 105 colonies in 100 mL of faecal coliforms. However, as expected, the analysis of pathogenic microorganisms after ozonation revealed the absence of bacteria such as E. coli with an ozone dose of 6.4 mg L−1 and a contact time of 180 min. On the other hand, total coliforms were completely removed from all samples and days analysed. This result is in line with that observed by Iakovides et al. [83] in a bubble reactor in which a WWTP effluent from a secondary treatment was ozonized. With a hydraulic retention time of 20 min, a dose of 0.75 g O3 g DOC−1 was able to completely inactivate E. coli. Ostoich et al. [84] completely inactivated faecal coliforms below the detection limit of <0.01 CFU mL−1 by ozonation in a WWTP, using ozone doses ranging from 10 to 15 mg L−1 and a contact time of 30 min. Another study focused on the inactivation of pathogenic microorganisms by ozonation with different contact times and ozone doses up to 50 mg L−1 and showed that more than 99% of heterotrophs, enterococci and total coliforms were removed in just 30 min [85].
Regarding the toxicity of the ozonated effluent, it was observed that after 180 min of reaction, the toxicity was <1 TU. This result is similar to that observed in the permeate stream of the c-UF. This corroborates the complete removal and mineralization of the identified CECs towards more biodegradable and lower molecular weight compounds [17].
3.2.2. Kinetic Analysis of the Ozonation Process
During the treatment of wastewater, SS is a crucial parameter for the analysis of the quality of water intended for reuse in agriculture. SS is a parameter that indicated the degradation of pollutants present in wastewater. In addition, they can act as a reservoir for pathogenic microorganisms due to their ease of adhering to particle surfaces [68]. Consequently, determining the relationship that SS could have with other global parameters, as other authors have done by associating suspended solids with turbidity, is necessary. According to Serajuddin et al. [86], SS are essentially constituted by organic matter. Currently, there is no correlation that relates SS with total organic carbon (TOC), unlike turbidity, for which there are already a few studies [68,87] concerning the waters of the Elbe River [86], despite finding significant seasonal variability. In this sense, considering dissolved organic carbon (DOC) could yield more reliable and accessible information for the monitoring of SS, which would include pathogenic microorganisms.
Considering that SS (mg L−1) is constituted by an oxidizable part (oxidizable suspended solid, OSS) and a non oxidizable part (NOSS), which persist at the end of oxidation, the initial concentration of the OSS (OSS0, mg L−1) can be quantified in terms of TOC according to the following expression:
Ozonation was monitored through the soluble organic matter (SOM) from the SS along with the existing SOM. The solubilization and subsequent degradation of OSS can be described through the following serial process:
In addition, taking into account the existence of an initial concentration of soluble organic matter (SOM0, mg L−1) and assuming first-order reactions in Equation (4), the variation in SOM throughout ozonation can be described according to Equation (5) [88].
The first-order constant k1 (min−1) of this model represents the appearance of SOM as a consequence of the dissolution of SS. On the other hand, a first-order constant k2 (min−1) corresponds to the degradation of the SOM due to the action of the NOSS until reaching the residual value. Figure 5 shows the evolutions of SS and SOM for the ozonized reject stream fitted to the first-order serial kinetic model previously proposed.
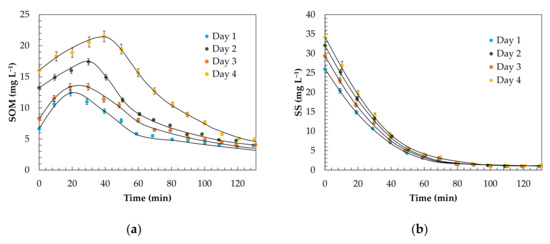
Figure 5.
Evolution of the kinetics of SOM removal (a) and SS removal (b) fit to the first-order serial model. Experimental conditions: FG = 1.6 mg min−1, P = 1.5 bar, = 6.4 mg L−1, pH0 = 9.0, T = 15.0 °C and V = 30.0 L.
The k1 and k2 (min−1) kinetic constants modelled in Table 7 were fit to the experimental values, minimizing the value of the mean standard deviation (σ), as shown in Equation (6) [89]:
where N is the number of experimental points and Cexp and Csim in mg L−1 are the experimental and simulated values obtained from simulating the first-order serial model, respectively. The mean standard deviation was less than 5.5% in all cases and demonstrates that through SOM monitoring, the removal of SS during an ozonation process can be extrapolated for WWTP streams.

Table 7.
Kinetic parameters determined from the serial kinetic model proposed for predicting the SS from the SOM.
According to the proposed model, the higher the constants k1 and k2 are, the greater the SS and SOM removal rates. Consequently, a better ozonation behaviour was found in the experiment corresponding to Day 1, with a constant k1 = 0.0438 min−1 and k2 = 0.0525 min−1. According to Table 7 and the evolutions shown in Figure 5, the observed divergences could be due to the variability in the nature of the pollutant load of the reject stream, typical of systems highly influenced by the habits of the population [17]. However, the proposed model showed its robustness under different scenarios.
To estimate the efficiency of the ozonation treatment, Table 7 shows the parameter (mg O3 mg SOM−1) proposed by Álvarez et al. [90], through Equation (7), which relates the ozone consumed per mg of SOM removed:
where FG is the gas stream (mg min−1), ,out (mg L−1) are the concentrations of ozone in the gas phase at the outlet of the reactor and V is the volume of treated water (L).
Ozone consumption was on the order of ≅20.0 mg O3 (mg SOM)−1, similar to that observed by other authors, such as Álvarez et al. [90], with 19 mg O3 (mg SOM)−1, or Ferreiro et al. [91], with 26.8 mg O3 (mg SOM)−1, for the treatment of wastewater streams. In view of the results shown in Table 7, the ozone dose used on Day 1 could be reduced given the lower organic load (Figure 5) of the waters of that experiment and the high value. However, for Days 2, 3 and 4, it would not be appropriate in any case to increase the ozone dose used, since an increase in the dose above 6.4 mg L−1 would make the reuse proposal unfeasible by increasing the costs of ozonation in exchange for increasing the mineralization of practically negligible organic matter [13]. On the other hand, according to Beltran et al. [78], an excess of oxidant could lead to the generation of hydroperoxyl radicals (HO2•) of lower oxidative capacity when the excess ozone reacts with the hydroxyl radicals produced.
3.3. Agriculture Reuse Aspects and Economic Estimation of the c-UF Process Coupled to Ozonation
3.3.1. Regulatory Aspects
In the reuse of water for direct use in agriculture, treatment with c-UF coupled to an ozonation system is proposed here to convert it into recycled water. The application of these reused waters is regulated by different criteria according to the country of destination. However, in the European Union (EU), to harmonize the regulation of each of the member states, a new regulation on water reuse has recently been proposed (EU 2020/741) [14]. This new regulation defines (i) types of crops, (ii) minimum quality requirements and (iii) quality control of the reclaimed water. In Table 8, the minimum criteria that the reclaimed waters must meet are detailed in the regulations of different governments for their use in all food crops, including tubers that could be consumed raw and food crops in which the edible part is in direct contact with the reclaimed waters, using any irrigation methods. Likewise, the average values obtained in this work were collected taking into account the c-UF process with a self-cleaning system and the coupled ozonation system for treating the reject stream.

Table 8.
Minimum quality requirements of recycled water from WWTPs established by different state regulations for use in irrigated agricultural activities [5,14,92].
According to Table 8, the new regulation incorporates stricter and more demanding parameters than other regulations adopted to date. For example, in comparison with the Royal Law 1620/2007 of Spain for E. coli, it is set at 100 CFU 100 mL−1, while in the European Regulation (EU 2020/741), it is set at 10 CFU 100 mL−1. The SS were set at 20 mg L−1, compared to 10 mg L−1. Finally, turbidity changed from 10 to 5 NTU with the new regulation.
In view of the results obtained, the reclaimed water obtained from the c-UF technology coupled to the ozonation system met the criteria established in the new European regulation in each and every one of the parameters specified by the regulations. By meeting these new limitations, it was ensured that the reclaimed waters were safe for irrigation in agricultural activities and that the environment was protected. In addition, a circular economy is promoted, since it would allow the reuse of more than half of the water flow of the WWTP effluent, avoiding the direct extraction of water from surface and underground sources. Additionally, this proposal would combat climate change and contribute to the fulfilling the SDG and Directive 2000/60/EC by promoting water savings.
3.3.2. Economic Comparison of c-UF Coupled to Ozonation Systems and a Conventional Tertiary Treatment
The costs related to reclaiming water through a conventional tertiary treatment and c-UF with ozonation are comparatively analysed. In both cases, the most representative costs were associated with the energy consumption of the different equipment used and the consumption of chemical reagents during some of the processes of the global operation.
Figure 6 shows the consumption derived from energy, as well as the reagents that were used in the tertiary treatment of the Galindo WWTP. Regarding the tertiary treatment (Figure 6a,b), the energy consumption was mainly attributed to the pumps (pumping raw water, pumps for the lamellar decanter for purging sludge, pumping for washing sand filters, bilge pumps for washing and treated water and chemical metering pumps), agitators (mixing tank, flocculation chamber and lamellar decanter), air blowers for washing the filters, helical fans and lamps for UV disinfection. It was estimated that the energy consumption of the Galindo WWTP was 161,110 kWh year−1.
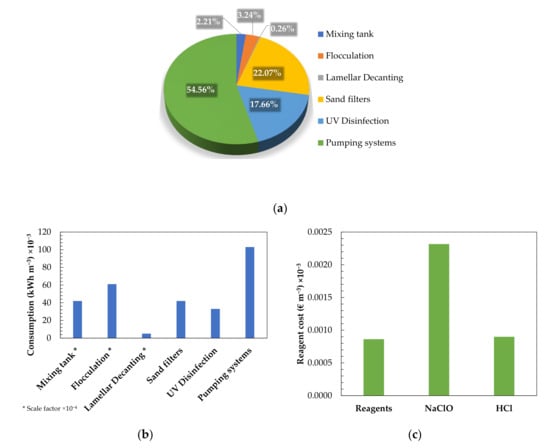
Figure 6.
Electrical consumption and cost of the chemical reagents used in the conventional tertiary treatment of the Galindo WWTP: (a) estimated percentage of the total energy consumption related to each process of the tertiary treatment; (b) energy consumption of each process involved in the tertiary treatment; and (c) cost relative to the reagents used in the oxidation and coagulation–flocculation chambers.
Regarding the cost attributed to the consumption of chemical reagents (Figure 6c), the use of sodium hypochlorite (NaClO) as an oxidizing agent and alumina sulphate (Al2 (SO4)3) as a coagulant were taken into account. In addition, the consumption of anionic polyelectrolyte as a flocculating agent was considered.
The energy consumption for the tertiary treatment plant estimated by the volume of the treated water was 0.1890 kWh m−3. Considering that the cost of energy for the industry in Spain is 0.0758 € kWh−1 [94], the energy cost per cubic metre of treated water obtained by tertiary treatment was 0.0143 € m−3.
For the estimation of the treatment cost with c-UF coupled to the ozonation system, the FLUKE 434/435 Tree Phase Power Quality Analysers (Fluke Ibérica S.L, Alcobendas, Spain) tool was used. This equipment monitors and records the energy consumption in the different stages of a filtration cycle.
The energy consumption required in the filtration stage was estimated at 0.6322 kWh. Additionally, it is necessary to consider the washing phase (flushing, backwashing and rinsing), which was 0.1191 kWh. Apart from the wash per cycle, it is necessary to consider the Cleaning-In-Place (CIP) and Chemical Enhanced Flushing (CEF) operations over an operating period of 30 days with a volume of treated water of 2300 m3. With all this, it was estimated that the energy consumption for a 30-day supercycle was 725 kWh, including the energy consumption associated with the self-cleaning system. Another aspect that was considered was the costs of consumption of chemical reagents for the CEF and CIP in the c-UF unit, in which 25% NaOH, 33% HCl and 13% NaClO were used. This estimation was made on the basis of the consumption of the reagents spent during both types of chemical cleaning.
The estimation of ozonation energy consumption was performed according to the criteria of Krichevskaya et al. [95], referring to a specific energy consumption of the ozone generator of 7 kWh kg−1 O3. Consequently, the energy consumed by the ozonation treatment was estimated at 0.054 kWh m−3.
In Figure 7, from the energy consumption, the cost per cubic metre of treated water by the c-UF coupled to ozonation process was estimated to be 0.0284 € m−3. The highest consumption comes from the c-UF unit, while the self-cleaning system turns out to be the smallest (0.00013 € m−3), but significant for the process efficiency based on the innumerable technical advantages mentioned above.
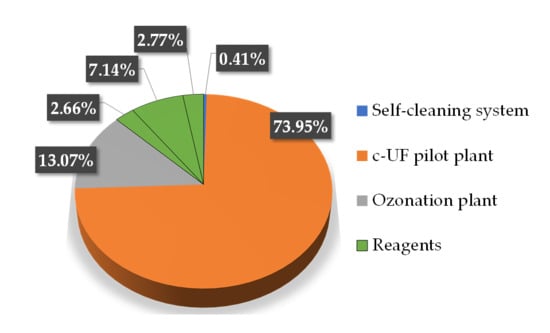
Figure 7.
Partial costs (€ m−3) of the proposed c-UF/ozonation treatment: c-UF (0.024), ozonation (0.0042) and reagents (0.00086), expressed as percent of the total cost of 0.0325 € m−3.
Finally, taking into account the economic costs related to energy and reagents of the conventional tertiary treatment and the c-UF system coupled to an ozonation process, a cost of 0.0325 € m−3 was determined for the proposed new system versus the cost of 0.0534 € m−3 for the conventional tertiary treatment, which would mean an economic savings of 39.1%. Authors such as Jodar-Abellan et al. [13] estimated a water reclamation cost of 0.06 € m−3 for the tertiary treatment in a WWTP in Valencia (Spain), a cost significantly higher than the alternative proposed in this work. In addition, the c-UF system coupled to ozonation allows us to obtain high-quality reclaimed water more than meets the minimum requirements established by the EU 2020/741 regulation and anticipates future restrictions on water reuse concerning the removal of CECs. This action would foster a circular economy between large cities and the agricultural sector through safe, sustainable and environmentally friendly food production, thus avoiding the discharge of urban wastewater into the water environment.
4. Conclusions
In this work, a novel continuous ultrafiltration system with self-cleaning capacity coupled to an ozonation process for water reclamation from a WWTP was studied for use in agriculture. The ability of c-UF to retain CECs of frequent appearance and pathogenic microorganisms was evaluated. During c-UF, it was observed that CECs such as amitriptyline (63.9%) and perfluorooctanesulfonamide (48.4%) performed better than others such as ciprofloxacin (16.7%) or sulfamethoxazole (10.2%). These differences in removal yields are associated with changing adsorption mechanisms according to each compound and determinant in the removal of this type of contaminant.
Regarding other physical–chemical parameters, it was observed that the self-cleaning system of the c-UF unit contributed to a reduction in turbidity of 95–96% and suspended solids of 90.1–100%. The incorporation of the self-cleaning system did not result in a significant reduction in the DOC (15–25%) compared to the c-UF unit without it.
The presence of E. coli bacteria, total coliforms and toxicity were studied as macroparameters indicative of the microbiological quality of the water. Regardless of whether the self-cleaning system was used in the c-UF unit, both E. coli and total coliforms were completely removed in the permeate stream.
Regarding the ozonation process following the c-UF, for treating the reject stream, ozonation was more effective with those CECs containing functional groups, such as amines, phenols and double bonds. All identified CECs were completely removed except for 2-hydroxybenzothiazole and caffeine, with 39.7% and 38.1%, respectively. Regarding the inactivation of pathogenic microorganisms with an ozone dose of 6.4 mg L−1 and a contact time of 180 min, the study revealed an absence of bacteria such as E. coli and a toxicity less than one. A first-order kinetic model of serial stages was proposed that describes the removal of SS with an error of less than 5%.
The water reclaimed with c-UF technology, followed by ozonation, meets the criteria established in the new European regulation (EU 2020/741) in each and every one of the parameters specified by the regulations.
Finally, taking into account the economic costs related to the energy and reagents of the conventional tertiary treatment system and the c-UF system with a self-cleaning system coupled to an ozonation process, a cost of 0.0325 € m−3 was determined for the novel system proposed versus a cost of 0.0534 € m−3 for the conventional tertiary treatment system.
Implementing this proposal would contribute to fighting against climate change and compliance with the SDG and Directive 2000/60/EC by addressing the problem of water scarcity, as well as promoting a circular economy between large cities and the agricultural sector. A further step in this research could focus on the effect that these regenerated waters rich in nutrients (nitrogen and phosphorous) could have on the fertilisation of crops.
5. Patents
The c-UF system used in this work has been patented in Spain under patent number ES201431341A.
Author Contributions
Conceptualization, J.I.L. and C.F.; methodology and analyses, J.I.L., C.F. and N.E.; model validation, A.d.L. and N.V.; formal analysis, N.V., A.d.L. and C.F.; investigation, C.F. and A.d.L.; original draft preparation, C.F., J.M.L. and A.d.L.; review and editing, C.F., J.I.L. and J.M.L.; supervised the experimentation, J.I.L. and N.E.; acquired the funding, J.I.L. and N.E. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the University of the Basque Country (UPV/EHU) for their financial support of this study through the PPGA20/33 project, and C. Ferreiro’s predoctoral PIF grant (PIF16/367).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
Authors are acknowledge to the Consorcio de Aguas Bilbao Bizkaia for their assistance in the sampling from Galindo’s WWTP and Fluytec S.A. for their c-UF unit used for this study.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature and Abbreviations
| σ | Mean standard deviation |
| Efficiency of the ozonation treatment, mg O3 mg SOM−1 | |
| BOD5 | Biochemical oxygen demand, mg O2 L−1 |
| C0 | Initian concentration of certain pollutant, mg L−1 |
| Cexp | Experimental concentration, mg L−1 |
| Ozone concentration at the reactor inlet, mg L−1 | |
| ,out | Ozone concentration in the gas phase at the outlet of the reactor, mg L−1 |
| Csim | Simulated concentration obtained from simulating the first-order serial model, mg L−1 |
| EC50 | Half maximal effective concentration refers to the concentration of pollutant, mg L−1 |
| FG | Ozone flow rate, mg min−1 |
| k | Kinetic oxidation constant, M−1 s−1 |
| k1 | First-order constant referred to the appearance of SOM as a consequence of the dissolution of SS, min−1 |
| k2 | First-order constant referred to the degradation of the SOM due to the action of the NOSS, min−1 |
| N | Number of experimental points |
| NOSS | Non oxidizable suspended solid, mg L−1 |
| OSS | Oxidizable suspended solid, mg L−1 |
| P | Pressure, bar |
| QA | Feed stream, m3 h−1 |
| QP | Permeate stream, m3 h−1 |
| QR | Reject stream, m3 h−1 |
| SOM | Soluble organic matter, mg L−1 |
| T | Temperature, °C |
| TOC | Total organic carbon, mg L−1 |
| Toxicity | Toxicity of the sample, TU |
| V | Reactor volume, L |
| Y | Recovery factor, % |
| AOP | Advanced Oxidation Process |
| CEC | Contaminant of emerging concern |
| CEF | Chemical Enhanced Flushing |
| CIP | Cleaning In Place |
| CNT | Carbon nanotube |
| c-UF | Continuous ultrafiltration |
| DOC | dissolved organic carbon |
| EDC | Endocrine disruptors |
| ESI | electrospray ionization source |
| G-L | Gas–liquid contact |
| LC-MS-MS | Liquid chromatography with tandem mass spectrometry equipment |
| MBR | Membrane bioreactor |
| MQ | Milli-Q quality water |
| PAN | Polyacrylonitrile |
| PES | Polyethersulfone |
| PPCP | Pharmaceutical and Personal Care Product |
| PVC | Polyvinylchloride |
| PVDF | Polyvinylidene fluoride |
| QqQ | Triple quadrupole |
| SDI | Silt density index |
| SPE | Solid-phase extraction |
| SS | Suspended solids |
| TU | Toxicity unit |
| WWTP | Wastewater treatment plant |
References
- Kookana, R.S.; Drechsel, P.; Jamwal, P.; Vanderzalm, J. Urbanisation and emerging economies: Issues and potential solutions for water and food security. Sci. Total Environ. 2020, 732, 139057. [Google Scholar] [CrossRef]
- Luthy, R.G.; Wolfand, J.M.; Bradshaw, J.L. Urban Water Revolution: Sustainable Water Futures for California Cities. J. Environ. Eng. 2020, 146, 04020065. [Google Scholar] [CrossRef]
- Aitken, D.; Rivera, D.; Godoy-Faúndez, A.; Holzapfel, E. Water Scarcity and the Impact of the Mining and Agricultural Sectors in Chile. Sustainability 2016, 8, 128. [Google Scholar] [CrossRef]
- Maiolo, M.; Pantusa, D. A proposal for multiple reuse of urban wastewater. J. Water Reuse Desalin. 2017, 8, 468–478. [Google Scholar] [CrossRef]
- Chojnacka, K.; Witek-Krowiak, A.; Moustakas, K.; Skrzypczak, D.; Mikula, K.; Loizidou, M. A transition from conventional irrigation to fertigation with reclaimed wastewater: Prospects and challenges. Renew. Sustain. Energy Rev. 2020, 130, 109959. [Google Scholar] [CrossRef]
- Visanji, Z.; Sadr, S.M.K.; Johns, M.B.; Savic, D.; Memon, F.A. Optimising wastewater treatment solutions for the removal of contaminants of emerging concern (CECs): A case study for application in India. J. Hydroinform. 2019, 22, 93–110. [Google Scholar] [CrossRef]
- Mijangos, L.; Ziarrusta, H.; Ros, O.; Kortazar, L.; Fernández, L.A.; Olivares, M.; Zuloaga, O.; Prieto, A.; Etxebarria, N. Occurrence of emerging pollutants in estuaries of the Basque Country: Analysis of sources and distribution, and assessment of the environmental risk. Water Res. 2018, 147, 152–163. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Contaminant Candidate List (4-CCL). Available online: https://www.epa.gov/ccl/contaminant-candidate-list-4-ccl-4-0 (accessed on 9 October 2020).
- Ferreiro, C.; Gómez-Motos, I.; Lombraña, J.I.; De Luis, A.; Villota, N.; Ros, O.; Etxebarria, N. Contaminants of Emerging Concern Removal in an Effluent of Wastewater Treatment Plant under Biological and Continuous Mode Ultrafiltration Treatment. Sustainability 2020, 12, 725. [Google Scholar] [CrossRef]
- Chowdhary, P.; Bharagava, R.N.; Mishra, S.; Khan, N. Role of Industries in Water Scarcity and Its Adverse Effects on Environment and Human Health. In Environmental Concerns and Sustainable Development; Springer: Singapore, 2019; pp. 235–256. ISBN 9789811358890. [Google Scholar]
- Jeong, H.; Bhattarai, R.; Adamowski, J.; Yu, D.J. Insights from socio-hydrological modeling to design sustainable wastewater reuse strategies for agriculture at the watershed scale. Agric. Water Manag. 2020, 231, 105983. [Google Scholar] [CrossRef]
- Rezaei, N.; Diaz-Elsayed, N.; Mohebbi, S.; Xie, X.; Zhang, Q. A multi-criteria sustainability assessment of water reuse applications: A case study in Lakeland, Florida. Environ. Sci. Water Res. Technol. 2018, 5, 102–118. [Google Scholar] [CrossRef]
- Jodar-Abellan, A.; López-Ortiz, M.I.; Melgarejo-Moreno, J. Wastewater Treatment and Water Reuse in Spain. Current Situation and Perspectives. Water 2019, 11, 1551. [Google Scholar] [CrossRef]
- European Parliament, Council of the EU. Regulation (EU) 2020/741 of the European Parliament and of the Council of 25 May 2020 on Minimum Requirements for Water Reuse; EU: Luxembourg, 2020; Volume 177, pp. 32–55. [Google Scholar]
- Tójar-Hurtado, J.-C.; Mena-Rodríguez, E.; Fernández-Jiménez, M.-Á. Spanish Agriculture and Water: Educational Implications of Water Culture and Consumption from the Farmers’ Perspective. Water 2017, 9, 964. [Google Scholar] [CrossRef]
- Silva, N.B.; Leonel, L.P.; Tonetti, A.L. UV-LED for Safe Effluent Reuse in Agriculture. Water Air Soil Pollut. 2020, 231, 1–10. [Google Scholar] [CrossRef]
- Chávez, A.M.; Ribeiro, A.R.; Moreira, N.F.F.; Silva, A.M.T.; Rey, A.; Álvarez, P.M.; Beltrán, F.J. Removal of Organic Micropollutants from a Municipal Wastewater Secondary Effluent by UVA-LED Photocatalytic Ozonation. Catalysts 2019, 9, 472. [Google Scholar] [CrossRef]
- Compagni, R.D.; Gabrielli, M.; Polesel, F.; Turolla, A.; Trapp, S.; Vezzaro, L.; Antonelli, M. Risk assessment of contaminants of emerging concern in the context of wastewater reuse for irrigation: An integrated modelling approach. Chemosphere 2020, 242, 125185. [Google Scholar] [CrossRef] [PubMed]
- Lamba, M.; Ahammad, S.Z. Performance comparison of secondary and tertiary treatment systems for treating antibiotic resistance. Water Res. 2017, 127, 172–182. [Google Scholar] [CrossRef]
- Benner, J.; Helbling, D.E.; Kohler, H.-P.E.; Wittebol, J.; Kaiser, E.; Prasse, C.; Ternes, T.A.; Albers, C.N.; Aamand, J.; Horemans, B.; et al. Is biological treatment a viable alternative for micropollutant removal in drinking water treatment processes? Water Res. 2013, 47, 5955–5976. [Google Scholar] [CrossRef]
- Bensalah, F.; Iddou, A.; Hentit, H.; Aziz, A.; Shishkin, A. Activated Carbon Design from Sludge to Remove Red Scarlet Nylosan “F3GL” in Aqueous Solution. Key Eng. Mater. 2018, 762, 87–92. [Google Scholar] [CrossRef]
- Dhangar, K.; Kumar, M. Tricks and tracks in removal of emerging contaminants from the wastewater through hybrid treatment systems: A review. Sci. Total Environ. 2020, 738, 140320. [Google Scholar] [CrossRef]
- Andriantsiferana, C.; Mohamed, E.F.; Delmas, H. Sequential adsorption-photocatalytic oxidation process for wastewater treatment using a composite material TiO2/activated carbon. Environ. Eng. Res. 2015, 20, 181–189. [Google Scholar] [CrossRef]
- Im, D.; Nakada, N.; Kato, Y.; Aoki, M.; Tanaka, H. Pretreatment of ceramic membrane microfiltration in wastewater reuse: A comparison between ozonation and coagulation. J. Environ. Manag. 2019, 251, 109555. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-Y.; Sungkyunkwan University; Kim, M.-G.; Lee, C.-H.; Kim, H.-S.; Kim, J.-H.; Lee, K.-I. A study on mitigation of membrane fouling by ozonation/coagulation in ultrafiltration. J. Korean Soc. Water Wastewater 2017, 31, 161–168. [Google Scholar] [CrossRef]
- Stackelberg, P.E.; Gibs, J.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Lippincott, R.L. Efficiency of conventional drinking water treatment processes in removal of pharmaceuticals and other organic compounds. Sci. Total Environ. 2007, 377, 255–272. [Google Scholar] [CrossRef]
- Cuartucci, M. Ultrafiltration, a cost-effective solution for treating surface water to potable standard. Water Pract. Technol. 2020, 15, 426–436. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Robinson, B.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Farraji, H.; Vakili, M. Pesticides in aquatic environments and their removal by adsorption methods. Chemosphere 2020, 253, 126646. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, H.; Feng, Y.; Yang, F.; Zhang, J. Removal of trace antibiotics from wastewater: A systematic study of nanofiltration combined with ozone-based advanced oxidation processes. Chem. Eng. J. 2014, 240, 211–220. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; José, R.-U. Ozonation of 1,3,6-naphthalenetrisulphonic acid catalysed by activated carbon in aqueous phase. Appl. Catal. B Environ. 2002, 39, 319–329. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Chen, H.; Zahraa, O.; Bouchy, M. Inhibition of the adsorption and photocatalytic degradation of an organic contaminant in an aqueous suspension of TiO2 by inorganic ions. J. Photochem. Photobiol. A Chem. 1997, 108, 37–44. [Google Scholar] [CrossRef]
- Hagen, K. Removal of particles, bacteria and parasites with ultrafiltration for drinking water treatment. Desalination 1998, 119, 85–91. [Google Scholar] [CrossRef]
- Acero, J.L.; Benítez, F.J.; Leal, A.I.; Real, F.J.; Teva, F. Membrane filtration technologies applied to municipal secondary effluents for potential reuse. J. Hazard. Mater. 2010, 177, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Benítez, F.J.; Real, F.J.; Acero, J.L.; Casas, F. Use of ultrafiltration and nanofiltration processes for the elimination of three selected emerging contaminants: Amitriptyline hydrochloride, methyl salicylate and 2-phenoxyethanol. Environ. Prot. Eng. 2017, 43, 125–141. [Google Scholar] [CrossRef]
- Dharupaneedi, S.P.; Nataraj, S.K.; Nadagouda, M.; Reddy, K.R.; Shukla, S.S.; Aminabhavi, T.M. Membrane-based separation of potential emerging pollutants. Sep. Purif. Technol. 2019, 210, 850–866. [Google Scholar] [CrossRef]
- Muhamad, M.S.; Salim, M.R.; Lau, W.J.; Yusop, Z.; Hadibarata, T. The Removal of Bisphenol A in Water Treatment Plant Using Ultrafiltration Membrane System. Water Air Soil Pollut. 2016, 227, 1–12. [Google Scholar] [CrossRef]
- Sui, Q.; Huang, J.; Deng, S.; Chen, W.; Yu, G. Seasonal Variation in the Occurrence and Removal of Pharmaceuticals and Personal Care Products in Different Biological Wastewater Treatment Processes. Environ. Sci. Technol. 2011, 45, 3341–3348. [Google Scholar] [CrossRef]
- Carr, D.L.; Morse, A.N.; Zak, J.C.; Anderson, T.A. Biological Degradation of Common Pharmaceuticals and Personal Care Products in Soils with High Water Content. Water Air Soil Pollut. 2011, 217, 127–134. [Google Scholar] [CrossRef]
- Chen, H.; Zhuang, R.; Yao, J.; Wang, F.; Qian, Y.; Masakorala, K.; Cai, M.; Liu, H. Short-term effect of aniline on soil microbial activity: A combined study by isothermal microcalorimetry, glucose analysis, and enzyme assay techniques. Environ. Sci. Pollut. Res. 2013, 21, 674–683. [Google Scholar] [CrossRef]
- Lahti, M.; Oikari, A. Microbial Transformation of Pharmaceuticals Naproxen, Bisoprolol, and Diclofenac in Aerobic and Anaerobic Environments. Arch. Environ. Contam. Toxicol. 2010, 61, 202–210. [Google Scholar] [CrossRef]
- Alresheedi, M.T.; Basu, O.D.; Barbeau, B. Chemical cleaning of ceramic ultrafiltration membranes—Ozone versus conventional cleaning chemicals. Chemosphere 2019, 226, 668–677. [Google Scholar] [CrossRef]
- Ferreiro, C.; Villota, N.; Lombraña, J.I.; Rivero, M.J.; Zúñiga, V.; Rituerto, J.M. Analysis of a Hybrid Suspended-Supported Photocatalytic Reactor for the Treatment of Wastewater Containing Benzothiazole and Aniline. Water 2019, 11, 337. [Google Scholar] [CrossRef]
- Noutsopoulos, C.; Mamais, D.; Mpouras, T.; Kokkinidou, D.; Samaras, V.; Antoniou, K.; Gioldasi, M. The role of activated carbon and disinfection on the removal of endocrine disrupting chemicals and non-steroidal anti-inflammatory drugs from wastewater. Environ. Technol. 2014, 35, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Von Sonntag, C.; Von Gunten, U. Chemistry of Ozone in Water and Wastewater Treatment: From Basic Principles to Applications; Iwa Publishing: London, UK, 2012; ISBN 978-1-84339-313-9. [Google Scholar]
- De Luis, A.; Lombraña, J.I. pH-Based Strategies for an Efficient Addition of H2O2 during Ozonation to Improve the Mineralisation of Two Contaminants with Different Degradation Resistances. Water, Air, Soil Pollut. 2018, 229, 372. [Google Scholar] [CrossRef]
- Gomes, J.; Matos, A.; Gmurek, M.; Quinta-Ferreira, R.M.; Martins, R.C. Ozone and Photocatalytic Processes for Pathogens Removal from Water: A Review. Catalysts 2019, 9, 46. [Google Scholar] [CrossRef]
- Martínez, P.O.; Echevarría, F.G. Equipo de Filtrado Mejorado y Procedimientos de Uso. ES201431341A, 24 March 2016. [Google Scholar]
- United States Environmental Protection Agency. Procedures for Collecting Wastewater Samples. Available online: https://www.epa.gov/quality/procedures-collecting-wastewater-samples (accessed on 27 November 2020).
- Black & Veatch Corporation. White’s Handbook of Chlorination and Alternative Disinfectant, 5th ed.; Wiley: Trenton, NJ, USA, 2010; ISBN 978-0-470-18098-3. [Google Scholar]
- Mijangos, L.; Ziarrusta, H.; Olivares, M.; Zuloaga, O.; Möder, M.; Etxebarria, N.; Prieto, A. Simultaneous determination of 41 multiclass organic pollutants in environmental waters by means of polyethersulfone microextraction followed by liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2017, 410, 615–632. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA; AWWA; WEF: Washington, DC, USA, 2005; ISBN 978-0875530475. [Google Scholar]
- Lopera, A.E.-C.; Ruiz, S.G.; Alonso, J.M.Q. Removal of emerging contaminants from wastewater using reverse osmosis for its subsequent reuse: Pilot plant. J. Water Process. Eng. 2019, 29, 100800. [Google Scholar] [CrossRef]
- Stavbar, S.; Hrnčič, M.K.; Premzl, K.; Kolar, M.; Turk, S. Šostar Sub- and super-critical water oxidation of wastewater containing amoxicillin and ciprofloxacin. J. Supercrit. Fluids 2017, 128, 73–78. [Google Scholar] [CrossRef]
- Egea-Corbacho, A.; Ruiz, S.G.; Alonso, J.M.Q. Removal of emerging contaminants from wastewater using nanofiltration for its subsequent reuse: Full–scale pilot plant. J. Clean. Prod. 2019, 214, 514–523. [Google Scholar] [CrossRef]
- Machado, K.C.; Grassi, M.T.; Vidal, C.; Pescara, I.C.; Jardim, W.F.; Fernandes, A.N.; Sodré, F.F.; Almeida, F.V.; Santana, J.S.; Canela, M.C.; et al. A preliminary nationwide survey of the presence of emerging contaminants in drinking and source waters in Brazil. Sci. Total Environ. 2016, 572, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Price, W.E. Occurrence of phytoestrogens in municipal wastewater and surface waters. J. Environ. Monit. 2009, 11, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Petrovic, M.; Barceló, D. Analysis of Emerging Contaminants of Municipal and Industrial Origin. In Emerging Contaminants from Industrial and Municipal Waste: Occurrence, Analysis and Effects; The Handbook of Environmental Chemistry; Barceló, D., Petrovic, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 37–104. ISBN 978-3-540-74795-6. [Google Scholar]
- Yu, Q.; Geng, J.; Zong, X.; Zhang, Y.; Xu, K.; Hu, H.; Deng, Y.; Zhao, F.; Ren, H. Occurrence and removal of progestagens in municipal wastewater treatment plants from different regions in China. Sci. Total Environ. 2019, 668, 1191–1199. [Google Scholar] [CrossRef]
- Loos, R.; Carvalho, R.; António, D.C.; Comero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef]
- Guedes-Alonso, R.; Montesdeoca-Esponda, S.; Pacheco-Juárez, J.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. A Survey of the Presence of Pharmaceutical Residues in Wastewaters. Evaluation of Their Removal using Conventional and Natural Treatment Procedures. Molecules 2020, 25, 1639. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ray, N.M.; Wan, J.; Khan, A.; Chakraborty, T.; Ray, M.B. Micropollutants in Wastewater: Fate and Removal Processes. Phys. Chem. Wastewater Treat. Resour. Recove. 2017. [Google Scholar] [CrossRef]
- Polesel, F.; Andersen, H.R.; Trapp, S.; Plósz, B.G. Removal of Antibiotics in Biological Wastewater Treatment Systems—A Critical Assessment Using the Activated Sludge Modeling Framework for Xenobiotics (ASM-X). Environ. Sci. Technol. 2016, 50, 10316–10334. [Google Scholar] [CrossRef]
- Fan, G.; Su, Z.; Lin, R.; Lin, X.; Xu, R.; Chen, W. Influence of Membrane Materials and Operational Modes on the Performance of Ultrafiltration Modules for Drinking Water Treatment. Int. J. Polym. Sci. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Bourgeous, K.N.; Darby, J.L.; Tchobanoglous, G. Ultrafiltration of wastewater: Effects of particles, mode of operation, and backwash effectiveness. Water Res. 2001, 35, 77–90. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, W.; Zhu, H.; Wei, D.; He, F.; Wang, D.; Du, B.; Wei, Q. Effect of turbidity on micropollutant removal and membrane fouling by MIEX/ultrafiltration hybrid process. Chemosphere 2019, 216, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yue, Q.; Gao, B.; Du, B. Impacts of organic coagulant aid on purification performance and membrane fouling of coagulation/ultrafiltration hybrid process with different Al-based coagulants. Desalination 2015, 363, 126–133. [Google Scholar] [CrossRef]
- De Oliveira, A.R.M.; Borges, A.C.; Matos, A.T.; Nascimento, M. Estimation on the Concentration of Suspended Solids from Turbidity in the Water of Two Sub-Basins in the Doce River Basin. Eng. Agríc. 2018, 38, 751–759. [Google Scholar] [CrossRef]
- Kim, S.; Chu, K.H.; Al-Hamadani, Y.A.; Park, C.M.; Jang, M.; Kim, D.-H.; Yu, M.; Heo, J.; Yoon, Y. Removal of contaminants of emerging concern by membranes in water and wastewater: A review. Chem. Eng. J. 2018, 335, 896–914. [Google Scholar] [CrossRef]
- Yang, M.; Chen, J.; Peng, B.; Yu, Z.; Chu, H.; Zhou, X. Performance and properties of coking nanofiltration concentrate treatment and membrane fouling mitigation by an Fe(II)/persulfate-coagulation-ultrafiltration process. RSC Adv. 2019, 9, 15277–15287. [Google Scholar] [CrossRef]
- Shaaban, A.M.F.; Hafez, A.I.; Abdel-Fatah, M.A.; Abdel-Monem, N.M.; Mahmoud, M.H. Process engineering optimization of nanofiltration unit for the treatment of textile plant effluent in view of solution diffusion model. Egypt. J. Pet. 2016, 25, 79–90. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: A review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar] [CrossRef]
- Mwabi, J.K.; Mamba, B.B.; Momba, M.N.B. Removal of Escherichia coli and Faecal Coliforms from Surface Water and Groundwater by Household Water Treatment Devices/Systems: A Sustainable Solution for Improving Water Quality in Rural Communities of the Southern African Development Community Region. Int. J. Environ. Res. Public Health 2012, 9, 139–170. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Colis, G.; Thalasso, F.; Marcela Ramirez-Lopez, E.; Rodriguez-Narciso, S.; Lilian Guerrero-Barrera, A.; Avelar-Gonzalez, F. Spatial-Temporal Evaluation of the Water Quality of the San Pedro River. Rev. Int. Contam. Ambient. 2011, 27, 89–102. [Google Scholar]
- Falsanisi, D.; Liberti, L.; Notarnicola, M. Ultrafiltration (UF) Pilot Plant for Municipal Wastewater Reuse in Agriculture: Impact of the Operation Mode on Process Performance. Water 2010, 2, 872–885. [Google Scholar] [CrossRef]
- Slavik, I.; Jehmlich, A.; Uhl, W. Impact of backwashing procedures on deep bed filtration productivity in drinking water treatment. Water Res. 2013, 47, 6348–6357. [Google Scholar] [CrossRef]
- Si, X.; Hu, Z.; Huang, S. Combined Process of Ozone Oxidation and Ultrafiltration as an Effective Treatment Technology for the Removal of Endocrine-Disrupting Chemicals. Appl. Sci. 2018, 8, 1240. [Google Scholar] [CrossRef]
- Beltran, F.J. Ozone Reaction Kinetics for Water and Wastewater Systems; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Valdés, H.; Zaror, C.A.; Jekel, M. Removal of Benzothiazole from Contaminated Waters by Ozonation: The Role of Direct and Indirect Ozone Reactions. J. Adv. Oxid. Technol. 2016, 19, 338–346. [Google Scholar] [CrossRef]
- Rosal, R.; Rodríguez, A.; Perdigón-Melón, J.A.; Petre, A.; García-Calvo, E.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. Degradation of caffeine and identification of the transformation products generated by ozonation. Chemosphere 2009, 74, 825–831. [Google Scholar] [CrossRef]
- Kuang, J.; Huang, J.; Wang, B.; Cao, Q.; Deng, S.; Yu, G. Ozonation of trimethoprim in aqueous solution: Identification of reaction products and their toxicity. Water Res. 2013, 47, 2863–2872. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Wang, H.; He, J.; Fan, G.; Pan, Z.; Tian, J.; Rong, H.; Li, G.; Yu, H. Tertiary treatment of secondary effluent using ultrafiltration for wastewater reuse: Correlating membrane fouling with rejection of effluent organic matter and hydrophobic pharmaceuticals. Environ. Sci. Water Res. Technol. 2019, 5, 672–683. [Google Scholar] [CrossRef]
- Iakovides, I.; Michael-Kordatou, I.; Moreira, N.; Ribeiro, A.; Fernandes, T.; Pereira, M.; Nunes, O.; Manaia, C.; Silva, A.; Fatta-Kassinos, D. Continuous ozonation of urban wastewater: Removal of antibiotics, antibiotic-resistant Escherichia coli and antibiotic resistance genes and phytotoxicity. Water Res. 2019, 159, 333–347. [Google Scholar] [CrossRef]
- Ostoich, M.; Serena, F.; Falletti, L.; Fantoni, A. Control of dangerous substances in discharges and microbiological abatement: European framework and a case study of an ozone disinfection system. Water Sci. Technol. 2013, 67, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.M.; Macedo, G.; Pedrosa, M.; Becerra-Castro, C.; Castro-Silva, S.; Pereira, M.F.R.; Silva, A.M.T.; Nunes, O.C.; Manaia, C.M. Ozonation and UV254nm radiation for the removal of microorganisms and antibiotic resistance genes from urban wastewater. J. Hazard. Mater. 2017, 323, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Serajuddin, M.; Chowdhury, A.I.; Haque, M.; Haque, E. Using Turbidity to Determine Total Suspended Solids in an Urban Stream: A Case Study. Int. J. Eng. Trends Technol. 2019, 67, 83–88. [Google Scholar] [CrossRef]
- Teixeira, L.C.; De Paiva, J.B.D.; Pereira, J.E.D.S.; Lisbôa, R.D.M. Relationship between Turbity and Suspended Sediment Concentration From a Small Hydrographic Basin in Santa Maria (Rio Grande Do Sul, Brazil). Int. J. River Basin Manag. 2016, 14, 1–10. [Google Scholar] [CrossRef]
- M. & E. Inc.; Tchobanoglous, G.; Burton, F.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse; McGraw-Hill Education: New York, NY, USA, 2002; ISBN 978-0-07-041878-3. [Google Scholar]
- Sanchez, M.; Rivero, M.J.; Ortiz, I. Kinetics of dodecylbenzenesulphonate mineralisation by TiO2 photocatalysis. Appl. Catal. B Environ. 2011, 101, 515–521. [Google Scholar] [CrossRef]
- Álvarez, P.M.; Pocostales, J.P.; Beltrán, F.J. Granular activated carbon promoted ozonation of a food-processing secondary effluent. J. Hazard. Mater. 2011, 185, 776–783. [Google Scholar] [CrossRef]
- Ferreiro, C.; Villota, N.; Lombraña, J.; Rivero, M.J. An efficient catalytic process for the treatment of genotoxic aniline wastewater using a new granular activated carbon-supported titanium dioxide composite. J. Clean. Prod. 2019, 228, 1282–1295. [Google Scholar] [CrossRef]
- Shoushtarian, F.; Negahban-Azar, M. Worldwide Regulations and Guidelines for Agricultural Water Reuse: A Critical Review. Water 2020, 12, 971. [Google Scholar] [CrossRef]
- Nutt, S.G.; Tran, J.; Vriezen, C.; Fuga, G.; Zaleski, A.; Miot, A.; Pagilla, K.; Lee, M.; Ross, M.; Palmer, T. Addressing BOD5 Limitations through TOC Correlations—A International Investigation. Proc. Water Environ. Fed. 2013, 2013, 2567–2577. [Google Scholar] [CrossRef]
- European Union Eurostat Regional Yearbook, 2018 ed.; Statistical books; Publications Office of the European Union: Luxembourg, 2018; ISBN 978-92-79-87876-3.
- Krichevskaya, M.; Klauson, D.; Portjanskaja, E.; Preis, S. The Cost Evaluation of Advanced Oxidation Processes in Laboratory and Pilot-Scale Experiments. Ozone Sci. Eng. 2011, 33, 211–223. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).