Foliar Spray Application of Chlorella vulgaris Extract: Effect on the Growth of Lettuce Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae Culture and Extract Preparation
2.2. Experimental Conditions
2.3. Morpho-Biometric and Physiologic Parameters in Lettuce Seedlings
2.4. Total Protein Extraction from Lettuce Tissues
2.5. Enzyme Activities in Lettuce Leaves
2.6. Statistical Analysis
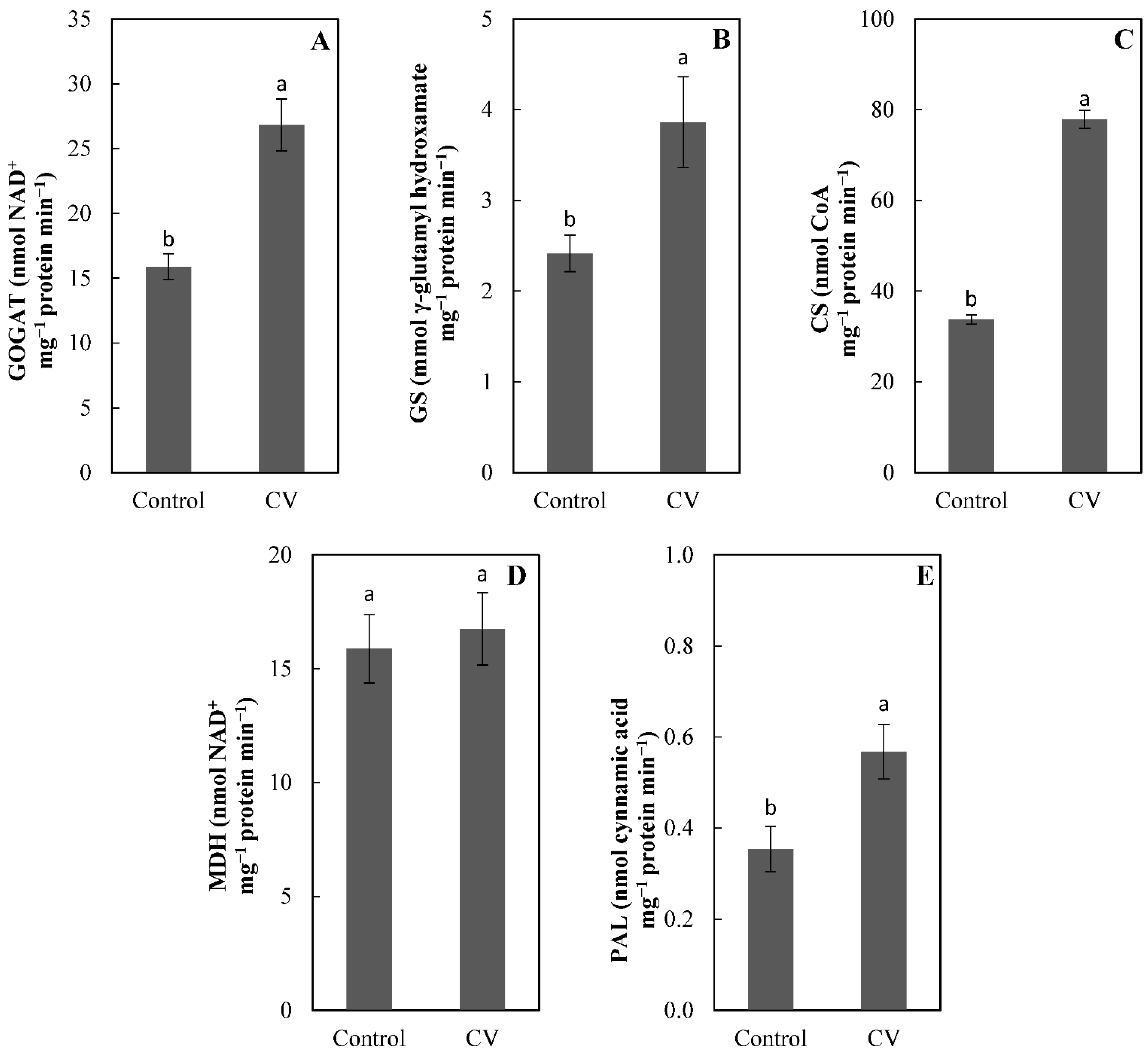
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Adiloğlu, S.; Eryılmaz Açıkgöz, F.; Solmaz, Y.; Çaktü, E.; Adiloğlu, A. Effect of vermicompost on the growth and yield of lettuce plant (Lactuca sativa L. var. crispa). Int. J. Plant Soil Sci. 2018, 21, 1–5. [Google Scholar] [CrossRef]
- du Jardin, P. The Science of Plant Biostimulants—A Bibliographic Analysis. Ad hoc Study Report to the European Commission DG ENTR. 2012. Available online: https://orbi.uliege.be/bitstream/2268/169257/1/Plant_Biostimulants_final_report_bio_2012_en.pdf (accessed on 27 January 2020).
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Caradonia, F.; Battaglia, V.; Righi, L.; Pascali, G.; Torre, A.L. Plant Biostimulant Regulatory Framework: Prospects in Europe and Current Situation at International Level. J. Plant Growth Regul. 2019, 38, 438–448. [Google Scholar] [CrossRef]
- Faheed, F.A.; Fattah, A.Z.E. Effect of Chlorella vulgaris as bio-fertilizer on growth parameters and metabolic aspects of lettuce plant. J. Agric. Soc. Sci. 2008, 4, 165–169. [Google Scholar]
- Elhafiz, A.A.; Elhafiz, A.A.; Gaur, S.S.; Hamdany, N.; Osman, M.; Lakshmi, T.V.R. Chlorella vulgaris and Chlorella pyrenoidosa live cells appear to be promising sustainable biofertilizer to grow rice, lettuce, cucumber and eggplant in the UAE soils. Recent Res. Sci. Technol. 2015, 7, 14–21. [Google Scholar]
- Zhang, J.; Wang, X.; Zhou, Q. Co-cultivation of Chlorella spp and tomato in a hydroponic system. Biomass Bioenergy 2017, 97, 132–138. [Google Scholar] [CrossRef]
- Barone, V.; Baglieri, A.; Stevanato, P.; Broccanello, C.; Bertoldo, G.; Bertaggia, M.; Cagnin, M.; Pizzeghello, D.; Moliterni, V.M.C.; Mandolino, G.; et al. Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L.). J. Appl. Phycol. 2018, 30, 1061–1071. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Barone, V.; Puglisi, I.; Fragalà, F.; Stevanato, P.; Baglieri, A. Effect of living cells of microalgae or their extracts on soil enzyme activities. Arch. Agron. Soil Sci. 2019, 65, 712–726. [Google Scholar] [CrossRef]
- Barone, V.; Puglisi, I.; Fragalà, F.; Piero, A.R.L.; Giuffrida, F.; Baglieri, A. Novel bioprocess for the cultivation of microalgae in hydroponic growing system of tomato plants. J. Appl. Phycol. 2019, 31, 465–470. [Google Scholar] [CrossRef]
- Puglisi, I.; Bella, E.L.; Rovetto, E.I.; Piero, A.R.L.; Baglieri, A. Biostimulant effect and biochemical response in lettuce seedlings treated with a Scenedesmus quadricauda extract. Plants 2020, 9, 123. [Google Scholar] [CrossRef]
- Puglisi, I.; Barone, V.; Fragalà, F.; Stevanato, P.; Baglieri, A.; Vitale, A. Effect of microalgal extracts from Chlorella vulgaris and Scenedesmus quadricauda on germination of beta vulgaris seeds. Plants 2020, 9, 675. [Google Scholar] [CrossRef]
- Kopta, T.; Pavlíková, M.; Sękara, A.; Pokluda, R.; Maršálek, B. Effect of bacterial-algal biostimulant on the yield and internal quality of Lettuce (Lactuca sativa L.) produced for spring and summer crop. Not. Bot. Horti Agrobot. Cluj Napoca 2018, 46, 615–621. [Google Scholar] [CrossRef]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.-C.; Rouphael, Y. Renewable sources of plant biostimulation: Microalgae as a sustainable means to improve crop performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef] [PubMed]
- Coppens, J.; Grunert, O.; van den Hende, S.; Vanhoutte, I.; Boon, N.; Haesaert, G.; de Gelder, L. The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J. Appl. Phycol. 2016, 28, 2367–2377. [Google Scholar] [CrossRef]
- Shaaban, M.M. Green microalgae water extract as foliar feeding to wheat plants. Pak. J. Biol. Sci. 2001, 4, 628–632. [Google Scholar]
- Shaaban, M.M. Nutritional status and growth of maise plants as affected by green microalgae as soil additives. J. Biol. Sci. 2001, 6, 475–479. [Google Scholar] [CrossRef]
- Hajnal-Jafari, T.; Seman, V.; Stamenov, D.; Đurić, S. Effect of Chlorella vulgaris on growth and photosynthetic pigment content in Swiss Chard (Beta vulgaris L. subsp. cicla). Pol. J. Microbiol. 2020, 69, 235–238. [Google Scholar] [CrossRef]
- Puglisi, I.; Barone, V.; Sidella, S.; Coppa, M.; Broccanello, C.; Gennari, M.; Baglieri, A. Biostimulant activity of humic like substances from agro-industrial waste on Chlorella vulgaris and Scenedesmus quadricauda. Eur. J. Phycol. 2018, 53, 433–442. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171. [Google Scholar] [CrossRef]
- Baglieri, A.; Sidella, S.; Barone, V.; Fragalà, F.; Silkina, A.; Nègre, M.; Gennari, M. Cultivating Chlorella vulgaris and Scenedesmus quadricauda microalgae to degrade inorganic compounds and pesticides in water. Environ. Sci. Pollut. Res. 2016, 23, 18165–18174. [Google Scholar] [CrossRef] [PubMed]
- Armon, D.I.; Hoagland, D.R. Crop production in artificial culture solutions and in soils with special reference to factors influencing yields and absorption of inorganic nutrients. Soil Sci. 1940, 50, 463–485. [Google Scholar]
- Gent, M.P.N. Factors affecting relative growth rate of lettuce and spinach in hydroponics in a greenhouse. HortScience 2017, 52, 1742–1747. [Google Scholar] [CrossRef]
- Vanni, A.; Anfossi, L.; Cignetti, A.; Baglieri, A.; Gennari, M. Degradation of pyrimethanil in soil: Influence of light, oxygen, and microbial activity. J. Environ. Sci. Health B 2006, 41, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Puglisi, I.; Petrone, G.; Piero, A.R.L. A kiwi juice aqueous solution as coagulant of bovine milk and its potential in Mozzarella cheese manufacture. Food Bioprod. Process. 2014, 92, 67–72. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Avila, C.; Rotella, J.R.; Canovas, F.M.; de Castro, I.N.; Valpuesta, V. Different characteristics of the two glutamate synthetases in green leaves of Lycopersicon esculentum. Plant. Physiol. 1987, 85, 1036–1039. [Google Scholar] [CrossRef]
- Canovas, F.M.; Canton, F.R.; Gallardo, F.; Garcia-Gutierrez, A.; de Vincente, A. Accumulation of glutamine synthetase during early development of maritime pine (Pinus pinaster) seedlings. Planta 1991, 185, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Ertani, A.; Nardi, S. Effects of an alfalfa protein hydrolysate on the gene expression and activity of enzymes of the tricarboxylic acid (TCA) cycle and nitrogen metabolism in Zea mays L. J. Agric. Food Chem. 2008, 56, 11800–11808. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Sakurai, M.; Sakuta, M. Effects of conditioned medium on activities of PAL, CHS, DAHP synthase (DS-Co and DS-Mn) and anthocyanin production in suspension cultures of Fragaria ananassa. Plant. Sci. 2001, 160, 355–360. [Google Scholar] [CrossRef]
- Barone, V.; Bertoldo, G.; Magro, F.; Broccanello, C.; Puglisi, I.; Baglieri, A.; Cagnin, M.; Concheri, G.; Squartini, A.; Pizzeghello, D. Molecular and morphological changes induced by leonardite-based biostimulant in Beta vulgaris L. Plants 2019, 8, 181. [Google Scholar] [CrossRef]
- Bohne, H.; Hasler, M. Relation between growth and nutrient content of field-grown tree nursery crops. Eur. J. Hortic. Sci. 2009, 74, 227–233. [Google Scholar]
- Spinelli, F.; Fiori, G.; Noferini, M.; Sprocatti, M.; Costa, G. A novel type of seaweed extract as a natural alter-native to the use of iron chelates in strawberry production. Sci. Hortic. 2010, 125, 263–269. [Google Scholar] [CrossRef]
- Fan, D.; Hodges, D.M.; Critchley, A.T.; Prithiviraj, B. A commercial extract of brown macroalga (Ascophyllum nodosum) affects yield and the nutritional quality of spinach in vitro. Commun. Soil Sci. Plant. Anal. 2013, 44, 1873–1884. [Google Scholar] [CrossRef]
- Kitajima, K.; Hogan, K.P. Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant. Cell Environ. 2003, 26, 857–865. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Sinauer Associates, Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Murchie, E.H.; Pinto, M.; Horton, P. Agriculture and the new challenges for photosynthesis research. New Phytol. 2009, 181, 532–552. [Google Scholar] [CrossRef]
- Lea, P.J. Nitrogen metabolism. In Plant Biochemistry and Molecular Biology; Lea, P.J., Leegood, R.C., Eds.; Wiley: New York, NY, USA, 1993; pp. 155–180. [Google Scholar]
- Gupta, N.; Gupta, A.K.; Gaur, V.S.; Kumar, A. Relationship of Nitrogen Use Efficiency with the Activities of Enzymes Involved in Nitrogen Uptake and Assimilation of Finger Millet Genotypes Grown under Different Nitrogen Inputs. Sci. World J. 2012, 2012, 625731. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J. Plant. Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Baglieri, A.; Cadili, V.; Tambone, F. Humic-like substances from agro-industrial residues affect growth and nitrogen assimilation in maize (Zea mays L.) plantlets. J. Geochem. Explor. 2013, 129, 103–111. [Google Scholar] [CrossRef]
- Hodges, M. Enzyme redundancy and the importance of 2-oxoglutarate in plant ammonium assimilation. J. Exp. Bot. 2002, 53, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]

| Shoot Height (cm) | Leaves (N°) | Shoot FW (g) | Shoot DW (g) | Root Length (cm) | Root FW (g) | Root DW (g) | |
|---|---|---|---|---|---|---|---|
| Ctr | 19.37 ± 0.89 b | 14.67 ± 1.15 b | 13.66 ± 1.05 b | 0.43 ± 0.04 b | 11.72 ± 0.56 a | 1.95 ± 0.20 a | 0.099 ± 0.02 b |
| CV | 23.53 ± 0.75 a | 18 ± 1.15 a | 16.85 ± 0.95 a | 0.60 ± 0.05 a | 11.33 ± 0.89 a | 1.89 ± 0.16 a | 0.154 ± 0.03 a |
| Root/Shoot FW Ratio | Root/Shoot DW Ratio | Shoot FW/DW | Root FW/DW | RGR | |
|---|---|---|---|---|---|
| Ctr | 0.14 ± 0.01 a | 0.23 ± 0.01 a | 31.77 ± 1.15 a | 19.70 ± 1.05 a | 0.035 ± 0.004 b |
| CV | 0.11 ± 0.01 b | 0.25 ± 0.02 a | 28.08 ± 1.04 b | 12.27 ± 1.25 b | 0.042 ± 0.002 a |
| Ch-a (mg g−1 DW) | Ch-b (mg g−1 DW) | C (mg g−1 DW) | Ch-a/Ch-b Ratio | |
|---|---|---|---|---|
| Ctr | 0.484 ± 0.042 b | 0.239 ± 0.024 b | 0.153 ± 0.010 b | 2.02 ± 0.10 b |
| CV | 0.699 ± 0.035 a | 0.282 ± 0.023 a | 0.282 ± 0.025 a | 2.48 ± 0.11 a |
| Shoot Protein Content (mg g−1 DW) | Root Protein Content (mg g−1 DW) | Shoot Ashes (%) | Root Ashes (%) | |
|---|---|---|---|---|
| Ctr | 92.10 ± 2.2 b | 51.93 ± 2.0 b | 18.55 ± 1.2 b | 6.48 ± 1.0 b |
| CV | 110.53 ± 2.3 a | 56.91 ± 2.1 a | 21.91 ± 1.5 a | 11.28 ± 2.0 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Bella, E.; Baglieri, A.; Rovetto, E.I.; Stevanato, P.; Puglisi, I. Foliar Spray Application of Chlorella vulgaris Extract: Effect on the Growth of Lettuce Seedlings. Agronomy 2021, 11, 308. https://doi.org/10.3390/agronomy11020308
La Bella E, Baglieri A, Rovetto EI, Stevanato P, Puglisi I. Foliar Spray Application of Chlorella vulgaris Extract: Effect on the Growth of Lettuce Seedlings. Agronomy. 2021; 11(2):308. https://doi.org/10.3390/agronomy11020308
Chicago/Turabian StyleLa Bella, Emanuele, Andrea Baglieri, Ermes Ivan Rovetto, Piergiorgio Stevanato, and Ivana Puglisi. 2021. "Foliar Spray Application of Chlorella vulgaris Extract: Effect on the Growth of Lettuce Seedlings" Agronomy 11, no. 2: 308. https://doi.org/10.3390/agronomy11020308
APA StyleLa Bella, E., Baglieri, A., Rovetto, E. I., Stevanato, P., & Puglisi, I. (2021). Foliar Spray Application of Chlorella vulgaris Extract: Effect on the Growth of Lettuce Seedlings. Agronomy, 11(2), 308. https://doi.org/10.3390/agronomy11020308









