Effect of Deposition Aids Tank-Mixed with Herbicides on Cotton and Soybean Canopy Deposition and Spray Droplet Parameters
Abstract
1. Introduction
2. Materials and Methods
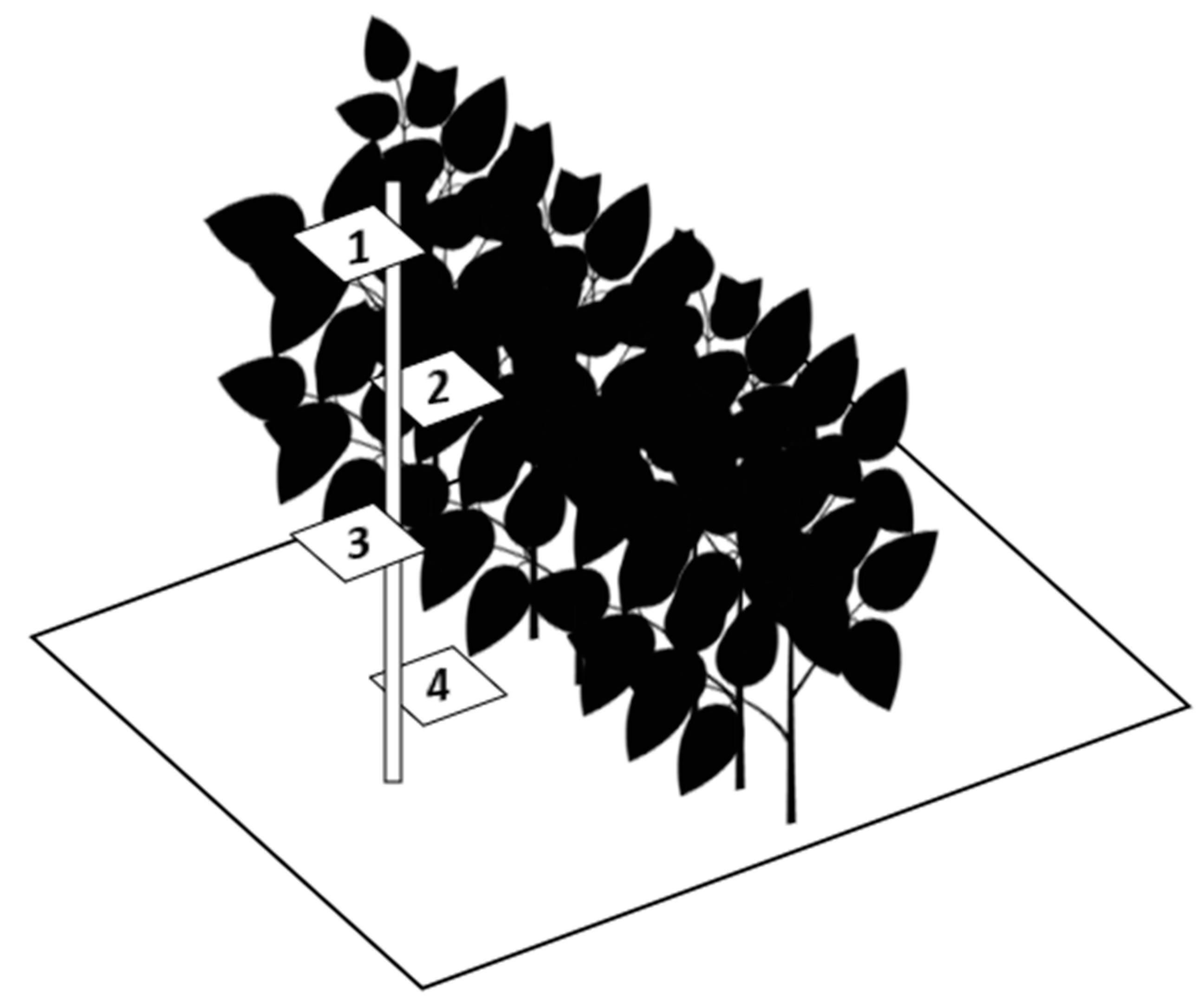
2.1. Spray Deposition Study
2.2. Fluorometric Analysis
2.3. Droplet Size Distribution Analysis
2.4. Statistical Analysis
3. Results
3.1. Cotton Field Study
3.2. Droplet Size Distributions of Herbicide Solutions Used in Cotton
3.3. Soybean Field Study
3.4. Droplet Size Distributions of Herbicide Solutions Used in Soybean
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giles, D.K.; Akesson, N.B.; Yates, W.E. Pesticide Application Technology: Research and Development and the Growth of the Industry. Trans. ASABE 2008, 51, 397–403. [Google Scholar] [CrossRef]
- Creech, C.F.; Henry, R.S.; Fritz, B.K.; Kruger, G.R. Influence of Herbicide Active Ingredient, Nozzle Type, Orifice Size, Spray Pressure, and Carrier Volume Rate on Spray Droplet Size Characteristics. Weed Technol. 2015, 29, 298–310. [Google Scholar] [CrossRef]
- Dorr, G.J.; Hewitt, A.J.; Adkins, S.W.; Hanan, J.; Zhang, H.; Noller, B. A Comparison of Initial Spray Characteristics Produced by Agricultural Nozzles. Crop Prot. 2013, 53, 109–117. [Google Scholar] [CrossRef]
- Hilz, E.; Vermeer, A.W.P. Spray Drift Review: The Extent to Which a Formulation Can Contribute to Spray Drift Reduction. Crop Prot. 2013, 44, 75–83. [Google Scholar] [CrossRef]
- Li, M.; Tank, H.; Kennedy, A.; Zhang, H.; Downer, B.; Ouse, D.; Liu, L. Enlist Duo Herbicide: A Novel 2,4-D Plus Glyphosate Premix Formulation with Low Potential for Off-target Movement. In Pesticide Formulation and Delivery Systems: 32 Volume, Innovating Legacy Products for New Uses; Bernards, M.L., Ed.; ASTM International: West Conshohocken, PA, USA, 2013; pp. 3–14. ISBN 978-0-8031-7544-0. [Google Scholar]
- Butts, T.R.; Butts, L.E.; Luck, J.D.; Fritz, B.K.; Hoffmann, W.C.; Kruger, G.R. Droplet Size and Nozzle Tip Pressure from a Pulse-Width Modulation Sprayer. Biosyst. Eng. 2019, 178, 52–69. [Google Scholar] [CrossRef]
- Vieira, B.C.; Butts, T.R.; Rodrigues, A.O.; Golus, J.A.; Schroeder, K.; Kruger, G.R. Spray Particle Drift Mitigation Using Field Corn (Zea mays L.) as a Drift Barrier. Pest Manag. Sci. 2018, 74, 2038–2046. [Google Scholar] [CrossRef]
- Lake, J.R.; Marchant, J.A. The Use of Dimensional Analysis in a Study of Drop Retention on Barley. Pestic. Sci. 1983, 14, 638–644. [Google Scholar] [CrossRef]
- Mueller, T.C.; Womac, A.R. Effect of Formulation and Nozzle Type on Droplet Size with Isopropylamine and Trimesium Salts of Glyphosate. Weed Technol. 1997, 11, 639–643. [Google Scholar] [CrossRef]
- Ramsdale, B.K.; Messersmith, C.G. Drift-Reducing Nozzle Effects on Herbicide Performance1. Weed Technol. 2001, 15, 453–460. [Google Scholar] [CrossRef]
- Bode, L.E.; Butler, B.J.; Goering, C.E. Spray Drift and Recovery as Affected by Spray Thickener, Nozzle Type, and Nozzle Pressure. Proc. Trans. Am. Soc. Agric. Eng. 1976, 19, 213–218. [Google Scholar] [CrossRef]
- Spanoghe, P.; Schampheleire, M.D.; der Meeren, P.V.; Steurbaut, W. Influence of Agricultural Adjuvants on Droplet Spectra. Pest Manag. Sci. 2007, 63, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Knoche, M. Effect of Droplet Size and Carrier Volume on Performance of Foliage-Applied Herbicides. Crop Prot. 1994, 13, 163–178. [Google Scholar] [CrossRef]
- McMullan, P.M. Utility Adjuvants. Weed Technol. 2000, 14, 792–797. [Google Scholar] [CrossRef]
- Jones, E.J.; Hanks, J.E.; Wills, G.D.; Mack, R.E. Effect of Two Polysaccharide Adjuvants on Glyphosate Spray Droplet Size and Efficacy. Weed Technol. 2007, 21, 171–174. [Google Scholar] [CrossRef]
- Akesson, N.B.; Steinke, W.E.; Yates, W.E. Spray Atomization Characteristics as a Function of Pesticide Formulations and Atomizer Design. J. Environ. Sci. Health Part B 1994, 29, 785–814. [Google Scholar] [CrossRef]
- Hazen, J. United States Patent 5,5500,224. Available online: https://patentimages.storage.googleapis.com/ed/1c/54/3a93181c06d709/US5550224.pdf (accessed on 1 February 2021).
- Hewitt, A. The Effect of Tank Mix and Adjuvants on Spray Drift. In Proceedings of the Fifth International Symposium on Adjuvants for Agrochemicals, Memphis, TN, USA, 17–21 August 1998; Volume 1, pp. 451–462. [Google Scholar]
- Chamberlain, P.; Rose, S.A. Effect of Non-Ionic Polyacrylamides on Spray Droplet Size, Spray Drift, and Pesticide Efficacy. In Proceedings of the Fifth International Symposium on Adjuvants for Agrochemicals, Memphis, TN, USA, 17–21 August 1998; Volume 1, pp. 463–467. [Google Scholar]
- Hall, F.R.; Downer, J.A.; Latting, J.A. Improving Herbicide Efficacy with Adjuvant/Ammonium Sulfate Combinations. In Proceedings of the Fifth International Symposium on Adjuvants for Agrochemicals, Memphis, TN, USA, 17–21 August 1998; Volume 1, pp. 475–480. [Google Scholar]
- ASTM. Terminology relating to agricultural tank mix adjuvants. In Annual Book of ASTM Standards. Biological Effects and Environmental Fate; Biotechnology, Pesticides; ASTM: Philadelphia, PA, USA, 1995; Volume 11.05, pp. 966–967. [Google Scholar]
- Farris, M.E. The Effect of Penetrator and Penetrator plus on Pesticide Deposition, Evaporation, and Foliage Residue in Cotton. In Proceedings of the Beltwide Cotton Conference, San Antonio, TX, USA, 8–12 January 1991; Volume 2, pp. 768–771. [Google Scholar]
- Richards, M.D.; Holloway, P.J.; Stock, D. Structure-Spray Retention Enhancement Relationships for Some Polymers and Polymeric Surfactants. In Proceedings of the Fifth International Symposium on Adjuvants for Agrochemicals, Memphis, TN, USA, 17–21 August 1998; Volume 1, pp. 79–84. [Google Scholar]
- Creech, C.F.; Henry, R.S.; Hewitt, A.J.; Kruger, G.R. Herbicide Spray Penetration into Corn and Soybean Canopies Using Air-Induction Nozzles and a Drift Control Adjuvant. Weed Technol. 2018, 32, 72–79. [Google Scholar] [CrossRef]
- Steckel, L.E. The Dioecious Amaranthus Spp.: Here to Stay. Weed Technol. 2007, 21, 567–570. [Google Scholar] [CrossRef]
- Alves, G.S.; Kruger, G.R.; da Cunha, J.P.A.R.; Vieira, B.C.; Henry, R.S.; Obradovic, A.; Grujic, M. Spray Drift from Dicamba and Glyphosate Applications in a Wind Tunnel. Weed Technol. 2017, 31, 387–395. [Google Scholar] [CrossRef]
- Vieira, B.C.; Butts, T.R.; Rodrigues, A.O.; Iii, J.J.S.; Fritz, B.K.; Kruger, G.R. Particle Drift Potential of Glyphosate plus 2,4-D Choline Pre-Mixture Formulation in a Low-Speed Wind Tunnel. Weed Technol. 2020, 34, 520–527. [Google Scholar] [CrossRef]
- Fritz, B.; Hoffmann, W.; Jank, P. A Fluorescent Tracer Method for Evaluating Spray Transport and Fate of Field and Laboratory Spray Applications. In Pesticide Formulations and Delivery Systems, 31 st Volume: Innovative Green Chemistries for the 21 st Century; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar] [CrossRef]
- Henry, R.S.; Kruger, G.R.; Fritz, B.K.; Hoffmann, W.C.; Bagley, W.E. Measuring the Effect of Spray Plume Angle on the Accuracy of Droplet Size Data. In Pesticide Formulation and Delivery Systems: 33rd Volume, “Sustainability: Contributions from Formulation Technology”; Sesa, C., Ed.; ASTM International: West Conshohocken, PA, USA, 2014; pp. 129–138. ISBN 978-0-8031-7578-5. [Google Scholar]
- Butts, T.R.; Samples, C.A.; Franca, L.X.; Dodds, D.M.; Reynolds, D.B.; Adams, J.W.; Zollinger, R.K.; Howatt, K.A.; Fritz, B.K.; Hoffmann, W.C.; et al. Spray Droplet Size and Carrier Volume Effect on Dicamba and Glufosinate Efficacy. Pest Manag. Sci. 2018, 74, 2020–2029. [Google Scholar] [CrossRef]
- Creech, C.F.; Moraes, J.G.; Henry, R.S.; Luck, J.D.; Kruger, G.R. The Impact of Spray Droplet Size on the Efficacy of 2,4-D, Atrazine, Chlorimuron-Methyl, Dicamba, Glufosinate, and Saflufenacil. Weed Technol. 2016, 30, 573–586. [Google Scholar] [CrossRef]
- Spillman, J.J. Spray Impaction, Retention and Adhesion: An Introduction to Basic Characteristics. Pestic. Sci. 1984, 15, 97–106. [Google Scholar] [CrossRef]
- Forster, W.A.; Kimberley, M.O.; Zabkiewicz, J.A. A Universal Spray Droplet Adhesion Model. Trans ASAE 2005, 48, 1321–1330. [Google Scholar] [CrossRef]
- Lake, J.R. The Effect of Drop Size and Velocity on the Performance of Agricultural Sprays. Pestic. Sci. 1977, 8, 515–520. [Google Scholar] [CrossRef]
- Nuti, R.C.; Viator, R.P.; Casteel, S.N.; Edmisten, K.L.; Wells, R. Effect of Planting Date, Mepiquat Chloride, and Glyphosate Application to Glyphosate-Resistant Cotton. Agron. J. 2006, 98, 1627–1633. [Google Scholar] [CrossRef]
- Reddy, V.R.; Baker, D.N.; Hodges, H.F. Temperature and Mepiquat Chloride Effects on Cotton Canopy Architecture. Agron. J. 1990, 82, 190–195. [Google Scholar] [CrossRef]
- Behringer, F.J.; Cosgrove, D.J.; Reid, J.B.; Davies, P.J. Physical Basis for Altered Stem Elongation Rates in Internode Length Mutants of Pisum. Plant Physiol. 1990, 94, 166–173. [Google Scholar] [CrossRef]
- Biles, S.P.; Cothren, J.T. Flowering and Yield Response of Cotton to Application of Mepiquat Chloride and PGR-IV. Crop Sci. 2001, 41, 1834–1837. [Google Scholar] [CrossRef]
- Yang, T.; Davies, P.J.; Reid, J.B. Genetic Dissection of the Relative Roles of Auxin and Gibberellin in the Regulation of Stem Elongation in Intact Light-Grown Peas. Plant Physiol. 1996, 110, 1029–1034. [Google Scholar] [CrossRef]
- Tagliapietra, E.L.; Streck, N.A.; da Rocha, T.S.M.; Richter, G.L.; da Silva, M.R.; Cera, J.C.; Guedes, J.V.C.; Zanon, A.J. Optimum Leaf Area Index to Reach Soybean Yield Potential in Subtropical Environment. Agron. J. 2018, 110, 932–938. [Google Scholar] [CrossRef]
- Ashley, D.A.; Doss, B.D.; Bennett, O.L. Relation of Cotton Leaf Area Index to Plant Growth and Fruiting. Agron. J. 1965, 57, 61–64. [Google Scholar] [CrossRef]
- Wolf, R.E.; Bretthauer, S. Improving Canopy Coverage by Using Deposition Aids in Low Volume Fungicide Applications in Corn. In Proceedings of the American Society of Agricultural and Biological Engineers, Saint Joseph, MN, USA, 29 June–2 July 2008; pp. 1–7. [Google Scholar]
| Crop | Herbicide Treatments | Products Used | Rate (kg ae/ai ha−1) |
|---|---|---|---|
| Cotton | glyphosate + dicamba | MON76832 | 1.1 + 0.6 |
| glyphosate + 2,4-D | Enlist DuoTM | 0.9 + 0.9 | |
| glufosinate + dicamba | Liberty® 280 SL + Xtendimax® | 0.6 + 0.6 | |
| glyphosate + dicamba + acetochlor | MON76832 + Warrant® | 1.1 + 0.6 + 1.3 | |
| glyphosate + dicamba + S-metolachlor | MON76832 + Dual Magnum | 1.1 + 0.6 + 1.38 | |
| Soybean | glufosinate + dicamba | Liberty® 280 SL + Xtendimax® | 0.6 + 0.6 |
| glyphosate + 2,4-D + glufosinate | Enlist DuoTM + Liberty® 280 SL | 0.9 + 0.9 + 0.6 | |
| glyphosate + fomesafen | Roundup Powermax + Flexstar® | 1.1 + 0.26 | |
| glufosinate + fomesafen | Liberty® 280 SL + Flexstar® | 0.6 + 0.26 | |
| glyphosate + dicamba fomesafen | MON76832 + Flexstar® | 1.1 + 0.6 + 0.26 |
| Effect | Degrees of Freedom | F-Value | p-Value |
|---|---|---|---|
| Deposition aid | 3 | 0.31 | 0.8195 |
| Position within canopy | 3 | 18.79 | <0.0001 |
| Deposition aid × canopy | 9 | 0.13 | 0.9988 |
| Canopy Position | Spray Deposition (RFU) b |
|---|---|
| 1 (Top canopy) | 1972.82 A |
| 2 (15 cm downward) | 1327.57 B |
| 3 (30 cm downward) | 1219.94 B |
| 4 (46 cm downward) | 913.02 C |
| Deposition Aid | Spray Deposition (RFU) b | ||
|---|---|---|---|
| Glyphosate + Dicamba + S-Metolachlor | Glyphosate + Dicamba + Acetochlor | Glyphosate + 2,4-D | |
| Guargam | 1407.27 AB | 2036.51 A | 798.29 B |
| No deposition aid | 807.43 C | 2238.85 A | 931.65 B |
| Oil | 916.19 BC | 668.24 B | 1389.74 A |
| Polymer | 1640.55 A | 1179.33 B | 695.39 B |
| Effect | D.F. A | DV0.1 B | DV0.5 C | DV0.9 D | RS E | <150 µm F |
|---|---|---|---|---|---|---|
| Herbicide | 4 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 |
| Deposition Aid | 3 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 |
| Herbicide × Deposition Aid | 12 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 |
| Herbicide Combinations | Deposition Aid | DV0.1 A | DV0.5 B | DV0.9 C | RS D | <150 µm E |
|---|---|---|---|---|---|---|
| ________________________ µm ________________________ | % | |||||
| glyphosate + dicamba | Alone | 154 M | 337 M | 566 IJ | 1.22 E | 9.37 C |
| Oil | 174 J | 346 L | 564 IJK | 1.13 I | 6.33 G | |
| Polymer | 240 F | 532 F | 848 E | 1.14 GHI | 2.90 M | |
| Guargum | 304 C | 660 C | 1031 B | 1.1 J | 1.36 P | |
| glyphosate + 2,4-D | Alone | 194 H | 357 K | 551 K | 1.00 L | 4.35 I |
| Oil | 187 I | 356 K | 565 IJ | 1.06 K | 5.08 H | |
| Polymer | 215 G | 457 I | 781 G | 1.24 CDE | 3.3 L | |
| Guargum | 310 B | 675 B | 1081 A | 1.14 GHI | 1.13 Q | |
| glufosinate + dicamba | Alone | 121 P | 289 P | 519 L | 1.38 A | 15.58 A |
| Oil | 146 O | 326 N | 553 JK | 1.25 BCD | 10.57 B | |
| Polymer | 217 G | 476 H | 802 F | 1.23 DE | 3.61 K | |
| Guargum | 365 A | 728 A | 1093 A | 1.00 L | 0.58 R | |
| glyphosate + dicamba + acetochlor | Alone | 164 K | 348 L | 568 I | 1.16 G | 7.75 F |
| Oil | 173 J | 345 L | 564 IJK | 1.13 HI | 6.46 G | |
| Polymer | 215 G | 484 G | 808 F | 1.23 DE | 4.00 J | |
| Guargum | 267 D | 599 D | 985 C | 1.20 F | 1.99 O | |
| glyphosate + dicamba + S-metolachlor | Alone | 150 N | 316 O | 513 L | 1.15 GH | 9.98 B |
| Oil | 158 L | 313 O | 495 M | 1.08 JK | 8.49 E | |
| Polymer | 197 H | 447 J | 763 H | 1.26 B | 4.94 H | |
| Guargum | 251 E | 569 E | 966 D | 1.27 BC | 2.53 N | |
| Effect | Degrees of Freedom | F-Value | p-Value |
|---|---|---|---|
| Deposition aid | 3 | 1.12 | 0.3402 |
| Position within canopy | 3 | 55.58 | <0.0001 |
| Deposition aid × canopy | 9 | 0.90 | 0.5249 |
| Canopy Position | Spray Deposition (RFU) b |
|---|---|
| 1 (Top canopy) | 2657.38 A |
| 2 (15 cm downward) | 2038.85 B |
| 3 (30 cm downward) | 951.15 C |
| 4 (46 cm downward) | 779.70 C |
| Deposition Aid | Spray Deposition (RFU) b | |
|---|---|---|
| Glyphosate + 2,4-D+ | Glufosinate + Fomesafen | |
| Glufosinate | ||
| Guargam | 1222.79 B | 1288.34 AB |
| No deposition aid | 1886.72 A | 984.92 B |
| Oil | 1307.67 B | 1535.04 A |
| Polymer | 1856.98 A | 1748.67 A |
| Effect | D.F. A | DV0.5 B | DV0.1 C | DV0.9 D | RS E | <150 µm F |
|---|---|---|---|---|---|---|
| Herbicide | 4 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 |
| Deposition Aid | 3 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 |
| Herbicide × Deposition Aid | 12 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 | ≤0.0001 |
| Herbicide Combinations | Deposition Aid | DV0.1 A | DV0.5 B | DV 0.9 C | R.S. D | <150 µm E |
|---|---|---|---|---|---|---|
| ________________________ µm ________________________ | % | |||||
| glufosinate + dicamba | Alone | 121 P | 289 O | 519 K | 1.38 A | 15.58 B |
| Oil | 146 M | 326 K | 553 J | 1.25 D | 10.57 E | |
| Polymer | 217 G | 476 G | 802 F | 1.23 DE | 3.61 K | |
| Guargum | 365 A | 728 A | 1093 A | 1.00 L | 0.58 P | |
| glufosinate + 2,4-D | Alone | 133 O | 310 L | 542 J | 1.32 B | 12.75 C |
| Oil | 135 O | 304 MN | 500 LM | 1.20 EFG | 12.55 C | |
| Polymer | 179 J | 407 J | 700 I | 1.28 C | 6.35 H | |
| Guargum | 352 B | 705 B | 1068 B | 1.01 L | 0.66 P | |
| glufosinate + fomesafen | Alone | 120 P | 281 P | 489 M | 1.31 B | 16.29 A |
| Oil | 121 P | 286 OP | 495 LM | 1.31 B | 15.81 B | |
| Polymer | 195 I | 432 I | 723 H | 1.22 DE | 5.06 I | |
| Guargum | 334 C | 689 C | 1072 B | 1.07 K | 0.92 O | |
| glyphosate + fomesafen | Alone | 158 L | 326 K | 543 J | 1.18 GH | 8.44 F |
| Oil | 163 K | 321 K | 524 K | 1.12 J | 7.47 G | |
| Polymer | 228 F | 497 F | 823 E | 1.20 EFG | 2.97 L | |
| Guargum | 292 D | 647 D | 1025 C | 1.13 IJ | 1.50 N | |
| glyphosate + dicamba + fomesafen | Alone | 139 N | 300 N | 496 LM | 1.19 FG | 12.00 D |
| Oil | 147 M | 310 LM | 505 L | 1.16 HI | 10.56 E | |
| Polymer | 202 H | 443 H | 739 G | 1.21 EF | 4.53 J | |
| Guargum | 264 E | 588 E | 982 D | 1.22 DE | 2.09 M | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samples, C.A.; Butts, T.R.; Vieira, B.C.; Irby, J.T.; Reynolds, D.B.; Catchot, A.; Kruger, G.R.; Dodds, D.M. Effect of Deposition Aids Tank-Mixed with Herbicides on Cotton and Soybean Canopy Deposition and Spray Droplet Parameters. Agronomy 2021, 11, 278. https://doi.org/10.3390/agronomy11020278
Samples CA, Butts TR, Vieira BC, Irby JT, Reynolds DB, Catchot A, Kruger GR, Dodds DM. Effect of Deposition Aids Tank-Mixed with Herbicides on Cotton and Soybean Canopy Deposition and Spray Droplet Parameters. Agronomy. 2021; 11(2):278. https://doi.org/10.3390/agronomy11020278
Chicago/Turabian StyleSamples, Chase Allen, Thomas R. Butts, Bruno C. Vieira, Jon Trenton Irby, Daniel B. Reynolds, Angus Catchot, Greg R. Kruger, and Darrin M. Dodds. 2021. "Effect of Deposition Aids Tank-Mixed with Herbicides on Cotton and Soybean Canopy Deposition and Spray Droplet Parameters" Agronomy 11, no. 2: 278. https://doi.org/10.3390/agronomy11020278
APA StyleSamples, C. A., Butts, T. R., Vieira, B. C., Irby, J. T., Reynolds, D. B., Catchot, A., Kruger, G. R., & Dodds, D. M. (2021). Effect of Deposition Aids Tank-Mixed with Herbicides on Cotton and Soybean Canopy Deposition and Spray Droplet Parameters. Agronomy, 11(2), 278. https://doi.org/10.3390/agronomy11020278






