Development and Characterization of Wheat-Agropyron cristatum Introgression Lines Induced by Gametocidal Genes and Wheat ph1b Mutant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
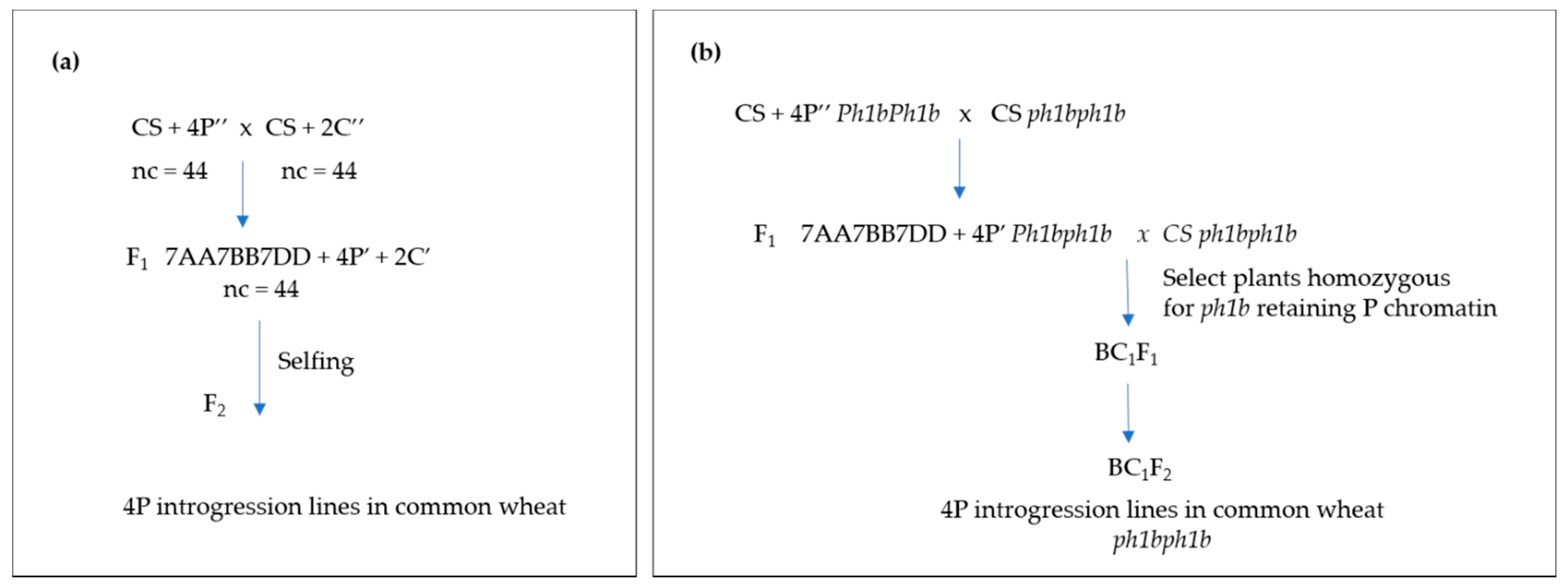
2.1.1. Gametocidal 2Cc Chromosome
2.1.2. Ph1b Mutant
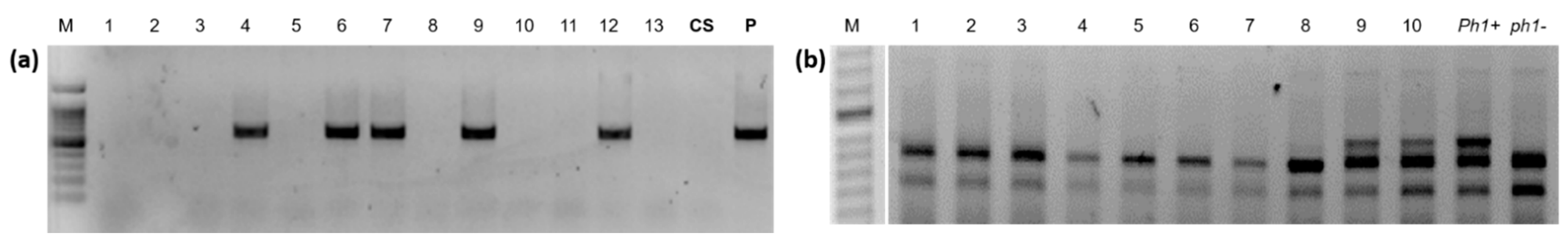
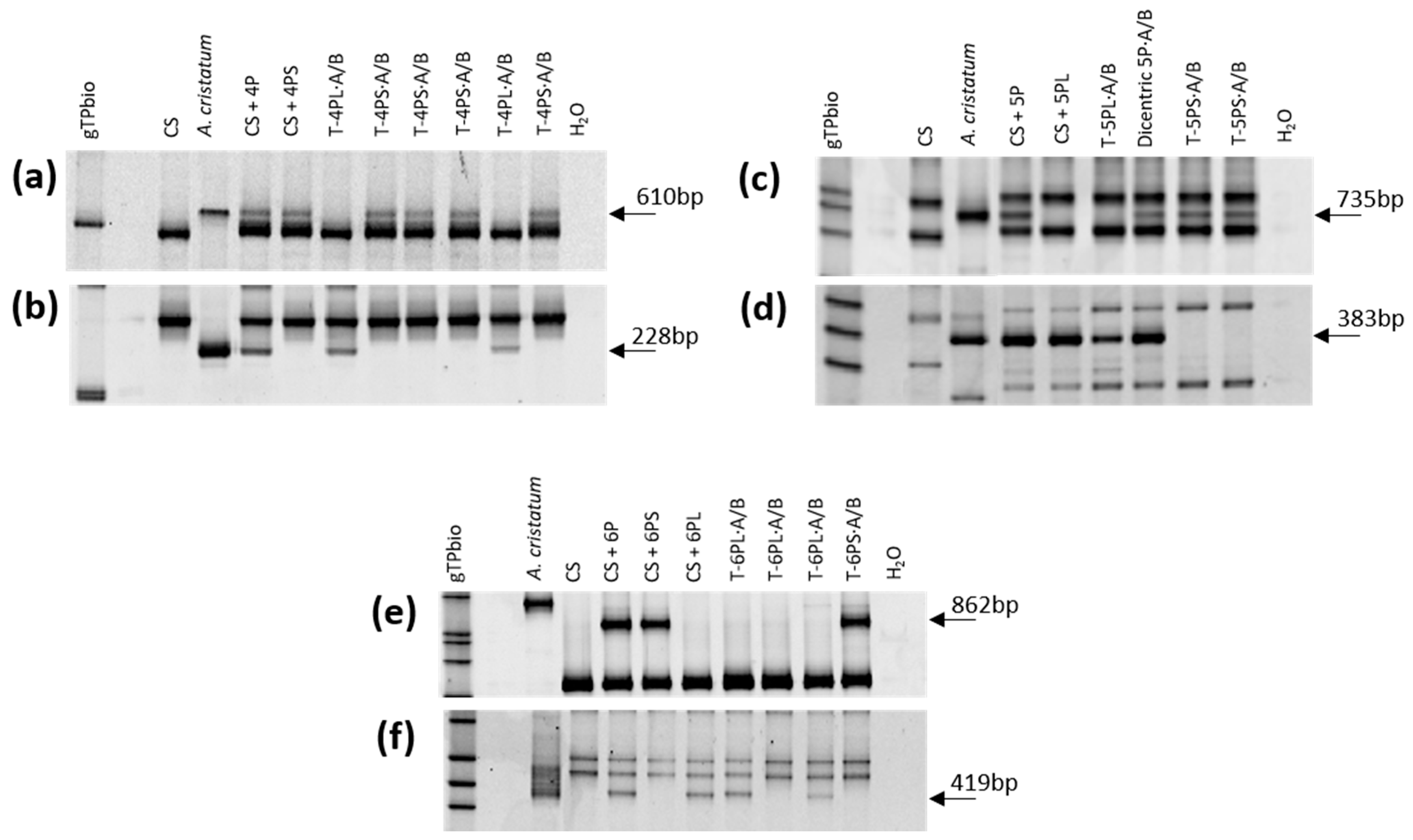
2.2. Molecular Marker Characterization
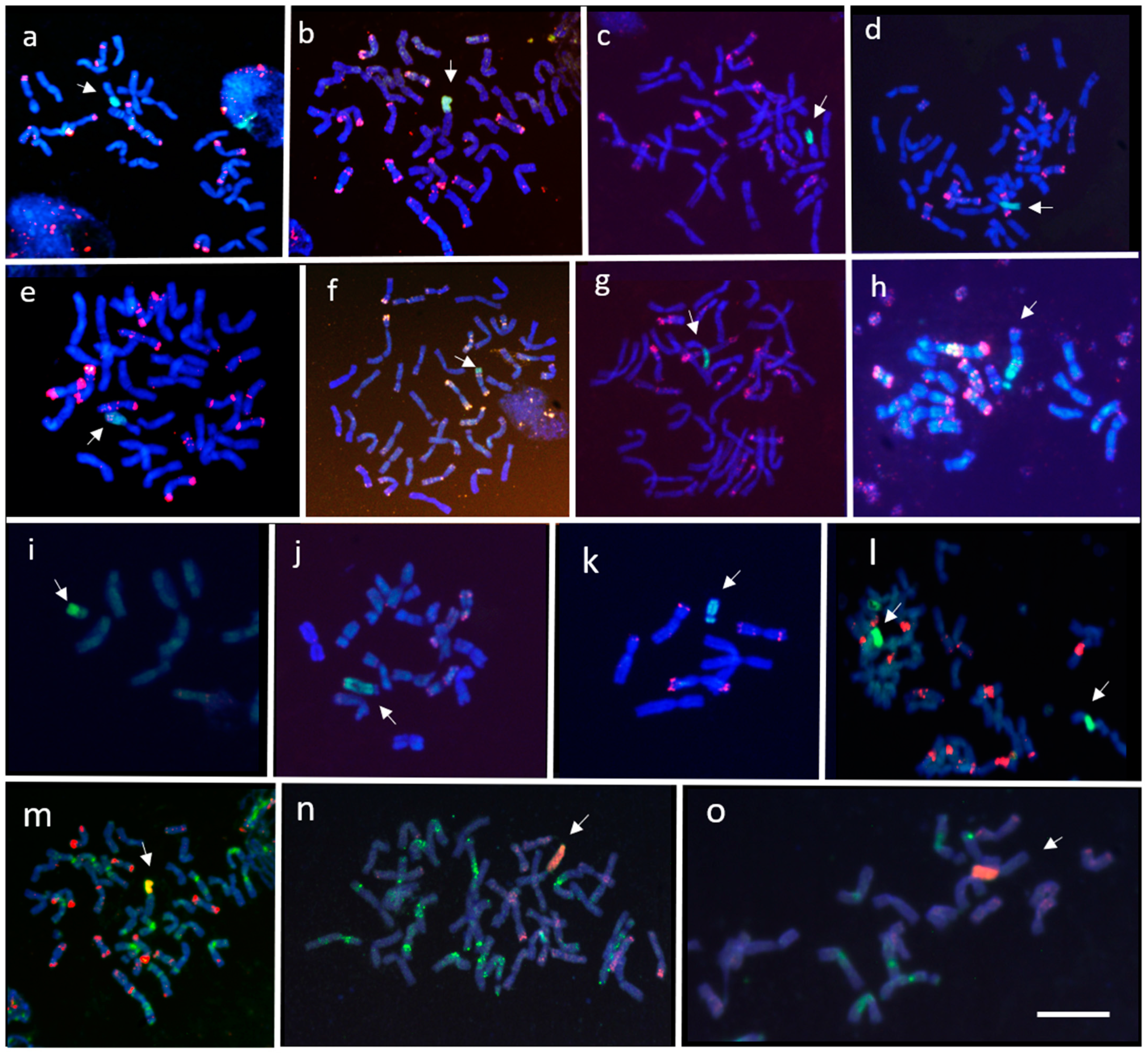
2.3. Fluorescence In Situ Hybridization (FISH)
3. Results
3.1. Induction of Wheat-A. cristatum Introgressions by Gametocidal 2Cc Chromosome
3.2. Induction of Wheat-A. cristatum Introgressions by ph1b Mutant
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dewey, D.R. The genomic system of classification as a guide to intergeneric hybridization with the perennial Triticeae. In Gene Manipulation in Plant Improvement; Gustafson, J.P., Ed.; Stadler Genetics Sympo, sium Series; Springer: Boston, MA, USA, 1984; pp. 209–279. [Google Scholar]
- Ochoa, V.; Madrid, E.; Said, M.; Rubiales, D.; Cabrera, A. Molecular and cytogenetic characterization of a common wheat-Agropyron cristatum chromosome translocation conferring resistance to leaf rust. Euphytica 2015, 201, 89–95. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, L.; Han, H.; Zhou, S.; Zhang, J.; Yang, X.; Li, X.; Liu, W.; Li, L. Physical localization of a locus from Agropyron cristatum conferring resistance to stripe rust in common wheat. Int. J. Mol. Sci. 2017, 18, 2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copete, A.; Cabrera, A. Chromosomal location of genes for resistance to powdery mildew in Agropyron cristatum and mapping of conserved orthologous set molecular markers. Euphytica 2017, 213, 189. [Google Scholar] [CrossRef]
- Li, H.; Jiang, B.; Wang, J.; Lu, Y.; Zhang, J.; Pan, C.; Yang, X.; Li, X.; Liu, W.; Li, L. Mapping of novel powdery mildew resistance gene(s) from Agropyron cristatum chromosome 2P. Theor. Appl. Genet. 2017, 130, 109–121. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Liu, W.; Han, H.; Lu, Y.; Yang, X.; Li, X.; Li, L. Introgression of Agropyron cristatum 6P chromosome segment into common wheat for enhanced thousand-grain weight and spike length. Theor. Appl. Genet. 2015, 128, 1827–1837. [Google Scholar] [CrossRef]
- McGuire, P.E.; Dvorák, J. High salt-tolerance potential in wheatgrasses. Crop Sci. 1981, 21, 702–705. [Google Scholar] [CrossRef]
- Bayat, H.; Nemati, H.; Tehranifar, A.; Gazanchian, A. Screening different crested wheatgrass (Agropyron cristatum (L.) Gaertner.) accessions for drought stress tolerance. Arch. Agron. Soil Sci. 2016, 62, 769–780. [Google Scholar] [CrossRef]
- Limin, A.E.; Fowler, D.B. An interspecific hybrid and amphiploid produced from Triticum aestivum crosses with Agropyron cristatum and Agropyron desertorum. Genome 1990, 33, 581–584. [Google Scholar] [CrossRef]
- Chen, Q.; Jahier, J.; Cauderon, Y. Production and cytogenetic analysis of BC1, BC2, and BC3 progenies of an intergeneric hybrid between Triticum aestivum (L.) Thell. and tetraploid Agropyron cristatum (L.) Gaertn. Theor. Appl. Genet. 1992, 84, 698–703. [Google Scholar] [CrossRef]
- Martín, A.; Rubiales, D.; Cabrera, A. Meiotic pairing in a trigeneric hybrid Triticum tauschii-Agropyron cristatum-Hordeum chilense. Hereditas 1998, 129, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Martín, A.; Cabrera, A.; Esteban, E.; Hernández, P.; Ramírez, M.C.; Rubiales, D. A fertile amphiploid between diploid wheat (Triticum tauschii) and crested wheatgrass (Agropyron cristatum). Genome 1999, 42, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.H.; Rubiales, D.; Cabrera, A. A Fertile Amphiploid between durum wheat (Triticum turgidum) and the ×Agroticum amphiploid (Agropyron cristatum × T. tauschii). Hereditas 2001, 135, 183–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, M.H.; Cabrera, A.; Sillero, J.C.; Rubiales, D. Genomic constitution and expression of disease resistance in Agropyron cristatum x durum wheat derivatives. Breed. Sci. 2007, 57, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Luan, Y.; Wang, X.; Liu, W.; Li, C.; Zhang, J.; Gao, A.; Wang, Y.; Yang, X.; Li, L. Production and identification of wheat-Agropyron cristatum 6P translocation lines. Planta 2010, 232, 501–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Lv, M.; Song, L.; Zhang, J.; Gao, A.; Li, L.; Liu, W. Production and identification of wheat-Agropyron cristatum 2P translocation lines. PLoS ONE 2016, 11, e0145928. [Google Scholar] [CrossRef] [Green Version]
- Sears, E.R. The transfer of leaf rust resistance from Aegilops umbellulata to wheat. Brookhaven Symp. Biol. 1956, 9, 1–22. [Google Scholar]
- Mukai, Y.; Friebe, B.; Hatchett, J.H.; Yamamoto, M.; Gill, B.S. Molecular cytogenetic analysis of radiation-induced wheat-rye terminal and intercalary chromosomal translocations and the detection of rye chromatin specifying resistance to Hessian fly. Chromosoma 1993, 102, 88–95. [Google Scholar] [CrossRef]
- Friebe, B.; Jiang, J.; Gill, B.S.; Dyck, P.L. Radiation-induced nonhomologous wheat Agropyrum intermedium chromosomal translocations conferring resistance to leaf rust. Theor. Appl. Genet. 1993, 86, 141–149. [Google Scholar] [CrossRef]
- Sharma, P.; Sheikh, I.; Kumar, S.; Verma, S.K.; Kumar, R.; Vyas, P.; Dhaliwal, H.S. Precise transfers of genes for high grain iron and zinc from wheat-Aegilops substitution lines into wheat through pollen irradiation. Mol. Breed. 2018, 38, 81. [Google Scholar] [CrossRef]
- Riley, R.; Chapman, V. Genetic control of the cytologically diploid behaviour of hexaploid wheat. Nature 1958, 182, 713–715. [Google Scholar] [CrossRef]
- Sears, E.R. An induced mutant with homoeologous pairing in common wheat. Can. J. Genet. Cytol. 1977, 19, 585–593. [Google Scholar] [CrossRef]
- Qi, L.L.; Friebe, B.; Zhang, P.; Gill, B.S. Homoeologous recombination, chromosome engineering and crop improvement. Chromosome Res. 2007, 15, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Friebe, B.; Jiang, J.; Raupp, W.J.; McIntosh, R.A.; Gill, B.S. Characterization of wheat-alien translocations conferring resistance to diseases and pests: Current status. Euphytica 1996, 91, 59–87. [Google Scholar] [CrossRef]
- Xin, Z.Y.; Zhang, Z.Y.; Chen, X.; Lin, Z.S.; Ma, Y.Z.; Xu, H.J.; Banks, P.M.; Larkin, P.J. Development and characterization of common wheat-Thinopyrum intermedium translocation lines with resistance to barley yellow dwarf virus. Euphytica 2001, 119, 163–167. [Google Scholar] [CrossRef]
- Hajjar, R.; Hodgkin, T. The use of wild relatives in crop improvement: A survey of developments over the last 20 years. Euphytica 2007, 156, 1–13. [Google Scholar] [CrossRef]
- Qi, L.L.; Pumphrey, M.O.; Friebe, B.; Chen, P.D.; Gill, B.S. Molecular cytogenetic characterization of alien introgressions with gene Fhb3 for resistance to Fusarium head blight disease of wheat. Theor. Appl. Genet. 2008, 117, 1155–1166. [Google Scholar] [CrossRef]
- Mullan, D.J.; Mirzaghaderi, G.; Walker, E.; Colmer, T.D.; Francki, M.G. Development of wheat-Lophopyrum elongatum recombinant lines for enhanced sodium exclusion during salinity stress. Theor. Appl. Genet. 2009, 119, 1313–1323. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, H.; Xiao, J.; Bie, T.; Cheng, S.; Jia, Q.; Yuan, C.; Zhang, R.; Cao, A.; Chen, P.; et al. Induction of 4VS chromosome recombinants using the CS ph1b mutant and mapping of the wheat yellow mosaic virus resistance gene from Haynaldia villosa. Theor. Appl.Genet. 2013, 126, 2921–2930. [Google Scholar] [CrossRef]
- Endo, T.R. Gametocidal chromosomes and their induction of chromosome mutations in wheat. Jpn. J. Genet. 1990, 65, 135–152. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.; Endo, T. Genetic induction of structural changes in barley chromosomes added to common wheat by a gametocidal chromosome derived from Aegilops cylindrica. Genes Genet. Syst. 1999, 74, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Friebe, B.; Kynast, R.G.; Gill, B.S. Gametocidal factor-induced structural rearrangements in rye chromosomes added to common wheat. Chromosome Res. 2000, 8, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cao, A.; Qi, Z.; Zhang, W.; Chen, P. Structural changes of 2V chromosome of Haynaldia villosa induced by gametocidal chromosome 3C of Aegilops triuncialis. Agric. Sci. China 2008, 7, 804–811. [Google Scholar] [CrossRef]
- Chen, P.D.; Liu, W.X.; Yuan, J.H.; Wang, X.; Zhou, B.; Wang, S.L.; Zhang, S.Z.; Feng, Y.G.; Yang, B.J.; Liu, G.X.; et al. Development and characterization of wheat- Leymus racemosus translocation lines with resistance to Fusarium Head Blight. Theor. Appl. Genet. 2005, 111, 941–948. [Google Scholar] [CrossRef]
- Said, M.; Cabrera, A. A physical map of chromosome 4Hch from H. chilense containing SSR, STS and EST-SSR molecular markers. Euphytica 2009, 167, 253–259. [Google Scholar] [CrossRef]
- Cherif-Mouaki, S.; Said, M.; Alvarez, J.; Cabrera, A. Sub-arm location of prolamin and EST-SSR loci on chromosome 1Hch from Hordeum chilense. Euphytica 2011, 178, 63–69. [Google Scholar] [CrossRef]
- Said, M.; Recio, R.; Cabrera, A. Development and characterisation of structural changes in chromosome 3Hch from Hordeum chilense in common wheat and their use in physical mapping. Euphytica 2012, 188, 429–440. [Google Scholar] [CrossRef]
- Liu, W.H.; Luan, Y.; Wang, J.C.; Wang, X.G.; Su, J.J.; Zhang, J.P.; Yang, X.M.; Gao, A.N.; Li, L.H. Production and identification of wheat—Agropyron cristatum (1.4P) alien translocation lines. Genome 2010, 53, 472–481. [Google Scholar] [CrossRef]
- Song, L.; Jiang, L.; Han, H.; Gao, A.; Yang, X.; Li, L.; Liu, W. Efficient induction of Wheat-Agropyron cristatum 6P translocation lines and GISH detection. PLoS ONE 2013, 8, e69501. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ma, H.; Zhang, J.; Zhou, S.; Han, H.; Liu, W.; Li, X.; Yang, X.; Li, L. Molecular cytogenetic characterization of an Agropyron cristatum 6PL chromosome segment conferring superior kernel traits in wheat. Euphytica 2018, 214, 198. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic. Acids. Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Zhang, J.P.; Wang, J.C.; Yang, X.M.; Gao, A.N.; Zhang, X.K.; Liu, W.H.; Li, L.H. Cloning and characterization of repetitive sequences and development of SCAR markers specific for the P genome of Agropyron cristatum. Euphytica 2010, 172, 363–372. [Google Scholar] [CrossRef]
- Quraishi, U.M.; Abrouk, M.; Bolot, S.; Pont, C.; Throude, M.; Guilhot, N.; Bortolini, F.; Praud, S.; Murigneux, A.; Charmet, G.; et al. Genomics in cereals: From genome-wide conserved orthologous set (COS) sequences to candidate genes for trait dissection. Funct. Integr. Genomics 2009, 9, 473–484. [Google Scholar] [CrossRef]
- Said, M.; Copete, A.; Gaál, E.; Molnár, I.; Cabrera, A.; Doležel, J.; Vrána, J. Uncovering macrosyntenic relationships between tetraploid Agropyron cristatum and bread wheat genomes using COS markers. Theor. Appl. Genet. 2019, 132, 2881–2898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Lai, J.; Liu, G.; Chen, F. Development of a Scar marker for the Ph1 locus in common wheat and its application. Crop Sci. 2002, 42, 1365–1368. [Google Scholar] [CrossRef]
- Cabrera, A.; Martín, A.; Barro, F. In-situ comparative mapping (ISCM) of Glu-1 loci in Triticum and Hordeum. Chromosome Res. 2002, 10, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Jubault, M.; Tanguy, A.M.; Abelard, P.; Coriton, O.; Dusautoir, J.C.; Jahier, J. Attempts to induce homoeologous pairing between wheat and Agropyron cristatum genomes. Genome 2006, 49, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yang, X.; Wang, R.; Gao, A.; Li, L.; Liu, W. The inhibiting effect of 1.4 recombinant P chromosome of wheat-Agropyron cristatum addition line on the Ph gene. Sci. Bull. 2010, 55, 153–157. [Google Scholar] [CrossRef]
- Jauhar, P.P. Chromosome pairing in hybrids between hexaploid bread wheat and tetraploid crested wheatgrass (Agropyron cristatum). Hereditas 1992, 116, 107–109. [Google Scholar] [CrossRef]
- Rey, M.-D.; Calderon, M.C.; Prieto, P. The use of the ph1b mutant to induce recombination between the chromosomes of wheat and barley. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, J.; Che, Y.; Liu, W.; Lu, Y.; Yang, X.; Li, X.; Jia, J.; Liu, X.; Li, L. Construction of Agropyron Gaertn. genetic linkage maps using a wheat 660K SNP array reveals a homoeologous relationship with the wheat genome. Plant Biotechnol. J. 2018, 16, 818–827. [Google Scholar] [CrossRef] [Green Version]
- Linc, G.; Gaál, E.; Molnár, I.; Icsó, D.; Badaeva, E.; Molnár-Láng, M. Molecular cytogenetic (FISH) and genome analysis of diploid wheatgrasses and their phylogenetic relationship. PLoS ONE 2017, 12, e0173623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Yan, B.; Li, F.; Zhang, J.; Zhang, J.; Ma, H.; Liu, W.; Lu, Y.; Yang, X.; Liu, X.; et al. RNA-Seq analysis provides first insights into the phylogenetic relationships and interespecific variation between Agropyron cristatum and wheat. Front. Plant Sci. 2017, 8, 1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrera, A.; Castellano, L.; Recio, R.; Alvarez, J.B. Chromosomal location and molecular characterization of three grain hardness genes in Agropyron cristatum. Euphytica 2019, 215, 165. [Google Scholar] [CrossRef]
- Cabrera, A.; Copete-Parada, A.; Madrid, E. Cloning and characterization of a putative orthologue of the wheat vernalization (VRN1) gene in perennial wheatgrass (Agropyron cristatum). Plant Breed. 2020. [Google Scholar] [CrossRef]
| Cross | No. of Plants | No. of Plants Retaining P Chromatin | No. of BC1F1 Plants Retaining P Chromatin and ph1ph1 | No. of BC1F2 Plants Evaluated | No. of BC1F2 Plants Retaining P Chromatin |
|---|---|---|---|---|---|
| (a) 2Cc Gametocidal | |||||
| CS + 4P × CS + 2Cc | 86 1 | 21 (24.4%) 1 | - | - | - |
| CS + 5P × CS + 2Cc | 90 1 | 32 (35.6%) 1 | - | - | - |
| CS + 6P × CS + 2Cc | 217 1 | 66 (30.4%) 1 | - | - | - |
| Total | 393 1 | 119 (30.3%) 1 | - | - | - |
| (b) CS ph1b mutant | |||||
| CS + 4P × CS ph1 | 97 2 | 36 (37.1%) 2 | 17 (47.2%) | 28 | 19 (67.8%) |
| CS + 5P × CS ph1 | 94 2 | 45 (47.8%) 2 | 24 (53.3%) | 56 | 24 (42.8%) |
| CS + 6P × CS ph1 | 140 2 | 57 (38.0%) 2 | 22 (38.6%) | 72 | 25 (34.7%) |
| Total | 331 2 | 138 (41.4%) 2 | 63 (45.6%) | 156 | 68 (43.6%) |
| Cross | No. of Plants | Complete Chromosome | Telosomic Chromosome | Ditelosomic Chromosome | Wheat-A. cristatum Translocation | |
|---|---|---|---|---|---|---|
| 2 Copies | 1 Copy | |||||
| (a) 2Cc Gametocidal | ||||||
| CS + 4P × CS + 2Cc | 21 | 2 (9.5%) | 13 (61.9%) | 2 (9.5%) | 0 | 4 (19.0%) |
| CS + 5P × CS + 2Cc | 32 | 1 (3.1%) | 25 (78.1%) | 2 (6.2%) | 0 | 4 (12.5%) |
| CS + 6P × CS + 2Cc | 66 | 2 (3.0%) | 57 (86.4%) | 1 (1.5%) | 1 (1.5%) | 5 (7.6%) |
| Total | 119 | 5 (4.2%) | 95 (79.8%) | 5 (4.2%) | 1 (0.8%) | 13 (10.9%) |
| (b) CS ph1b mutant | ||||||
| CS + 4P × CS ph1 | 19 | 1 (5.3%) | 13 (68.4%) | 2 (10.5%) | 0 | 3 (15.8%) |
| CS + 5P × CS ph1 | 24 | 4 (16.7%) | 16 (66.67%) | 2 (8.4%) | 1 (4.2%) | 1 (4.2%) |
| CS + 6P × CS ph1 | 25 | 4 (16.0%) | 15 (60.0%) | 2 (8.0%) | 2 (8.0%) | 2 (8.0%) |
| Total | 68 | 9 (13.2%) | 44 (64.7%) | 6 (8.8%) | 3 (4.4%) | 6 (8.8%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Copete-Parada, A.; Palomino, C.; Cabrera, A. Development and Characterization of Wheat-Agropyron cristatum Introgression Lines Induced by Gametocidal Genes and Wheat ph1b Mutant. Agronomy 2021, 11, 277. https://doi.org/10.3390/agronomy11020277
Copete-Parada A, Palomino C, Cabrera A. Development and Characterization of Wheat-Agropyron cristatum Introgression Lines Induced by Gametocidal Genes and Wheat ph1b Mutant. Agronomy. 2021; 11(2):277. https://doi.org/10.3390/agronomy11020277
Chicago/Turabian StyleCopete-Parada, Alejandro, Carmen Palomino, and Adoración Cabrera. 2021. "Development and Characterization of Wheat-Agropyron cristatum Introgression Lines Induced by Gametocidal Genes and Wheat ph1b Mutant" Agronomy 11, no. 2: 277. https://doi.org/10.3390/agronomy11020277
APA StyleCopete-Parada, A., Palomino, C., & Cabrera, A. (2021). Development and Characterization of Wheat-Agropyron cristatum Introgression Lines Induced by Gametocidal Genes and Wheat ph1b Mutant. Agronomy, 11(2), 277. https://doi.org/10.3390/agronomy11020277






