Abstract
The research was conducted between 2014 and 2016 at the Agricultural Experimental Station of the University of Environmental and Life Sciences in Wrocław. In the experiment, Poa pratensis and Lolium perenne in pure stand and in mixtures were used as Factor A. Two substances that stimulate grass growth and development constituted Factor B. The first was a biostimulant produced from brown algae (Phaeophyceae), containing various essential chemical compounds including amino acids, vitamins, alginic acid, microelements, and other unexplored biologically active components. The other was water treated with low-pressure glow plasma (LPGP). The seeds were sown in well-mixed light, alluvial loamy sand soil. During three growing seasons, a 9-point scale was used to evaluate grass density, leaf fineness, susceptibility to disease, and lawn overwintering. The lawns were mown every 14 days at a height of 4 cm. The substances with a stimulating effect significantly affected lawn grass features, including turf density and overwintering. Additionally, the biostimulant and plasma water reduced the incidence of fungal diseases. Better transport of water in plants after its low-pressure glow plasma treatment may be due to the effect of its declustered structure, its higher oxygen concentration, and its better solubility of the biostimulant.
1. Introduction
Low biomass production, slow growth rate, and high aesthetic value during intensive shoot formation are the basic features of lawn grass [1]. Application of biological stimulants improves turf visual value and grass overall condition, reducing negative effects of stress factors [2,3,4,5]. Products containing humic substances and-silicon and amino acids have been used successfully to improve plant growth and development. Calvo et al. [6] point out that biostimulants improve the natural immunity of plants under stress and activate their positive genetic potential. According to other authors, a wide range of such products increase plant nutrient uptake and tolerance to abiotic stress [7]. Posmyk and Szafrańska [8] provide a list of hormones that can modify the natural development of plants.
Du Jardin [7] defines biostimulants as substances or microorganisms applied to plants to improve nutrient efficiency and tolerance to abiotic stress. Regulation (EU) 2019/1009 of the European Parliament and of the Council (EC) defines biostimulant as “a product stimulating plant nutrition processes independently of the product’s nutrient content with the aim of improving one or more of the following characteristics of the plant or the plant rhizosphere: (1) nutrient use efficiency, (2) tolerance to abiotic stress, (3) quality traits, or (4) availability of confined nutrients in the soil or rhizosphere”. This definition was adopted by the European Biostimulation Industry Cuncil (EBIC). Apart from improving the efficiency of traditional fertilizes, biostimulants can become an alternative to the synthetic chemicals used in plant protection. In Europe, they are used on more than 4 million hectares, but some sources determine the area of crops treated with biostimulants as more than 6 million hectares [6]. Caradonia et al. (2018) point out that the assessment of plant biostimulants should be harmonized as far as possible, to avoid fragmentation and ensure a level, reliable football pitch. It is essential that a common market for these substances should be created [9].
According to Beaudreau [10], the use of biostimulants will increase due to their potential. An important study on the role of biostimulants in the plant world was published by Yakhin et al. [11]. According to Du Jardin [7], biostimulants positively affect plant metabolism with physiological, biochemical, and hormonal processes. Providing they are not used in high doses, they are environmentally friendly [11,12]. Chojnacka [13] considers biostimulants to be innovative bioproducts used against abiotic stress and harmful biopesticides, while Hamza and Shuger [14], Torre et al. [15], and Halpern et al. [12] argue that the mechanisms of their action proves that they should not be included in the group of pesticides or fertilizers. Bulgari et al. [16] point out that biostimulants contain bioactive compounds of humus substances and have a synergistic effect that triggers the physiological responses of plants. Rose et al. [17] highlight a lack of recent research and information on the organic structure of the humic acids applied to crops. Those acids are also naturally present in the soil, together with fulvic and humin acids [7,18]. Humic acids are the main and most active component of humic substances [19]. They are obtained from a variety of sources and are currently used in many lawn treatment products [2,20].
Analyzing the effects of biostimulants on lawn quality, Mueller et al. [21] confirmed their positive effects on grass color and density. The authors related positive effect of biostimulants on bentgrass visual quality, despite summer stress, to decreasing areas of localized dry spots (LDSs). The surfactant properties of biostimulants seemed to play a major role in improving turf appearance. This did not exclude the fact that seaweed extracts and humic acids in biostimulants might have improved grass tolerance to heat stress after LDSs had been formed. Thus, LDS area reduction increased turfgrass visual quality.
Daneshvar et al. [22] attribute improvements of ryegrass characteristics to the impact of exogenous plant hormones. The authors suggest that humic acid (HA) foliar application might have enhanced ryegrass root development and the uptake of some nutrients, possibly leading to improved drought resistance. They conclude that humic acid may play an important role in increasing plant growth. However, further research may be needed to obtain more information about its mechanism and impact on grass and to determine the optimal concentration for improving turf growth and quality.
Other studies have clearly demonstrated that application of exogenous biostimulant had a positive effect on pigment characteristics in grapes. An increase in anthocyanin content was associated with increased levels of expression of genes in the anthocyanin biosynthesis pathway and with increased enzyme activities [23].
The development of nanotechnology makes it possible to produce biotechnological materials with different physicochemical and biological properties [24]. However, there is a threat arising from their application [25]. With the development of nanotechnology, there is a problem of health risk resulting from incomplete knowledge of the negative effects of substances containing nanoparticles on living organisms [26]. Specific nanostructures can be obtained by mechanical, chemical, or biological synthesis and by inducing self-organization of atoms and molecules in treated materials. One of the effects of these processes is nanotechnology structured water, which in turn, in a technological process, can be declustered (HxOx) by destroying complex clusters of molecules [27]. In 2009, a patent was claimed [28] for equipment to treat water with low-pressure glow plasma (LPGP). LPGP appeared specific because in contrast to other kinds of plasma, [29,30,31,32,33,34], it could not break valence bonds. Thus, it could not cause any chemical reaction. Therefore, the treatment of water with LPGP in at mospheric conditions [35] as well as under molecular oxygen [36] did not create singlet oxygen atoms, hydrogen peroxide, or ozone. Following the invention of LPGP and a generator suitable for treatment of water [37,38], a number of papers were published. They dealt with specific physical and physicochemical properties of water treated with LPGP in atmospheric conditions (LPGPA) [35], but also under oxygen-free nitrogen (LPGPN) [38], ammonia (LPGPAM) [39], methane (LPGPM) [40], carbon dioxide (LPGPC) [34], and molecular oxygen (LPGPO) [38].
In every case, the organized macrostructure of water disintegrates and smaller structural (H2O2)n units are generated. They quickly return to their original macrostructure if the LPGP treated water does not contain dissolved gases mentioned above. During the treatment, oxygen molecules dissolved in water undergo excitation from their ground triplet state into a short living singlet state. Excited molecules provoke building aqueous clathrates hosting excited singlet molecules. Under basic and neutral conditions such clathrates are stable for several months. Aqueous clathrates hosting excited nitrogen molecules and ammonia are also known. In the case of the LPGP treatment of water in the presence of methane, carbon dioxide, and molecular oxygen, corresponding macrostructures are formed that incorporate these gaseous molecules into niches [39,40].
Tested in many experiments, functional properties of plasma treated water could be of use in agriculture, and-various branches of industry as well as in the prevention and treatment of human and animal diseases. LPGPA stimulate reproduction and pathogenicity of entomopathogenic fungi employed as biopesticides [41]. Similarly, fermentative microorganisms are stimulated by LPGPA, providing quality improvement of brewery barley and malt [42]. Murawski et al. [43] and Szymanowicz et al. [44] demonstrated successful application of LPGPA in animal breeding. Ram and boar semen preserved in plasma treated water provided more efficient insemination. Watering herbs with LPGP treated kinds of water significantly influenced the yield and composition of essential oils, as demonstrated in the case of peppermint [45], lavender [46], basil [47], and Greek oregano.
This study was undertaken to test the hypothesis that the use of a biostimulant based on seaweed extracts and plasma water can improve not only turf density, leaf fineness and grass susceptibility to diseases, but also winter survival.
The aim of the research was to assess the impact of a biostimulant, the basic component of a commercial product (Bio-Algeen S90), plasma water, and mineral fertilizers on the density, leaf fineness, overwintering, and susceptibility to disease of grass grown on light soil. Both the biostimulant and plasma water used in the experiment were natural and not harmful to the environment, while the latter was an innovative product in Europe.
2. Materials and Methods
In spring 2014 in the Agricultural Experimental Station of the Wrocław University of Environmental and Life Sciences, a two-factor split-plot experiment with four replications was founded on micro plots of 1 m2 (1 m × 1 m). Mixtures of P. pratensis (var. Niweta [48] and Liberlin (everris.co)) and of L. perenne (var. Info [49] and Libronco [50]) were planted with a seeding rate of 250 kg·ha−1. The seeds were sown at a depth of 1 cm into well mixed light, alluvial loamy sand soil. Grass mixtures constituted Factor A, while Factor B was grass treatments (Table 1).

Table 1.
Factors used in lawn experiment.
During the growing season, three doses of slow-release mineral fertilizer were used. The Professional Spring-Summer fertilizer (an NPK ratio of 17-6-11 +MgO+S+B) was used for the first time in April, then in June, while Professional Autumn was applied at the end of September (an NPK ratio of 5-10-25 +S+Ca+Fe+B). Total amounts of minerals applied during the growing season were (kg·ha−1): N—96.5; P—55; K—118; Mg—6; S—87.5; B—10.5; Ca—25; Fe—1.2 (Table 2).

Table 2.
Mineral fertilizer doses.
Spring-Summer Professional and Autumn-Professional autumn are fertilizers (Hortnas Ltd., Góra, Poland) containing nitrogen mainly in slow-release forms: ammonium and, partially, amide. Spring-Summer Professional contains 13.5% NH4-N ammonium nitrogen and 3.5% amide nitrogen ((NH2)CO2)-N, while Autumn Professional contains 4.0% and 1.0%, respectively. This makes the risk of nitrogen loss due to the leaching of mobile NO3 ions negligible. The prolonged transition of nitrogen into plant-absorbable forms makes this fertilizer ideal for treatment of grass, with its highest uptake of nutrients in later months.
Selected plots were irrigated with plasma water (50,000 L per ha) immediately after sowing. The biostimulant was applied as a spray at a dose of 1 L·ha−1 in 300 L of water, three times each year (spring, summer, autumn). In 2014, the biostimulant and plasma water were not applied until the summer, when the turf was fully developed.
Plasma water was also used three times during the growing season as a spray, in a volume corresponding to the volume of the spraying liquid applied with the biostimulant dose. On selected plots a combination of the biostimulant with plasma water was applied. The control plots were not treated.
2.1. Biostimulants
Bio-Algeen S90 is a natural extract from Ascophyllum nodosum algae. It is obtained using special extraction methods to preserve the physicochemical properties of the seaweed, supporting and accelerating natural processes instead of damaging or inhibiting them. The algae are collected by boats cutting them off from the sea bed in such a way as to ensure the growth of mother plants. On the same day, they are dried with geothermal heat and then ground. The described method guarantees that the cellular components of the sea algae will not be damaged by overheating or natural decomposition and will keep their quality. This makes an important condition for the quality of its products. The beneficial effects of seaweed and sea algae extracts have been known for a long time. However, the extensive application of natural extracts from brown algae (one of the oldest plants on earth) started 10 years ago. Bio-Algeen S90 is produced by the German company Schulze & Hermsen GmbH. Used in the experiment, it is a product with wide applications. Obtained from brown algae, it contains about 90 groups of chemical compounds, including amino acids, vitamins, alginic acid and other unexplored biologically active compounds. Alginic acid (Figure 1) is a block copolymer consisting of D-mannuronic and L-guluronic acids with β-(1,4)-glycosidic bonds. The most important elements present in the product are: nitrogen—0.02%; phosphorus—0.006%; potassium—0.096%; calcium—0.31%; magnesium—0.021%, boron—16 mg kg−1; iron—6.3 mg kg−1; copper—0.2 mg kg−1; manganese—0.6 mg kg−1; and zinc—1.0 mg kg−1. In addition, it contains molybdenum and selenium.
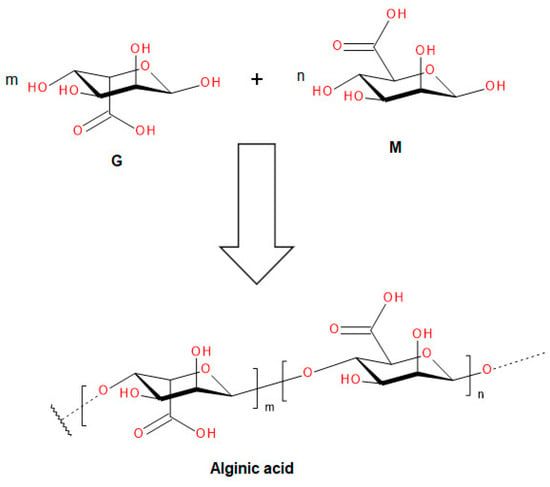
Figure 1.
Alginic acid structure.
2.2. Plasma Water (LPGP Treatment)
Tap water was treated with low temperature plasma [51] for 30 min. Plasma of 38 °C was generated at 5 × 10−3 mbar, 600 V, 50 mA, and 10 KHz frequency. Plasma water was stored at room temperature in a closed container. Samples of tap water and LPGP-treated water were evaluated by UV-VIS spectroscopy. Spectra were recorded using a Shimadzu 2101 spectrophotometer in the range of 200–800 nm in 10 mL quartz cuvettes. FTIR spectra were recorded over a scan range of 4000–700 cm−1, using a Mattson 3000 FT-IR spectrophotometer (Madison, WI, USA) equipped with a 30 SPEC—30 degree specular reflectance accessory and MIRacle ATR accessory produced by PIKE Technologies Inc. (Madison, WI, USA). Conductivity was measured at 25 °C with an Elmetron CPC-505 equipped with an Elmetron EC-60 sensor. Surface tension was determined with the Du Nouy ring method using a STA-1 tensiometer. The results were in line with those provided by Bialopiotrowicz et al. [35].
The schedule of pratotechnical procedures was moderately intensive. The first mowing at 6 cm was carried out when the grass was 8 cm tall, the next at 5 cm, and all others at 4 cm.
2.3. Weather Conditions
Wrocław is located in a lowland area surrounded by the Trzebnickie Hills, with the Sudeten Foothills in the south. With 167 days of precipitation per year, the 1993–2013 multiannual average annual rainfall was 596 mm. The average rainfall during the experimental period (2014–2016) of 554 mm per year was lower than the multiannual (Table 3). The average annual air temperature in Wrocław for the 1993–2013 period was 9.3 °C, but during the experiment (2014–2016) it was higher, with 10.5 °C. The greatest variability in air temperature was in winter months, but in the summer it was lower.

Table 3.
Monthly rainfall, average daily air temperatures during the experiment, and multiannual temperature (according to the Agro-Hydrometeorology Observatory Wrocław-Swojec, Wrocław University of Environmental and Life Sciences).
2.4. Soil Granulometric Composition
The experiment was established on alluvial loamy sand soil. The share of the sand fraction prevailed, with particles of 0.25–0.1 mm diameter representing about 60% of its amount (Table 4).

Table 4.
Granulometric composition of topsoil (0–15 cm).
2.5. Soil Chemical Properties
The chemical properties of the soil were determined each year, with samples collected from topsoil up to 0.15 m deep. Soil pH was determined with a potentiometer in 1 moldm–3 KCl solution; total nitrogen was determined with the Kjeldahl method; available phosphorus with the Egner-Riehm method; available potassium with flame photometry; organic carbon with elemental analysis; available magnesium with spectrophotometric determination by titan yellow and iron with atomic absorption spectrometry (AAS).
It was observed that the content of available forms of phosphorus and potassium in the soil varied throughout the experiment and was the highest in the last year of the research. The average content of available phosphorus was high, with low amounts of potassium and nitrogen. The average content of available forms of magnesium in the soil was very high and iron was moderate, while soil humus content was low. Soil pH was alkaline, but its value decreased in subsequent years (Table 5).

Table 5.
Soil pH and the content of nitrogen, phosphorus, and potassium as well as soil organic carbon, magnesium, and iron content in topsoil (0–15 cm).
2.6. Visual Assessment of Turf Functional Value
The assessment of the functional value of sports turf was carried out in accordance with the COBORU methodology [1]. Visual assessment of grass features with a 9-point scale was performed three times during the growing season (spring, summer, autumn). The following characteristics were assessed: density, susceptibility to disease, leaf fineness, and overwintering. To assess turf density with a 9-point scale the following criteria were used: 1—bad, with 0–5% grass coverage; 2—bad to weak, with 6–15% coverage; 3—weak, with 16–25% coverage; 4—weak to sufficient, with 26–40% coverage; 5—sufficient, with 41–60% coverage; 6—sufficient to good, with 61–75% coverage; 7—good, with 76–85% coverage; 8—good to very good, with 86–95% coverage; 9—very good, with 96–100% coverage.
2.7. Statistical Analysis
Firstly, the normality of distribution of the traits was tested using the Shapiro-Wilk normality test. Non-normal traits were transformed using the power (Box-Cox) transformation with lambda (λ) parameter at interval from −2 to 2. Having the variables transformed and normally distributed, it was assumed that the data followed the multivariate normal distribution. Two-way analysis of variance (ANOVA) was applied to determine the grass mixture and NPK fertilizer effects as well as grass mixtures and NPK fertilizer interaction effects on the observed traits. To analyze differences between variables, post-hoc tests were applied, comparing multiple group means at α = 0.05 [52].
3. Results
3.1. Density
Analysis of variance indicated that the main effects of grass mixtures and NPK fertilizers, as well as the interaction between grass mixtures and NPK fertilizers, were statistically significant for all the traits.
Turf density ratings varied throughout the research years, but seasonal changes in the same growing period were also recorded (Figure 2 and Figure 3). For two years (2014 and 2015), the parameter was rated as good or very good. A significant reduction in surface coverage was noted in 2016, with sufficient and good ratings. Across annual seasons, the highest value was recorded in the autumn (good and very good), which significantly differed from the assessment made in the spring and summer (good). During the 2014 and 2016 periods, the degree of turf density varied depending on the season. In the first year, it was at a high level in all three seasons, with the highest value in the autumn. It was also high in the second year (2015), but it decreased significantly in 2016, when it was rated as sufficient in the spring and summer and good in autumn. Throughout the experiment, ratings differed depending not only on annual seasons but also on treatment.
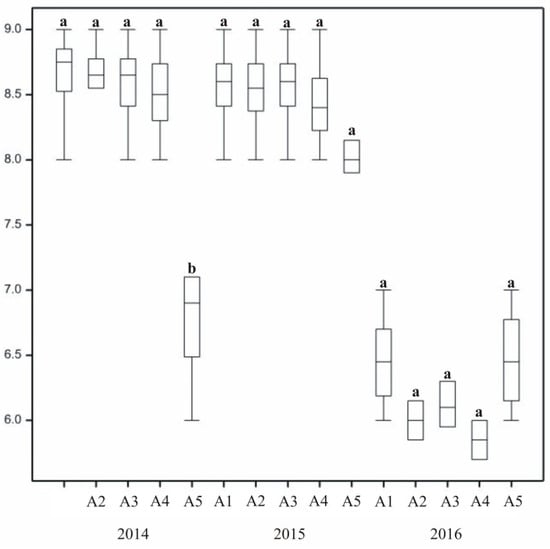
Figure 2.
The effect of grass mixtures on turf density. In columns, means followed by the same letters are not significantly different.
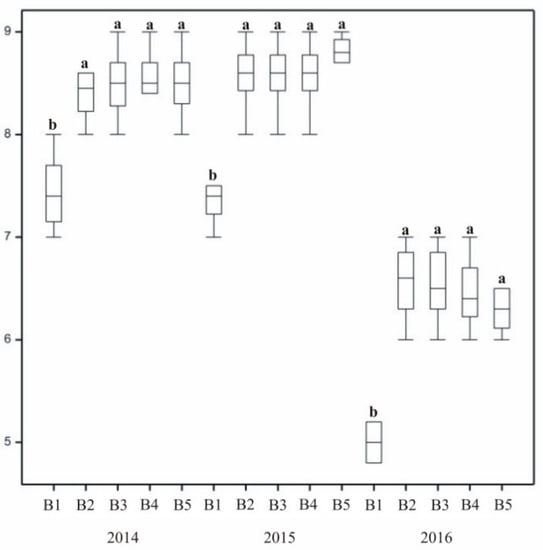
Figure 3.
The effect of stimulating substances on turf density. In columns, means followed by the same letters are not significantly different.
The treatments used in the studies affected lawn density (Figure 3). On the control plot (B1), it differed from the density of the lawns with treatment applied. Rated as sufficient, control grass produced poor-quality turf. In the case of lawns with treatment combinations (Mixtures B2–B5), the density parameter was at a similar but high level. In 2016, the most difficult weather conditions for grass growth were recorded. During that growing season, density on plots with Factor B5 (NPK fertilizers + biostimulant + plasma water) decreased, while on those with plasma water and mineral fertilizers it remained favourable.
The highest degree of density was assigned to lawns with the dominance of L. perenne in mixture composition (Mixtures A1–A2) as well as to the two-species turf with L. perenne and P. pratensis (A3). The bluegrass mixture (A5) was of the lowest density. The varied composition of lawn mixtures differentiated density (Figure 2). Difference between the scores assigned to different mixtures were statistically significant in the first year of research. In the same year the highest value of density was for ryegrass mixtures (A1–A3), with ratings reaching 9. Lower density was assigned to Mixture A5 with bluegrass varieties. On average, bluegrass and ryegrass two-species mixtures were rated lower than single-species ones.
3.2. Susceptibility to Disease
During the research, grass health was at a very high level, but there were slight symptoms of plant infection by pathogens, determined as small to very small (Figure 4 and Figure 5). Susceptibility to disease was low, indicating only some signs of infection in the spring and autumn of 2014 and 2016, with no recording of grass disease in the summer.
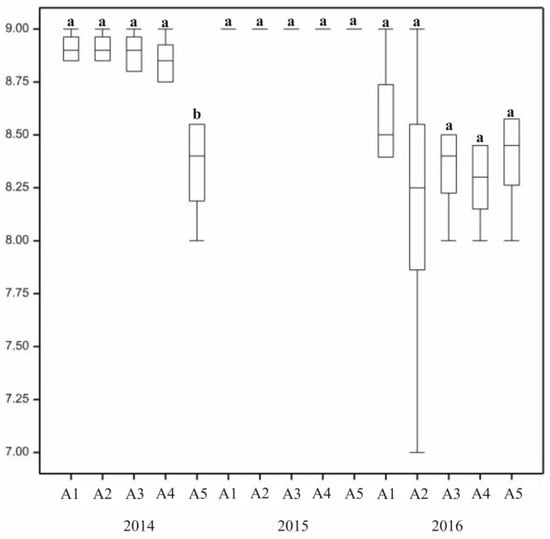
Figure 4.
The effect of grass mixtures on turf susceptibility to disease. In columns, means followed by the same letters are not significantly different.
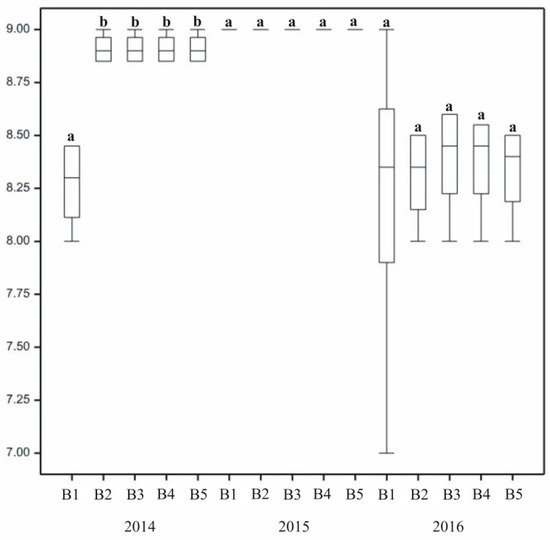
Figure 5.
The effect of stimulating substances on turf susceptibility to disease. In columns, means followed by the same letters are not significantly different.
In the first year there were traces of plant infection by crown rust (Puccinia spp.) in the autumn, but with low susceptibility of grass mixtures to the pathogen, they rated, on average, above 8 (Figure 4). In 2016, statistically significant differences in susceptibility to disease between annual seasons were noted, with clear symptoms of snow mould (Microdochium nivale) recorded in the spring. The susceptibility of grass mixtures to disease varied. Snow mold (Microdochium nivale), observed in 2016, affected single-species bluegrass and perennial ryegrass, both in pure stand, but also two-species mixtures with bluegrass and perennial ryegrass. In 2014, single-species turf with bluegrass varieties and the control lawn (B1) were most affected by crown rust. Bluegrass turf (A5) was affected the strongest and was, assigned lower ratings than other mixtures. Snow mould intensity in spring 2016 did not vary across treatments.
Compared to others, lawns treated with plasma water (B3) and biostimulant (B4–B5) were in the best condition (Figure 5). In the first year susceptibility to disease was dependent on the treatment. Control turf on which no treatment was used (B1) exhibited reduced resistance to disease. The highest resistance to infection was noted for mixtures treated with plasma water, and was slightly lower for turf treated with the biostimulant.
3.3. Leaf Fineness
Grass leaf fineness ratings varied across the years of research and annual seasons (Figure 6). The average value of this parameter was at a high level, with grass producing delicate long leaves. Throughout the experiment, leaf fineness was assigned high scores during summer assessment. In 2014, the autumn assessment was more favourable than the spring assessment, while the most favorable value was recorded in the summer. In 2016, all three ratings across three seasons differed statistically. In autumn 2016, leaf fineness ratings reached the lowest level.
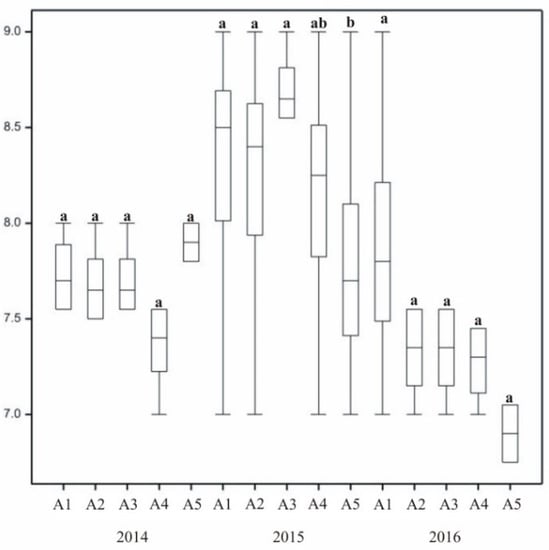
Figure 6.
The effect of grass mixtures on grass leaf fineness. In columns, means followed by the same letters are not significantly different.
Throughout the research leaves were the most narrow in the second year (2015). The width of the leaf blade varied throughout the experiment. Leaf width varied across grass mixtures (Figure 6), and bluegrass (A5) produced the widest leaves. It was assessed as being significantly lower, but produced leaf blades of considerable fineness. Leaf fineness also varied across grass mixtures in 2014 and 2016, but there were no clear differences between the seasons of 2015. However, regarding the whole growing season of 2015, significant differences were noted between the mixtures, with the most favorable leaf fineness noted for the one with two species: bluegrass and perennial ryegrass. Bluegrass in pure stand (A5) was rated lower than two-species mixtures.
Treatment lowered ratings slightly, with the turf on the control plot (B1) assessed the most favorably. Mineral fertilizers decreased leaf fineness as well, but plasma water and biostimulant applied on their own and together positively affected the formation of highly rated leaves. In all three seasons, leaf fineness of control lawns (Factor B1) was rated high (Figure 7). In the last year (2016), out of three kinds of treatment, only the biostimulant affected leaf fineness (B4).
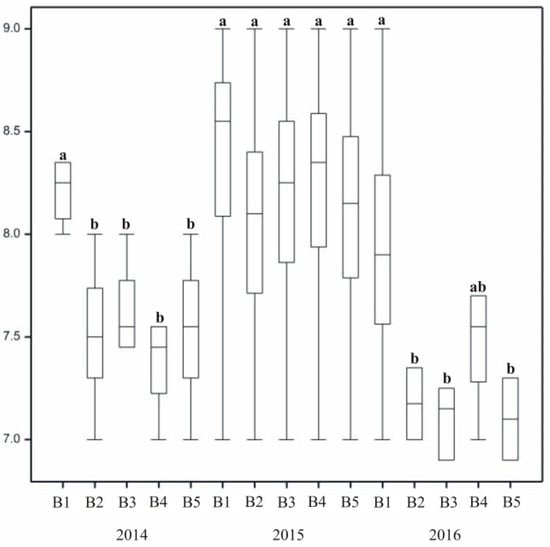
Figure 7.
The effect stimulating substances on grass leaf fineness. In columns, means followed by the same letters are not significantly different.
3.4. Overwintering
The degree of grass winter survival varied across subsequent years of research. The most favourable value was recorded in 2014, with overwintering assessed as very good (Figure 8 and Figure 9). In 2015 and 2016, the values decreased, with satisfactory or good ratings. Significant differences were recorded between the winter survival of untreated control turf (B1) and lawns where treatment was applied. A low overwintering score of the control lawn (B1) indicated that treatment of plants positively affected their winter survival. Furthermore, statistically significant differences in overwintering ratings between different forms of treatment were recorded. In the second and third years, plots treated with mineral fertilizers were rated 2–3 points higher than control turf. The two other substances applied to grass also had a positive effect on lawn overwintering.
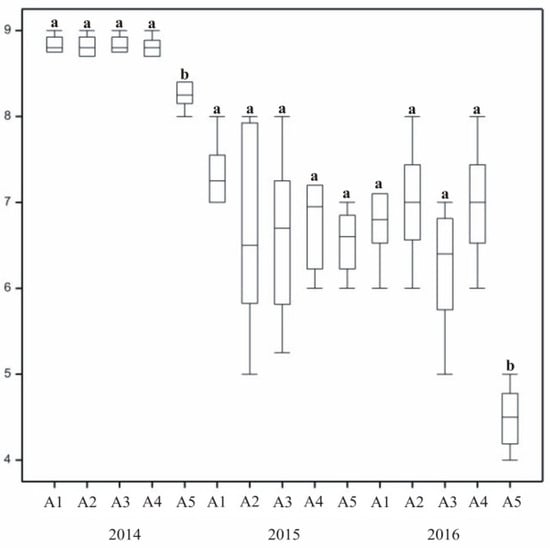
Figure 8.
The effect of grass mixtures on turf overwintering. In columns, means followed by the same letters are not significantly different.
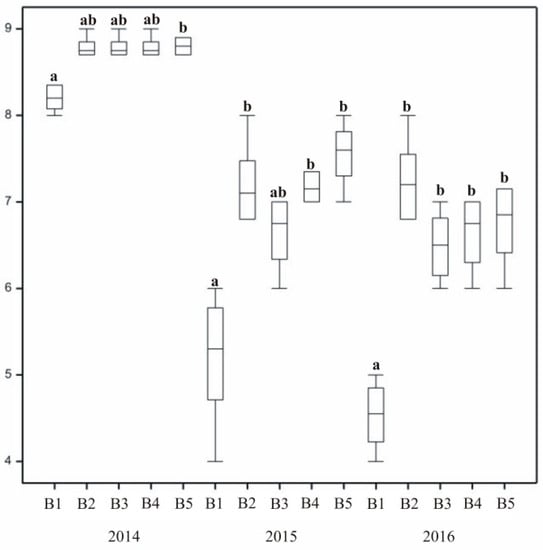
Figure 9.
The effect of stimulating substances on turf overwintering. In columns, means followed by the same letters are not significantly different.
In 2015 and 2016, overwintering ratings of lawns with different varieties of the same species were at a similar level (Figure 8). When interpreting the results presented graphically, slight differences were observed across mixtures. In the third year (2017), the bluegrass mixture (A5) was in a significantly worse condition after winter than the others. Overwintering of the turf was also dependent on its species composition, with the lowest overwintering ratings assigned to single-species mixtures. Statistically significant differences between grass mixtures in their overwintering assessment were recorded in 2015 and 2017.
4. Discussion
In the three-year experiment, the functional value of lawns of different species composition and treated with substances of stimulating nature was assessed. Both the research factors and the grass growth environment affected the characteristics of ryegrass-bluegrass and single-species turf in many ways. The density of ryegrass in pure stand and in turf with its dominant share was assessed higher than that of the bluegrass mixture. This has been confirmed by other authors studying the impact of perennial ryegrass on turf visual quality [53]. Jankowski et al. [54] reported that bluegrass turf appearance was rated significantly lower and found that density assessment varied across single-species and multispecies lawns, in the latter case depending on mixture components. Russi et al. [55] confirmed this and recorded an increase in lawn visual quality in subsequent years, concluding that their findings were similar to those of Brede’s [56]. Visual appearance is a synthetic characteristic, closely related to the overwintering of plants, their color and surface density, and, to a lesser extent, to leaf fineness [54]. Prończuk and Prończuk [57] also pointed to the susceptibility of grasses to disease as an important parameter affecting turf overall appearance. According to Laudański et al. [58], the assessment of turf overall appearance over the years were difficult to interpret and to draw clear conclusions from, due to high weather variability in the research seasons. Zhang et al. [20] confirmed the positive effect of humic acids in combination with fertilizers on turf visual quality. Daneshvar et al. [22] reported that biostimulant increased turf visual quality and the iron content of plant tissues, and it positively affected root system formation of perennial ryegrass.
With adverse weather conditions in the second and third years of the present experiment, the biostimulant and plasma water applied separately increased the ratings of grass parameters in relation to the control by a considerable extent. The functions of plasma water are quite complex and more research and data are probably needed to develop the most advantageous application procedure. The favorable effects of plasma water may be due to such factors as its structure resulting in its better penetration into the plant, its higher oxygen concentration, and better solubility of biostimulants. However, the differences observed in overwintering assessment may be due to some changes in osmotic pressure and in the freezing point of plasma water. During their studies on the effects of plasma water on Mentha L., Pisulewska et al. [45] found that plasma water irrigation reduced chlorophyll a content, while stimulating the synthesis of chlorophyll b to such an extent that, as a result the total chlorophyll content of plants increased [59,60]. Lower fungal infection rates of plants treated with plasma water can be explained by its higher concentration of oxygen [35]. Finally, synergistic antimicrobial effects of plasma-treated water was observed by Schnabel et al. [61]. The effect of LPGP-treated water may be due to its better penetration into the plant because of its declustering, higher oxygen concentration, and better solubility of the biostimulant. The fact that fungal diseases infected plants treated with plasma water less could be explained by its higher concentration of oxygen. However, the differences observed in the assessment of overwintering may be due to slight changes in osmotic pressure and in the freezing point of plasma water.
Seaweed extracts contain phytohormones such as indole-acetic acid, cytokines, gibberellic acid, polyamine, and abscisic acid [62]. Many genes involved in plant growth and development are regulated by phytohormones, which can discern cis-motifs in the promoter sequences of the genes [63].
The results indicated that Bio-Algeen S90 might increase the expression of the genes involved in anthocyanin biosynthesis, possibly by stimulating the promoter activity of the genes. The changes in lawn characteristics may be due to mucopolysaccharides, also known as glycosaminoglycans (GAGs), biologically active compounds present in biostimulants and made up of amino sugars and uronic acids. The most common glycosaminoglycans are: hyaluronic acid and chondroitin sulfate, alginic acid and its salts-mannitol and sorbitol-caragenates and agar-unsaturated fatty acids (EFA—Essential Fatty Acid): arachidonic, eicosapentaenoic, and γ-linolenic (GLA); and polyunsaturated fatty acids (PUFA) series ω 3 and ω 6.
The biostimulant may have contributed to the activation of precursors of active gibberellin compounds, affecting growth and development processes throughout the plant life cycle, including the lengthening of shoots and changing of leaf shape [64,65]. Therefore, plants respond with increased growth to the use of biostimulants rich in active or activated compounds [66], which has been proven in scientific research [67]. The authors point out that biostimulants affect many metabolic pathways at the molecular level, including gibberellin biosynthesis, mainly regulated by three 2-oxoglutarate-dependent dioxygenases, i.e., GA 20-oxidase, GA 3-oxidase, and GA 2-oxidase [68,69]. The first two enzymes catalyze the last two stages of the synthesis of bioactive gibberellin, while the third enzyme converts bioactive gibberellins and their precursors into inactive 2-hydroxylated forms. This demonstrates that both an increase in GA 20-oxidase regulation and the suppression of GA2-oxidase significantly determine an increase in endogenous bioactive levels of gibberellin, leading to increased synthesis and increased content of fiber in the walls of plant cells [70,71,72,73,74,75,76,77,78,79]. According to Bai et al. [67], increased gibberellin production induces the expression of sucrose synthesis and secondary cell wall deposition. In addition, by regulating the expression of sucrose synthesis, gibberellins can modify carbon flux into the secondary cell wall and cellulose synthesis.
5. Conclusions
The biostimulating substances used in the experiment significantly affected the characteristics of Poa pratensis and Lolium perenne, both planted together and separately. Plasma water and a biostimulator reduced the incidents of fungal diseases, positively affecting turf density and overwintering. The Bio-Algeen S90 biostimulant dissolved in LPGP-treated water was more efficient than the biostimulant applied on its own. Weather conditions had a significant impact on the value of turf characteristics. Strong drought in the second year of research and severe soil moisture deficit in 2016 resulted in a decrease in lawn values (density, leaf fineness). The visual quality of lawn mixtures based on bluegrass and perennial ryegrass and single-species ones varied depending on the species composition, and a higher share of L. perenne ensured high ratings of the turf.
Author Contributions
Conceptualization, M.T.-K. and K.W.; methodology, M.T.-K., K.W. and K.K.; software, M.T.-K., K.W., H.B. and J.B.; validation, K.W., A.R. and J.B.; formal analysis, K.W. and J.B.; investigation, M.T.-K. and K.W.; resources, K.W. and J.B.; data curation, M.T.-K., A.R. and J.B.; writing—original draft preparation, M.T.-K., K.W., A.R. and J.B.; writing—review and editing, A.R. and J.B.; visualization, J.B.; supervision, A.R. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
The authors thank the Janusz Urbański for his great contribution in the implementation of the research topic.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wolski, K.; Talar-Krasa, M.; Dradrach, A.; Szymura, M.; Biernacik, M.; Świerszcz, S. Ocena użytkowa murawy piłkarskiej na przykładzie KŚ AZS we Wrocławiu. Łąkarstwo w Polsce (Grassl. Sci. Pol.) 2015, 18, 241–254. [Google Scholar]
- Chen, Y.; Clapp, C.E.; Magen, H. Mechanisms of plant growth stimulation by humic substances: The role of organo-iron complexes. Soil Sci. Plant Nutr. 2004, 50, 1089–1095. [Google Scholar] [CrossRef]
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef] [PubMed]
- Radkowski, A.; Radkowska, I.; Wolski, K. Effect of silicon foliar application on the functional value of lawns. J. Elem. 2018, 23, 1257–1270. [Google Scholar] [CrossRef]
- Radkowski, A.; Radkowska, I.; Bocianowski, J.; Sladkovska, T.; Wolski, K. The effect of foliar application of an amino acid based biostimulant on lawn functional value. Agronomy 2020, 10, 1656. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostumulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, maincategories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Szafrańska, K. Biostimulators: A new trend towards solving an old problem. Front. Plant Sci. 2016, 7, 748. [Google Scholar] [CrossRef]
- Caradonia, F.; Battaglia, V.; Righi, L.; Pascali, G.; La Torre, A. Plant biostimulant regulatory framework: Prospects in europe and current situation at international level. J. Plant Growth Regul. 2019, 38, 438–448. [Google Scholar] [CrossRef]
- Beaudreau, D.G. Biostimulants in Agriculture: Their Current and Future Role in a Connected Agricultural Economy; Biostimulant Coalition: Washington, DC, USA, 2013; Available online: www.biostimulantcoalition.org (accessed on 11 February 2017).
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar] [CrossRef]
- Chojnacka, K. Innovative bio-products for agriculture. Open Chem. 2015, 1, 932–937. [Google Scholar] [CrossRef]
- Hamza, B.; Suggars, A. Biostimulants: Myths and realities. TurfGrass Trends 2001, 8, 6–10. [Google Scholar]
- Torre, L.A.; Battaglia, V.; Caradonia, F. An overview of the current plant biostimulant legislations in different European Member States. J. Sci. Food Agric. 2016, 96, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulats and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Cavagnaro, T.R. A meta analysis and review of plant-growth response to humic substances: Practical implications for agriculture. Adv. Agron. 2014, 124, 37–89. [Google Scholar] [CrossRef]
- Schmidt, R.E.; Ervin, E.H.; Zhang, X. Questions and answers about biostimulants. Golf Course Manag. 2003, 71, 91–94. [Google Scholar]
- Liu, H.; Cooper, R.J. Humic substances influence creeping bentgrass growth. Golf Course Manag. 2000, 49–53. Available online: https://jhbiotech.com/docs/Humic-Acids-on-BentGrass.pdf (accessed on 28 January 2021).
- Zhang, X.; Ervin, E.H.; Schmidt, R.E. Physiological effects of liquid applications of a seaweed extract and humic acid on Creeping Bentgrass. J. Am. Soc. Hortic. Sci. 2003, 128, 492–496. [Google Scholar] [CrossRef]
- Mueller, S.R.; Kussow, W.R. Biostimualnt influences on turfgrass microbial communities and creeping bentgrass putting green quality. HortScience 2005, 40, 1904–1910. [Google Scholar] [CrossRef]
- Daneshvar, N.; Maibodi, H.; Kafi, M.; Nikbakht, A.; Rejali, F. Effect of foliar applications of humic acid on growth, visual quality, nutrients content and root parameters of Perennial Ryegrass (Lolium perenne L.). J. Plant Nutr. 2015, 38, 224–236. [Google Scholar] [CrossRef]
- Deng, Q.; Xia, H.; Lin, L.; Wang, J.; Yuan, L.; Li, K.; Zhang, J.; Lv, X.; Liang, D. SUNRED, a natural extract-based biostimulant, application stimulates anthocyanin production in the skins of grapes. Sci. Rep. 2019, 9, 2590. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.; Chen, H. Nanoscale science and engineering for agriculture and food systems. IB in Depth–Special Section on Nanobiotechnology, Part 2. Ind. Biotechnol. 2012, 9, 17–18. [Google Scholar] [CrossRef]
- Mrowiec, B. Nanomateriały–nowe zagrożenie środowiska. Inżynieria Środowiska 2017, 18, 105–110. [Google Scholar]
- Świderski, F.; Waszkiewicz-Robak, B. Nanotechnologia–teraźniejszość i przyszłość. Postępy Techniki Przetwórstwa Spożywczego 2006, 16, 55–57. [Google Scholar]
- Kryża, K.; Szczepanik, G. Zastosowanie techniki zimnej plazmy jako nowoczesna technologia zabezpieczania surowców żywnościowych; Zachodniopomorski Universytet Technologiczny: Szczecin, Poland, 2010. [Google Scholar]
- Oszczęda, Z.; Elkin, I.; Stręk, W. Equipment for Treatment of Water with Plasma. Polish Patent PL 216025 B1, 28 February 2014. [Google Scholar]
- Donsbach, K.W.; Cazares, R. Process for Making Highly Oxygenated Drinking Water and Drinking Water Made by the Process. U.S. Patent 5587191A, 24 December 1996. [Google Scholar]
- Zelenak, Z.M.; Berzsenyi, L.; Abramo, F. Oxygen Enriched Liquids, Method and Apparatus for Making, and Applications Thereof. U.S. Patent 581422A, 29 September 1998. [Google Scholar]
- DeWald, J.J. Method and Apparatus for Adding Oxygen to Drinking Water. U.S. Patent 69361179B2, 30 August 2005. [Google Scholar]
- Lascoste, C.; Brunner, S.; Jimenez, L.; Klein, A. Method for Enriching Water with Oxygen by an Electrolytic Process, Oxygen Enriched Water or Beverage and Uses Thereof. U.S. Patent 8,709,231, 19 December 2008. [Google Scholar]
- Messer Americas, FARMOX. Drop-in Oxygenation Apparatus. New, Easy-to-Deploy, Highly Efficient Solution for Oxygenation of Water. Available online: https://cdn2.hubspot.net/hubfs/189660/Messer%20US%20Website_2019/Resources/MESS-3024_FARMOX_Dropln_datasheet.pdf (accessed on 25 June 2020).
- Ciesielska, A.; Ciesielski, W.; Khachatryan, K.; Koloczek, H.; Kulawik, D.; Oszczeda, Z.; Soroka, J.A.; Tomasik, P. Structure and physicochemical properties of water treated under carbon dioxide with low-temperature low-pressure glow plasma of low frequency. Water 2020, 12, 1920. [Google Scholar] [CrossRef]
- Bialopiotrowicz, T.; Ciesielski, W.; Domanski, J.; Doskocz, M.; Khachatryan, K.; Fiedorowicz, M.; Graz, K.; Koloczek, H.; Kozak, A.; Oszczeda, Z.; et al. Structure and Physicochemical Properties of Water Treated w ith Low-Temperature Low-Frequency Glow Plasma. Curr. Phys. Chem. 2016, 6, 312–320. [Google Scholar] [CrossRef]
- Chwastowski, J.; Ciesielski, W.; Khachatryan, K.; Kołoczek, H.; Kulawik, D.; Oszczęda, Z.; Soroka, J.A.; Tomasik, P.; Witczak, M. Water of increased content of molecular oxygen. Water 2020, 12, 2488. [Google Scholar] [CrossRef]
- Reszke, E.; Yelkin, I.; Oszczęda, Z. Plasming Lamp with Power Supply. Polish Patent PL 227530 B1, 26 October 2017. [Google Scholar]
- Chwastowski, J.; Ciesielska, K.; Ciesielski, W.; Khachatryan, K.; Kołoczek, H.; Kulawik, D.; Oszczeda, Z.; Tomasik, P.; Witczak, M. Structure and Physicochemical Properties of Water Treated under Nitrogen with Low-Temperature Glow Plasma. Water 2020, 12, 1314. [Google Scholar] [CrossRef]
- Ciesielska, A.; Ciesielski, W.; Kołoczek, H.; Kulawik, D.; Kończyk, J.; Oszczęda, Z.; Tomasik, P. Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low-frequency. Glob. J. Chem. 2020, 18, 1195–1206. [Google Scholar] [CrossRef]
- Ciesielska, A.; Ciesielski, W.; Khachatryan, K.; Kołoczek, H.; Kulawik, D.; Oszczęda, Z.; Soroka, J.A.; Tomasik, P. Structure and physicochemical properties of water treated under methane with low-temperature glow plasma of low frequency. Water 2020, 12, 1638. [Google Scholar] [CrossRef]
- Jaworska, M.; Oszczęda, Z.; Tomasik, P. Water treated with low-temperature, low-pressure, low-frequency glow plasma as a stimulant of pathogenicity and reproduction of biopesticides Part I. Entomopathogenic fungi. Pol. J. Nat. Sci. 2018, 33, 561–568. [Google Scholar]
- Pater, A.; Zdaniewicz, M.; Satora, P.; Khachatryan, G.; Oszczęda, Z. Application of Water Treated with Low-Temperature Low-Pressure Glow Plasma for Quality Improvement of Barley and Malt. Biomolecules 2020, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Murawski, M.; Schwarz, T.; Grygier, J.; Patkowski, K.; Oszczęda, Z.; Jelkin, I.; Kosiek, A.; Gruszecki, T.M.; Szymanowska, A.; Skrzypek, T.; et al. The utilityof nanowater for ram semen cryopreservation. Exp. Biol. Med. 2014, 240, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Szymanowicz, J.; Schwarz, M.; Murawski, M.; Małopolska, M.; Oszczęda, Z.; Tuz, R.; Nowicki, J.; Bartlewski, P.M. Storage, of bear semen at 16–18 °C in the long term commercial extender prepared with deionized water or nanowater. Anim. Reprod. 2019, 16, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Pisulewska, E.; Ciesielski, W.; Jackowska, M.; Gąstoł, M.; Oszczęda, Z.; Tomasik, P. Cultivation of peppermint (Mentha piperita rubescens) using water treated with low-pressure, low-temperature glow plasma of low frequency. Electron. J. Pol. Agric. Univ. 2018, 21, 01. [Google Scholar] [CrossRef]
- Ciesielska, K.; Ciesielski, W.; Girek, T.; Kołoczek, H.; Oszczęda, Z.; Tomasik, P. Reaction of Lavandula angustifolia Mill. to water treated with low-temperature, low-pressure glow plasma of low frequency. Water 2020, 12, 3168. [Google Scholar] [CrossRef]
- Ciesielski, W.; Gąstoł, M.; Kulawik, D.; Oszczęda, Z.; Pisulewska, E.; Tomasik, P. Specific controlling essential oil composition of basil (Ocimum basilicum L.) involving low-temperature, low-pressure glow plasma of low frequency. Water 2020, 12, 3332. [Google Scholar] [CrossRef]
- Małopolska Hodowla Traw. 2017. Available online: http://hbp.pl/pl/trawy-gazonowe_85/wiechlina-lakowa_135 (accessed on 28 March 2017).
- Małopolska Hodowla Traw. 2017. Available online: http://hbp.pl/pl/trawy-gazonowe_85/zycica-trwala_136 (accessed on 28 March 2017).
- Turgrass Seeds. 2017. Available online: https://www.bspb.co.uk/sg_userfiles/BSPB_Turfgrass_2017.pdf (accessed on 28 January 2021).
- Khachatryan, G.; Khachatryan, K.; Krystyjan, M.; Pardus, L.; Oszczęda, Z. Preparation and properties of gels and foils from starch and water treated with low-temperature low-frequency glow plasma (LPGP). In Proceedings of the 14th International Conference on Polysaccharides-Glycoscience, Prague, Czech Republic, 7–9 November 2018; Radmila, Ř., Andrea, H., Jana, Č., Eds.; Czech Chemical Society: Praha, Czech Republic, 2018; pp. 196–199, ISBN 978-80-86238-80-7. [Google Scholar]
- Bocianowski, J.; Kozak, M.; Liersch, A.; Bartkowiak-Broda, I. A heuristic method of searching for interesting markers in terms of quantitative traits. Euphytica 2011, 181, 89–100. [Google Scholar] [CrossRef]
- Starczewski, K.; Affek-Starczewska, A. Wpływ udziału życicy trwałej w mieszankach trawnikowych na aspekt ogólny terenów zadarnionych. Fragm. Agron. 2011, 28, 60–69. [Google Scholar]
- Jankowski, K.; Sosnowski, J.; Jankowska, J.; Kowalczyk, R. Impact of hydrogel and kind of soil cover on the compactness of turf lawns. Inżynieria Ekologiczna 2012, 30, 249–256. (In Polish) [Google Scholar]
- Russi, L.; Annicchiarico, P.; Martiniello, P.; Tomasoni, C.; Piano, E.; Veronesi, F. Turf quality cool season grasses at low imputs: Reliability across years, seasons and sites of evaluation. Acta Hortic. 2004, 661, 387–392. [Google Scholar] [CrossRef]
- Brede, D. Multi-Way Kentucky bluegrass blends and their effect on turfgrass quality. Acta Hortic. 2008, 783, 19–27. [Google Scholar] [CrossRef]
- Prończuk, S.; Prończuk, M. Evaluation of the response of perennial ryegrass (Lolium perenne L.) cultivars to temporary shading in turf maintenance. Biul. IHAR 2008, 248, 134–135. [Google Scholar]
- Laudański, Z.; Prończuk, M.; Prończuk, M. Proposition of turf characters synthesis for assessment of Festuca spp. cultivars value. Biul. IHAR 2004, 233, 181–192. [Google Scholar]
- Szulc, P.; Bocianowski, J. Monitoring of biogenic element concentrations in drainage water of the production field. Bulg. J. Agric. Sci. 2013, 19, 472–478. [Google Scholar]
- Gromadzka, K.; Waśkiewicz, A.; Świetlik, J.; Bocianowski, J.; Goliński, P. The role of wastewater treatment in reducing pollution of surface waters with zearalenone. Arh. Hig. Rada Toksikol. 2015, 66, 159–164. [Google Scholar] [CrossRef][Green Version]
- Schnabel, U.; Handorf, O.; Yarova, K.; Zessin, B.; Zechlin, S.; Sydow, D.; Zellmer, E.; Stachowiak, J.; Andrasch, M.; Below, H.; et al. Plasma-treated air and water—Assessment of synergistic antimicrobial effects for sanitation of food processing surfaces and environment. Foods 2019, 8, 55. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Yamamoto, Y.Y.; Yoshioka, Y.; Hyakumachi, M.; Maruyama, K.; Yamaguchi-Shinozaki, K.; Tokizawa, M.; Koyama, H. Prediction of transcriptional regulatory elements for plant hormone responses based on microarray data. BMC Plant Biol. 2011, 11, 39. [Google Scholar] [CrossRef]
- Hooley, R. Gibberellins: Perception, transduction and responses. Plant Mol. Biol. 1994, 26, 1529–1555. [Google Scholar] [CrossRef] [PubMed]
- Kende, H.; Zeevaart, J. The five “classical” plant hormones. Plant Cell 1997, 9, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Dayan, J.; Schwarzkopf, M.; Avni, A.; Aloni, R. Enhancing plant growth and fiber production by silencing GA 2-oxidase. Plant Biotechnol. J. 2010, 8, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.Q.; Xiao, Y.H.; Zhao, J.; Song, S.Q.; Hu, L.; Zeng, J.Y.; Li, X.B.; Hou, L.; Luo, M.; Li, D.M.; et al. Gibberellin overproduction promotes sucrose synthase expression and secondary cell wall deposition in cotton fibers. PLoS ONE 2014, 9, e96537. [Google Scholar] [CrossRef]
- Hedden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Ward, D.A.; Hedden, P.; Phillips, A.L.; Power, J.B.; Davey, M.R. Engineering gibberellin metabolism in Solanum nigrum L. by ectopic expression of gibberellin oxidase genes. Plant Cell 2012, 31, 945–953. [Google Scholar] [CrossRef]
- Gou, J.; Ma, C.; Kadmiel, M.; Gai, Y.; Strauss, S.; Jiang, X.; Busov, V. Tissue-specific expression of Populus C19 GA 2-oxidases differentially regulate above- and below-ground biomass growth through control of bioactive GA concentrations. New Phytol. 2011, 192, 626–639. [Google Scholar] [CrossRef]
- Xiao, Y.-H.; Li, D.-M.; Yin, M.-H.; Li, X.-B.; Zhang, M.; Wang, Y.-J.; Dong, J.; Zhao, J.; Luo, M.; Luo, X.-Y.; et al. Gibberellin 20-oxidase promotes initiation and elongation of cotton fibers by regulating gibberellin synthesis. J. Plant Physiol. 2010, 167, 829–837. [Google Scholar] [CrossRef]
- Vidal, A.M.; Gisbert, C.; Talón, M.; Primo-Millo, E.; López-Díaz, I.; García-Martínez, J.L. The ectopic overexpression of a citrus gibberellin 20-oxidase enhances the non-13-hydroxylation pathway ofgibberellin biosynthesis and induces an extremely elongated phenotype in tobacco. Physiol. Plant. 2001, 112, 251–260. [Google Scholar] [CrossRef]
- Eriksson, M.E.; Israelsson, M.; Olsson, O.; Moritz, T. Increased gibberellins biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nat. Biotechnol. 2000, 18, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Grabber, J.H.; Ralph, J.; Lapierre, C.; Barrière, Y. Genetic and molecular basis of grass cell-wall degradability. I. Lignin–cell wall matrix interactions. C. R. Biol. 2004, 327, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Anterola, A.M.; Lewis, N.G. Trends in lignin modification: A comprehensive analysis of the effects ofgenetic manipulations/mutations on lignification and vascular integrity. Phytochemistry 2002, 61, 221–294. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Goffner, D.; Joffroy, I.; Grima-Pettenati, J.; Halpin, C.; Knight, M.E.; Schuch, W.; Boudet, A.M. Purification and characterization of isoforms of cinnamyl alcohol dehydrogenase from Eucalyptus xylem. Planta 1992, 188, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Shi, W.J.; Hu, W.R.; Hao, X.Y.; Wang, D.M.; Yuan, H.; Yan, H.Y. Molecular and biochemical evidence for phenylpropanoid synthesis and presence of wall-linked phenolics in cotton fibers. J. Integr. Plant Biol. 2009, 51, 626–637. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).