Mulching with Leaf Litter from Municipal Green Waste Favours Predatory Mononchid Nematodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Open-Field Experiment
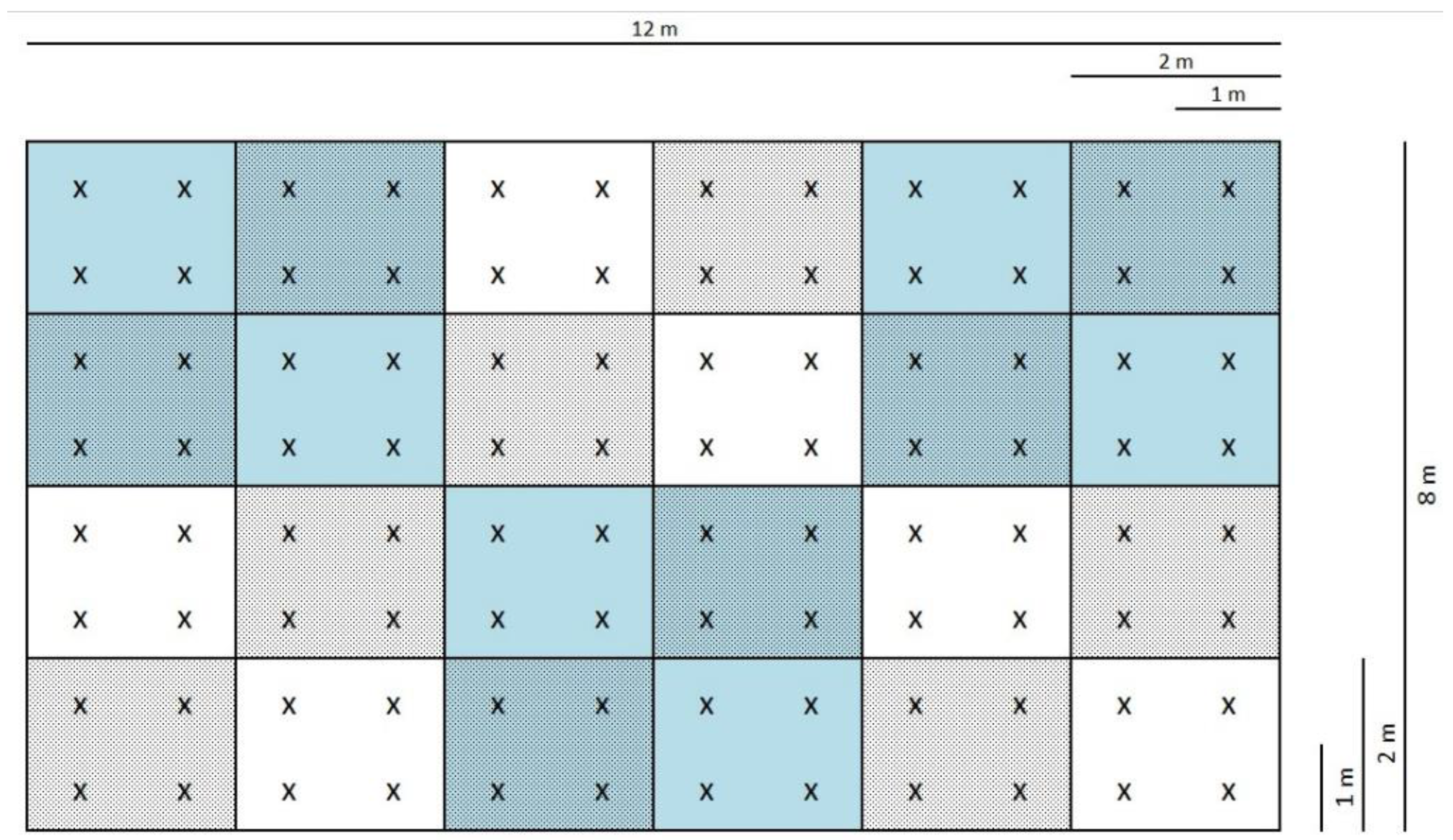
2.2. The Design of Microplots
2.2.1. Plant Materials
2.2.2. Mulching Protocol
2.2.3. Plot Maintenance
2.2.4. Irrigation Protocol
2.3. Soil Sample Collection
2.4. Identification of Mononchids
2.5. Data Analysis
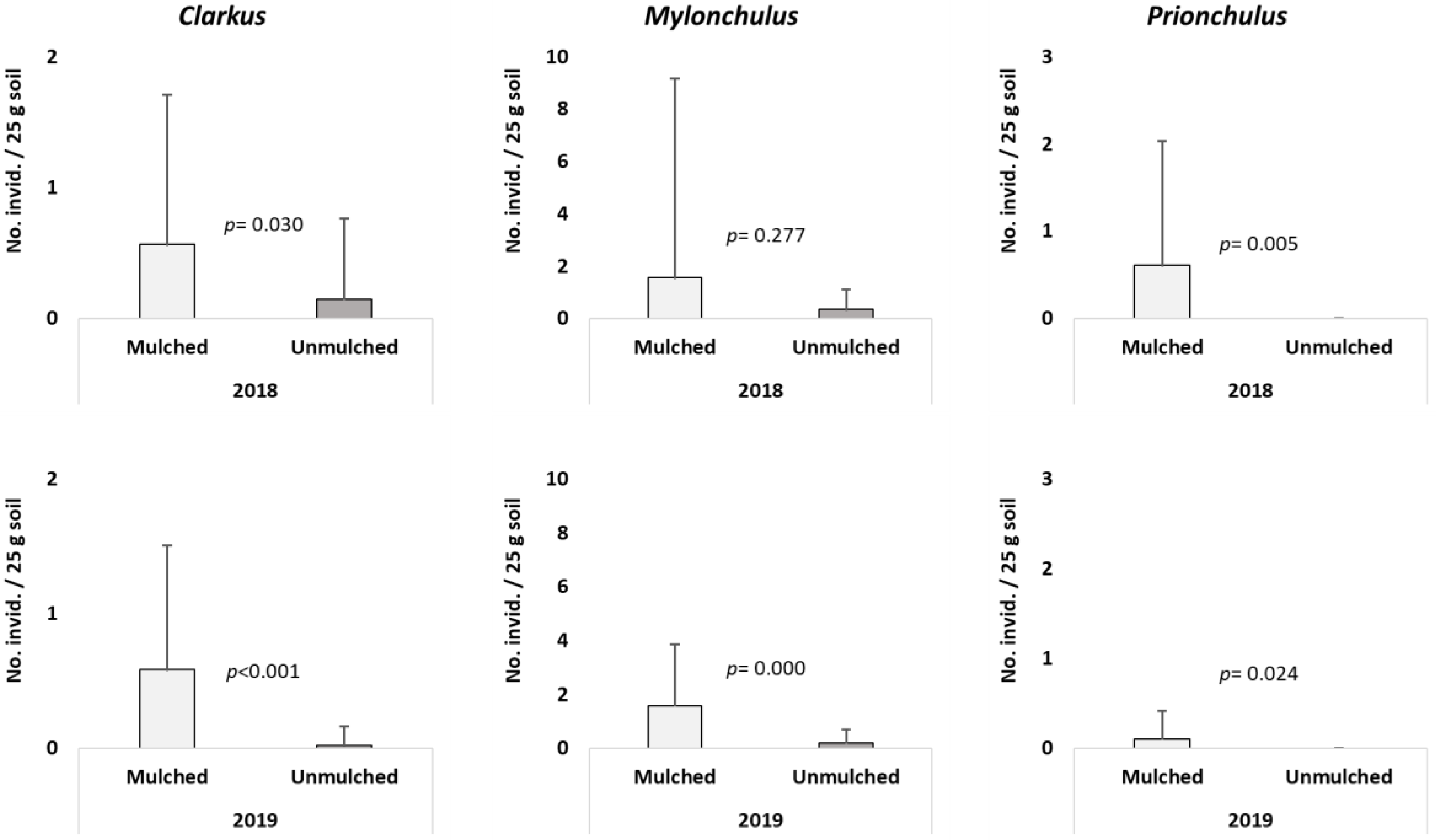
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
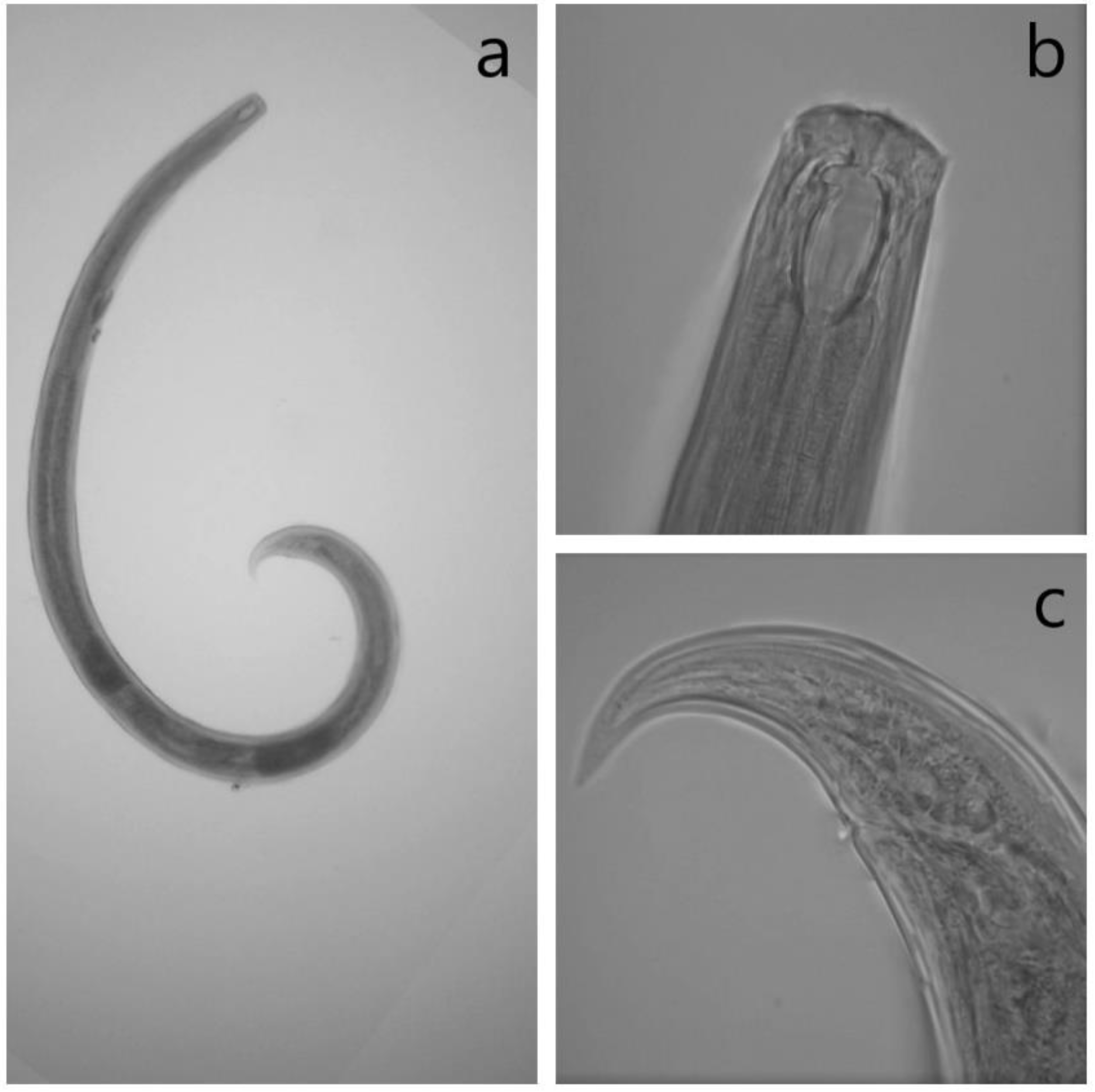
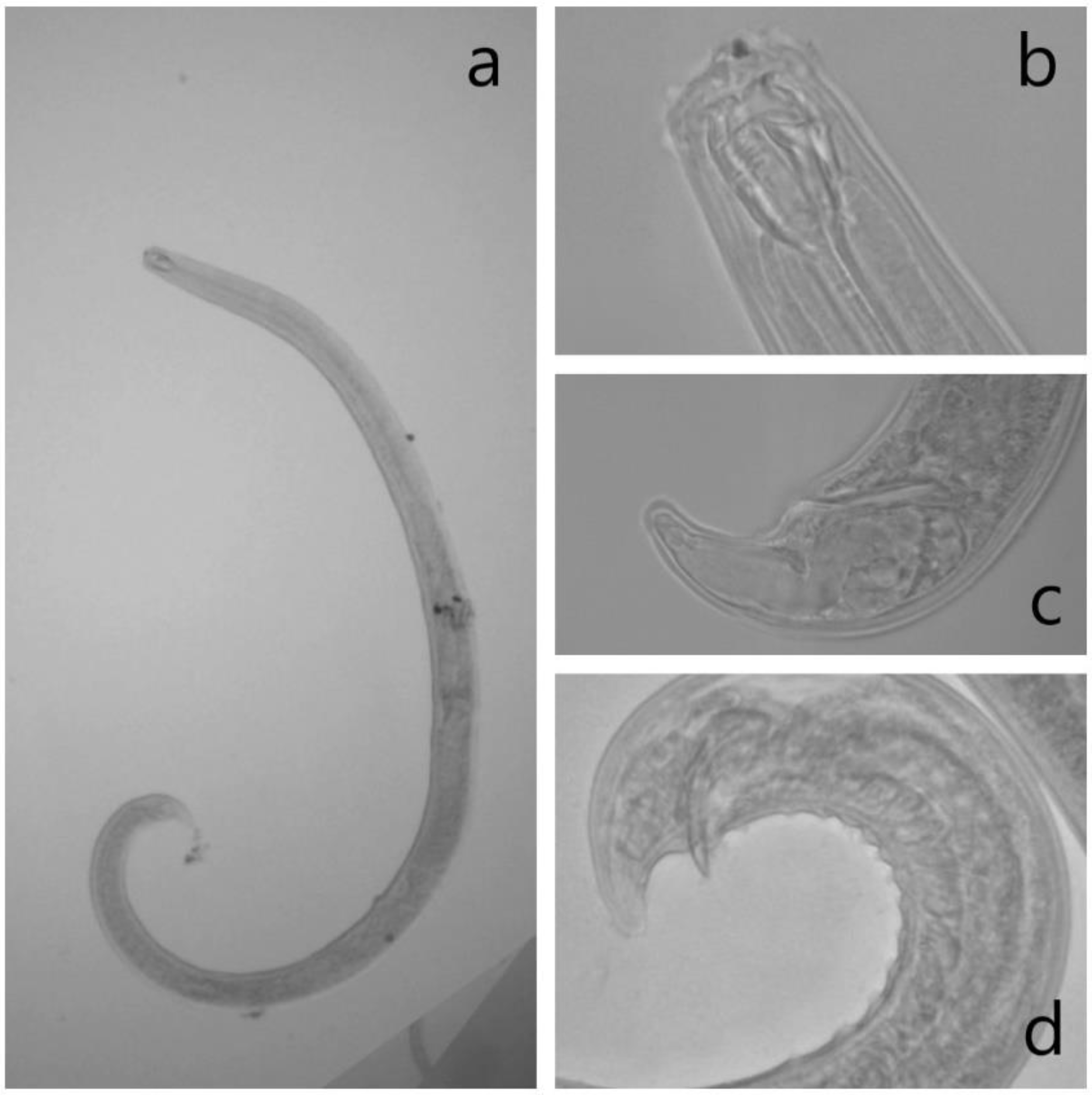
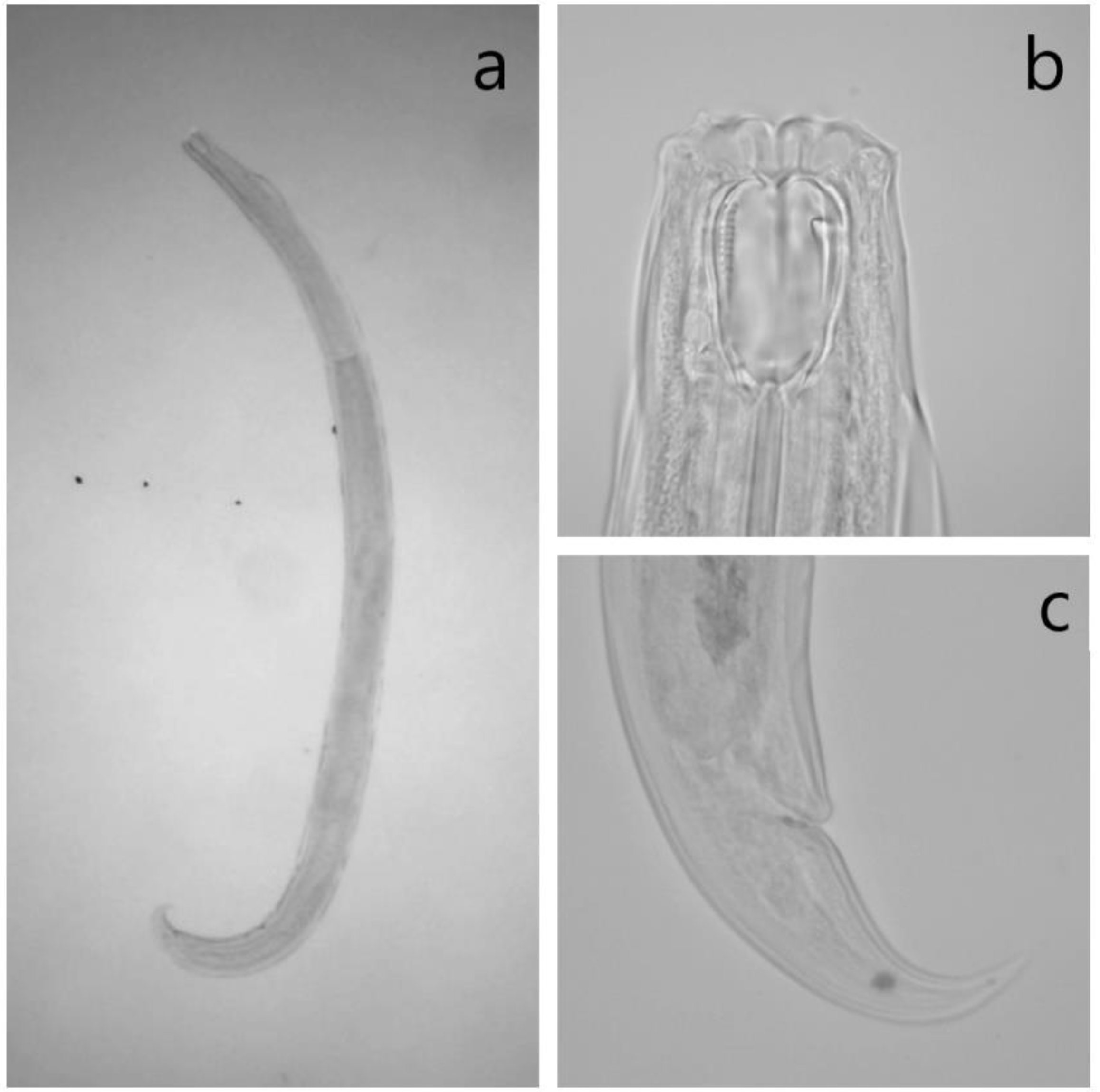
References
- Communication from the Commission to the European Parliament, the Council; The European Economic and Social Committee and the Committee of the Regions. A New Circular Economy Action Plan—For a Cleaner and More Competitive Europe; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Soobhany, N. Assessing the physicochemical properties and quality parameters during composting of different organic constituents of Municipal Solid Waste. J. Environ. Chem. Eng. 2018, 6, 1979–1988. [Google Scholar] [CrossRef]
- Rolewicz-Kalińska, A.; Lelicińska-Serafin, K.; Manczarski, P. The Circular Economy and Organic Fraction of Municipal Solid Waste Recycling Strategies. Energies 2020, 13, 4366. [Google Scholar] [CrossRef]
- Van, D.P.; Fujiwara, T.; Tho, B.L.; Toan, P.P.S.; Minh, G.H. A review of anaerobic digestion systems for biodegradable waste: Configurations, operating parameters, and current trends. Environ. Eng. Res. 2019, 25, 1–17. [Google Scholar] [CrossRef]
- Eades, P.; Kusch-Brandt, S.; Heaven, S.; Banks, C.J. Estimating the Generation of Garden Waste in England and the Differences between Rural and Urban Areas. Resources 2020, 9, 8. [Google Scholar] [CrossRef]
- Eurostat. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Municipal_waste_statistics (accessed on 9 October 2021).
- Stavi, I. On-Site Use of Plant Litter and Yard Waste as Mulch in Gardening and Landscaping Systems. Sustainability 2020, 12, 7521. [Google Scholar] [CrossRef]
- Bogdányi, F.T.; Pullai, K.B.; Doshi, P.; Erdős, E.; Gilián, L.D.; Lajos, K.; Leonetti, P.; Nagy, P.I.; Pantaleo, V.; Petrikovszki, R.; et al. Composted Municipal Green Waste Infused with Biocontrol Agents to Control Plant Parasitic Nematodes—A Review. Microorganisms 2021, 9, 2130. [Google Scholar] [CrossRef]
- Lehec, E. Alternative Techniques to Large Urban Networks: The Misunderstandings about the Success of On-Site Composting in Paris. J. Urban Technol. 2020, 27, 93–113. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Xu, S.; Wang, J.; Zhou, Q.; Li, Y.; Tong, X. Rapid in-situ composting of household food waste. Process. Saf. Environ. Prot. 2020, 141, 259–266. [Google Scholar] [CrossRef]
- Ndegwa, P.; Thompson, S.; Merka, W. A Dynamic Simulation Model ofin-situComposting Of Caged Layer Manure. Compos. Sci. Util. 2000, 8, 190–202. [Google Scholar] [CrossRef]
- Martínez-Gallardo, M.R.; López, M.J.; Jurado, M.M.; Suárez-Estrella, F.; López-González, J.A.; Sáez, J.A.; Moral, R.; Moreno, J. Bioremediation of Olive Mill Wastewater sediments in evaporation ponds through in situ composting assisted by bioaugmentation. Sci. Total. Environ. 2019, 703, 135537. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, L.; Cadena, E.; Martínez-Blanco, J.; Gasol, C.M.; Rieradevall, J.; Gabarrell, X.; Gea, T.; Sort, X.; Sanchez, A. Recovery of organic wastes in the Spanish wine industry. Technical, economic and environmental analyses of the composting process. J. Clean. Prod. 2009, 17, 830–838. [Google Scholar] [CrossRef]
- Dudás, P.; Gedeon, C.; Menyhárt, L.; Ambrus, G.; Tóth, F. The effect of mulching on the abundance and diversity of ground beetle assemblages in two hungarian potato fields. Columella: J. Agric. Environ. Sci. 2016, 3. [Google Scholar] [CrossRef]
- Jafari, F.; Kartoolinejad, D.; Amiri, M.; Shayanmehr, M.; Akbarian, M. Long term effect of oil mulch on richness and biodiversity of soil macro-fauna and vegetation in Jask, Iran. Arid. Biome 2017, 7, 27–38. [Google Scholar] [CrossRef][Green Version]
- Forge, T.; Kempler, C. Organic mulches influence population densities of root-lesion nematodes, soil health indicators, and root growth of red raspberry. Can. J. Plant Pathol. 2009, 31, 241–249. [Google Scholar] [CrossRef]
- Stirling, G.R.; Halpin, N.V.; Bell, M.J. A surface mulch of crop residues enhances suppressiveness to plant parasitic nematodes in sugarcane soils. Nematropica 2011, 41, 109–121. [Google Scholar]
- Pane, C.; Piccolo, A.; Spaccini, R.; Celano, G.; Villecco, D.; Zaccardelli, M. Agricultural waste-based composts exhibiting suppressivity to diseases caused by the phytopathogenic soil-borne fungi Rhizoctonia solani and Sclerotinia minor. Appl. Soil Ecol. 2013, 65, 43–51. [Google Scholar] [CrossRef]
- Sinkevičienė, A.; Jodaugienė, D.; Pupalienė, R.; Urbonienė, M. The influence of organic mulches on soil properties and crop yield. Agron. Res. 2009, 7, 485–491. [Google Scholar]
- Ni, X.; Song, W.; Zhang, H.; Yang, X.; Wang, L. Effects of Mulching on Soil Properties and Growth of Tea Olive (Osmanthus fragrans). PLOS ONE 2016, 11, e0158228. [Google Scholar] [CrossRef]
- Simsek, U.; Erdel, E.; Barik, K. Effect of mulching on soil moisture and some soil characteristics. Fresenius Environ. Bull 2009, 26, 7437–7443. [Google Scholar]
- Young, I.M.; Ritz, K. Can there be a contemporary ecological dimension to soil biology without a habitat? Soil Biol. Biochem. 1998, 30, 1229–1232. [Google Scholar] [CrossRef]
- Kouser, Y.; Shah, A.A.; Rasmann, S. The functional role and diversity of soil nematodes are stronger at high elevation in the lesser Himalayan Mountain ranges. Ecol. Evol. 2021, 11, 13793–13804. [Google Scholar] [CrossRef]
- Neher, D.A. Role of nematodes in soil health and their use as indicators. J. Nematol. 2001, 33, 161–168. [Google Scholar] [PubMed]
- Nielsen, U.N.; Ayres, E.; Wall, D.H.; Li, G.; Bardgett, R.D.; Wu, T.; Garey, J. Global-scale patterns of assemblage structure of soil nematodes in relation to climate and ecosystem properties. Glob. Ecol. Biogeogr. 2014, 23, 968–978. [Google Scholar] [CrossRef]
- Ferris, H. Form and function: Metabolic footprints of nematodes in the soil food web. Eur. J. Soil Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; Goede, R. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Kergunteuil, A.; Campos-Herrera, R.; Sánchez-Moreno, S.; Vittoz, P.; Rasmann, S. The Abundance, Diversity, and Metabolic Footprint of Soil Nematodes Is Highest in High Elevation Alpine Grasslands. Front. Ecol. Evol. 2016, 4, 84. [Google Scholar] [CrossRef]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Devi, G.; George, J. Predatory nematodes as bio-control agent against plant-parasitic nematode—A review. Agric. Rev. 2018, 38, 55–61. [Google Scholar] [CrossRef]
- Wardle, D.A.; Yeates, G.W. The dual importance of competition and predation as regulatory forces in terrestrial ecosystems: Evidence from decomposer food-webs. Oecologia 1993, 93, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, R.; Patil, J.; Yadav, S. Prospects of using predatory nematodes in biological control for plant parasitic nematodes—A review. Biol. Control. 2021, 160, 104668. [Google Scholar] [CrossRef]
- Sharma, G.C.; Gupta, R. Effect of edaphic factors on mononchids in the rhizosphere of polyhouse grown flower crops. Indian J Nematol 2015, 45, 12–15. [Google Scholar]
- Akhtar, M.; Mahmood, I. Effect of Mononchus aquaticus and organic amendments on Meloidogyne incognita development on chilli. Nematol. Mediterr. 1993, 21, 251–252. [Google Scholar]
- Akhtar, M.; Mahmood, I. Control of plant-parasitic nematodes with organic and inorganic amendments in agricultural soil. Appl. Soil Ecol. 1996, 4, 243–247. [Google Scholar] [CrossRef]
- Bulluck, L.R., III; Barker, K.R.; Ristaino, J.B. Influences of organic and synthetic soil fertility amendments on nematode trophic groups and community dynamics under tomatoes. Appl. Soil Ecol. 2002, 21, 233–250. [Google Scholar] [CrossRef]
- Langat, J.; Kimenju, J.; Mutua, G.; Muiru, W.; Otieno, W. Response of Free-Living Nematodes to Treatments Targeting Plant Parasitic Nematodes in Carnation. Asian, J. Plant Sci. 2008, 7, 467–472. [Google Scholar] [CrossRef][Green Version]
- Steel, H.; Vandecasteele, B.; Willekens, K.; Sabbe, K.; Moens, T.; Bert, W. Nematode communities and macronutrients in composts and compost-amended soils as affected by feedstock composition. Appl. Soil Ecol. 2012, 61, 100–112. [Google Scholar] [CrossRef]
- Forge, T.A.; Hogue, E.; Neilsen, G.; Neilsen, D. Effects of organic mulches on soil microfauna in the root zone of apple: Implications for nutrient fluxes and functional diversity of the soil food web. Appl. Soil Ecol. 2003, 22, 39–54. [Google Scholar] [CrossRef]
- Bilgrami, A.L. Biological control potentials of predatory nematodes. In Integrated Management and Biocontrol of Vegetable and Grain Crops Nematodes; Ciancio, A., Mukerji, K.G., Eds.; Springer: Heidelberg, The Netherlands, 2008; pp. 3–28. [Google Scholar]
- Zhao, C.; Li, Y.; Zhang, C.; Miao, Y.; Liu, M.; Zhuang, W.; Shao, Y.; Zhang, W.; Fu, S. Considerable impacts of litter inputs on soil nematode community composition in a young Acacia crassicapa plantation. Soil Ecol. Lett. 2021, 3, 145–155. [Google Scholar] [CrossRef]
- FAO. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Petrikovszki, R.; Zalai, M.; Tóthné Bogdányi, F.; Tóth, F. The effect of organic mulching and irrigation on the weed species composition and the soil weed seed bank of tomato. Plants 2020, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Helyes, L.; Varga, G. Irrigation demand of tomato according to the results of three decades. Acta Hortic. 1994, 323–328. [Google Scholar] [CrossRef]
- Baermann, G. A simple method for the detection of Ankylostomum (nematode) larvae in soil tests. In Mededelingen uit het Geneeskundig Laboratorium te Weltevreden; Baermann, C., Ed.; Javasche Boekhandel & Drukkerij: Batavia, The Netherlands, 1917; pp. 41–47. [Google Scholar]
- Szakálas, J.; Kröel-Dulay, G.; Kerekes, I.; Seres, A.; Ónodi, G.; Nagy, P. Extrém szárazság és a növényzeti borítottság hatása szabadon élő fonálféreg együttesek denzitására. Effects of extreme drought manipulation on free-living nematode densities. Termved. Közlemé. 2015, 21, 293–300. [Google Scholar]
- Andrássy, I. Free-living nematodes of Hungary (Nematoda errantia). Vol. III; Pedozoologica Hungarica No. 5; Hungarian Natural History Museum and Systematic Zoology Research Group of the Hungarian Academy of Sciences: Budapest, Hungary, 2009; p. 608. [Google Scholar]
- Ahmad, W.; Jairajpuri, M.S. Mononchida: The Predatory Soil Nematodes. Nematology Monographs and Perspectives, Volume 7; Brill Leiden-Boston: Leiden, The Netherlands, 2010; p. 320. [Google Scholar]
- Stubbs, T.L.; Kennedy, A.C.; Schillinger, W. Soil Ecosystem Changes During the Transition to No-Till Cropping. J. Crop. Improv. 2004, 11, 105–135. [Google Scholar] [CrossRef]
- Yeates, G.W.; Shepherd, T.G.; Francis, G.S. Contrasting response to cropping of populations of earthworms and predacious nematodes in four soils. Soil Tillage Res. 1998, 48, 255–264. [Google Scholar] [CrossRef]
- Steel, H.; Ferris, H. Soil nematode assemblages indicate the potential for biological regulation of pest species. Acta Oecologica 2016, 73, 87–96. [Google Scholar] [CrossRef]
- Thorne, G. The life history, habits and economic importance of some mononchs. J. Agric. Res. 1927, 34, 265–286. [Google Scholar]
- Khan, Z.; Kim, Y.H. A review on the role of predatory soil nematodes in the biological control of plant parasitic nematodes. Appl. Soil Ecol. 2007, 35, 370–379. [Google Scholar] [CrossRef]
- Arpin, P. Etude preliminaire d’un facteur écologique important pour les nematodes: L’humidité actuelle du sol. Rev. Ecol. Biol. Sol. 1969, 6, 429–435. [Google Scholar]
- Arpin, P. Sur quelques aspects des interactions sol-nématodes dans des biocénoses forestières ou herbacées. Rev. Ecol. Biol. Sol. 1975, 12, 57–67. [Google Scholar]
- Small, R. The Effects of Predatory Nematodes On Populations of Plant Parasitic Nematodes in Pots. Nematologica 1979, 25, 94–103. [Google Scholar] [CrossRef]
- Jabran, K. Role of Mulching in Pest Management and Agricultural Sustainability; Springer International Publishing: Cham, Germany, 2019. [Google Scholar] [CrossRef]
- Bilgrami, A.L.; Brey, C. Potential of predatory nematodes to control plant-parasitic nematodes. In Nematodes as biocontrol agents; Grewal, P.S., Ehlers, R.U., Shapiro-Ilan, D.I., Eds.; CABI: Wallingford, UK, 2005; pp. 447–464. [Google Scholar]
- De Corato, U. Agricultural waste recycling in horticultural intensive farming systems by on-farm composting and com-post-based tea application improves soil quality and plant health: A review under the perspective of a circular economy. Sci. Total Environ. 2020, 738, 139840. [Google Scholar] [CrossRef] [PubMed]

| Year | 2016 | 2017 | 2018 | 2019 |
|---|---|---|---|---|
| Planting (Beginning of season) | 2 June | 12 May | 9 May | 30 May |
| End of growing season | 30 August | 19 September | 26 September | 4 October |
| Mulching | 18 March | 17 March and 18 July | 9 May | 3 April and 23 August |
| Collection of soil samples | 30 August | 18 September | 25 September | 5 September |
| Rainfall | 213 mm | 299.5 mm | 370.5 mm | 166 mm |
| Irrigation water | 153 mm | 303.2 mm | 193.4 mm | - |
| Average temperature | 21.0 °C | 21.1 °C | 21.6 °C | 20.8 °C |
| Minimum temperature | 8.6 °C | 7.0 °C | 0.0 °C | 3.0 °C |
| Maximum temperature | 35.0 °C | 38.0 °C | 35.0 °C | 36.0 °C |
| Character | Clarkus papillatus | Mylonchulus brachyuris | Prionchulus punctatus | |
|---|---|---|---|---|
| n | 20 ♀ | 17 ♀ | 1 ♂ | 7 ♀ |
| L (µm) | 1212.2 ± 43.4 | 1089.2 ± 44.4 | 1198.2 | 1689 ± 132.3 |
| 1012.8–1395.2 | 948–1288.2 | – | 1521.1–1991.1 | |
| a | 24.3 ± 2.2 | 28.7 ± 1.8 | 31.6 | 21.6 ± 1.1 |
| 18.6–40.3 | 22.1–34.7 | – | 19.6–23.6 | |
| b | 3.7 ± 0.1 | 3.7 ± 0.1 | 3.8 | 4 ± 0.3 |
| 3.4–4 | 3.4–3.9 | – | 3.7–4.8 | |
| c | 16.6 ± 0.5 | 31.7 ± 1 | 31.9 | 17.6 ± 0.7 |
| 15.1–18.4 | 28.1–35.5 | – | 16.5–19.1 | |
| V (%) | 62 ± 0.5 | 60.2 ± 0.6 | – | 63.7 ± 1.3 |
| 60.5–65.8 | 57.1–62.2 | – | 62–66.5 | |
| Buccal cavity length (μm) | 24.9 ± 0.5 | 20.6 ± 0.7 | 21.1 | 33.5 ± 1.4 |
| 22.5–26.3 | 16.4–22.6 | - | 30.6–36.8 | |
| Buccal cavity width (μm) | 12.9 ± 0.5 | 13.7 ± 0.9 | 12.4 | 21.4 ± 1.3 |
| 10.1–15.2 | 12.1–16.3 | – | 18–22.9 | |
| Maximum body width (μm) | 51.2 ± 3.6 | 38.4 ± 2.3 | 38 | 78 ± 3 |
| 30.7–68.7 | 29.6–47.3 | – | 73.2–85.3 | |
| Anal body width (μm) | 28.8 ± 1.1 | 25 ± 1.4 | 31.3 | 41 ± 2.6 |
| 23.7–33.3 | 19.5–31 | – | 37.4–45.7 | |
| Tail length (μm) | 73.1 ± 2.8 | 34.5 ± 1.6 | 37.5 | 96.3 ± 7.5 |
| 63.6 –85.8 | 28.6–41.2 | – | 86.5–115.5 | |
| Supplements | - | - | 12 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrikovszki, R.; Zalai, M.; Tóthné Bogdányi, F.; Tóth, F.; Nagy, P.I. Mulching with Leaf Litter from Municipal Green Waste Favours Predatory Mononchid Nematodes. Agronomy 2021, 11, 2522. https://doi.org/10.3390/agronomy11122522
Petrikovszki R, Zalai M, Tóthné Bogdányi F, Tóth F, Nagy PI. Mulching with Leaf Litter from Municipal Green Waste Favours Predatory Mononchid Nematodes. Agronomy. 2021; 11(12):2522. https://doi.org/10.3390/agronomy11122522
Chicago/Turabian StylePetrikovszki, Renáta, Mihály Zalai, Franciska Tóthné Bogdányi, Ferenc Tóth, and Péter István Nagy. 2021. "Mulching with Leaf Litter from Municipal Green Waste Favours Predatory Mononchid Nematodes" Agronomy 11, no. 12: 2522. https://doi.org/10.3390/agronomy11122522
APA StylePetrikovszki, R., Zalai, M., Tóthné Bogdányi, F., Tóth, F., & Nagy, P. I. (2021). Mulching with Leaf Litter from Municipal Green Waste Favours Predatory Mononchid Nematodes. Agronomy, 11(12), 2522. https://doi.org/10.3390/agronomy11122522








