Influence of Substrate Composition and Container Size on the Growth of Tissue Culture Propagated Apple Rootstock Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growing Conditions
2.2. Treatments
2.2.1. Substrate Composition (Experiment 1)
2.2.2. Container Size (Experiment 2)
2.3. Measurement of Plant Growth Parameters
2.4. Statistical Analysis
3. Results and Discussion
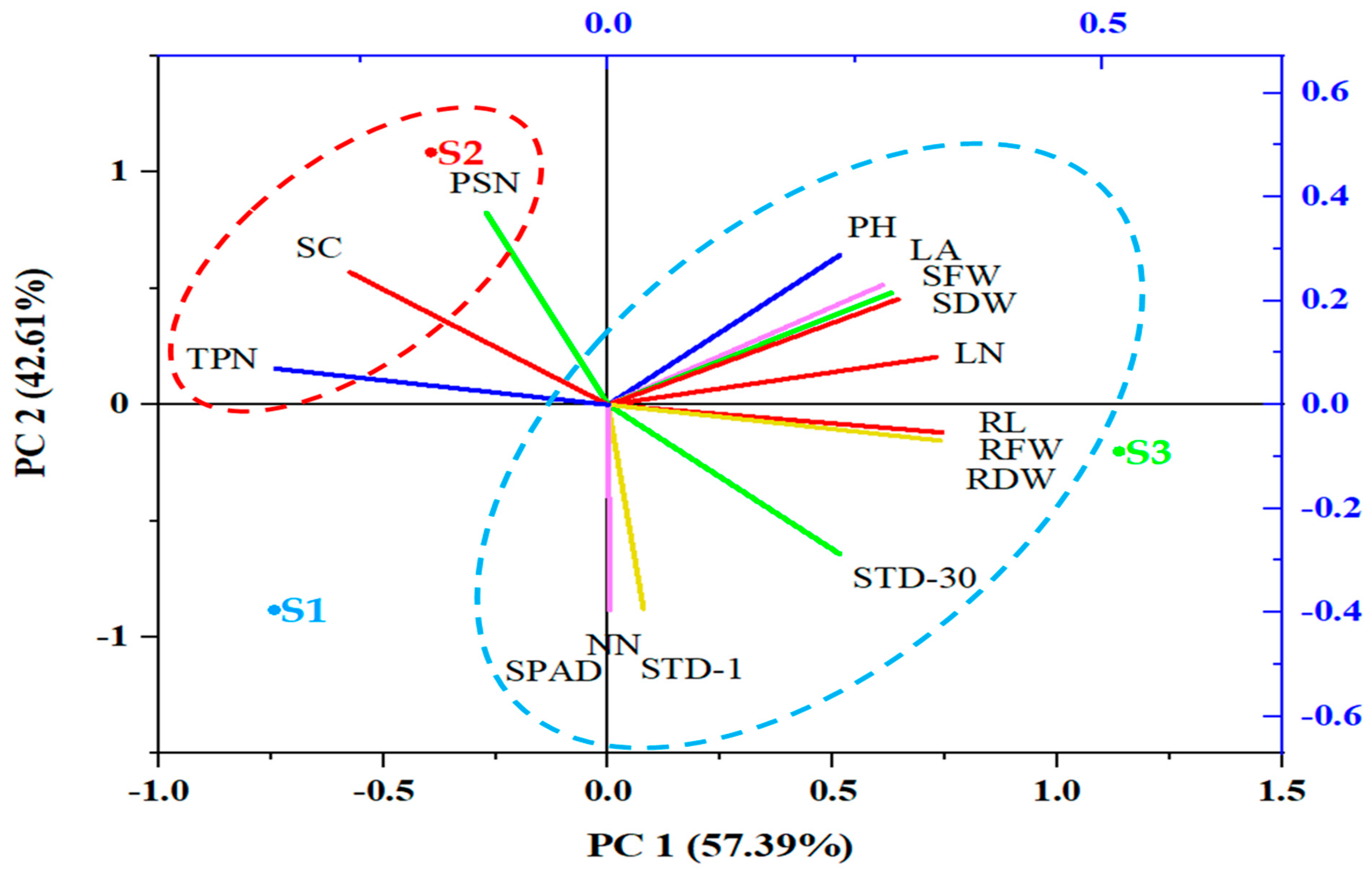
3.1. Effect of Substrate Composition (Experiment 1)
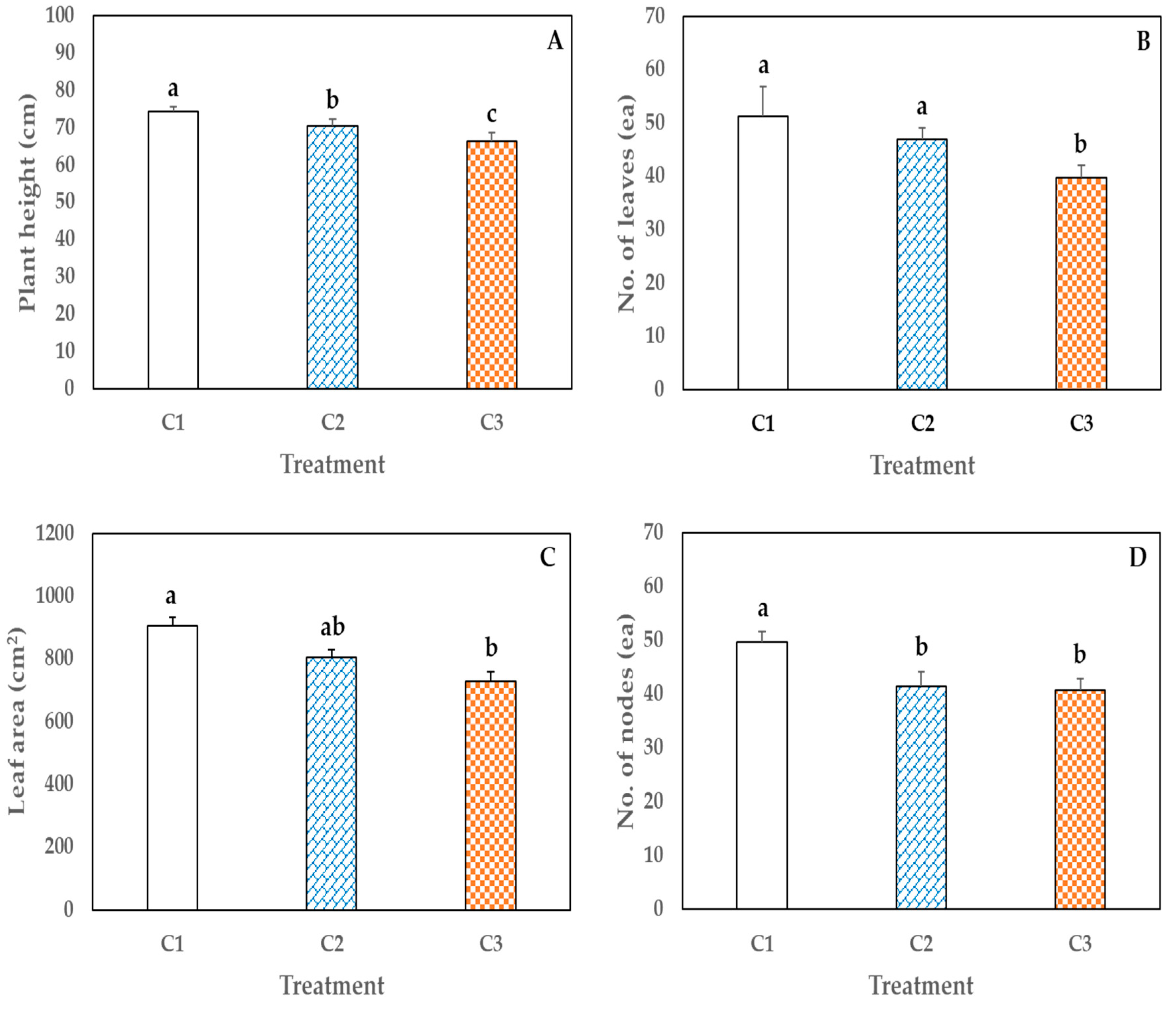
3.2. Effect of Container Size (Experiment 2)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, S.; Hinkle, A.F. South Korea: 2017 Apple Report- Revised. Gain Report No. KS1733. 2017. Available online: https://www.fas.usda.gov/data/south-korea-2017-apple-report-revised (accessed on 23 November 2018).
- Wang, Y.; Li, W.; Xu, X.; Qiu, C.; Wu, T.; Wei, Q.; Ma, F.; Han, Z. Progress of apple rootstock breeding and its use. Hortic. Plant. J. 2019, 5, 183–191. [Google Scholar] [CrossRef]
- Valverdi, N.A.; Cheng, L.; Kalcsits, L. Apple scion and rootstock contribute to nutrient uptake and partitioning under different belowground environments. Agronomy 2019, 9, 415. [Google Scholar] [CrossRef] [Green Version]
- Vahdati, K.; Sarikhani, S.; Arab, M.M.; Leslie, C.A.; Dandekar, A.M.; Aletà, N.; Bielsa, B.; Gradziel, T.M.; Montesinos, Á.; Rubio-Cabetas, M.J.; et al. Advances in rootstock breeding of nut trees: Objectives and strategies. Plants 2021, 10, 2234. [Google Scholar] [CrossRef]
- Yoon, T.M.; Kim, K.R.; Uhm, J.Y.; Byun, J.K. The extension results of the cultivation systems for dwarf apple trees and following tasks. In Proceedings of the 23rd Apple Festival for the Establishment of Dwarf Apple Orchards, North Kyungsang Province and Daegu Kyungbuk Apple Cooperative, Daegu, Korean, 2000; pp. 9–235. [Google Scholar]
- Hajnajari, H.; Chashnidel, B.; Vahdati, K.; Ebrahimi, M.; Nabipour, A.; Fallahi, E. Heritability of morphological traits in apple early-ripening full-sib and half-sib offspring and its potential use for assisted selection. HortScience 2012, 47, 328–333. [Google Scholar] [CrossRef]
- Choi, B.H.; Bhusal, N.; Jeong, W.T.; Park, I.H.; Han, S.G.; Yoon, T.M. Waterlogging tolerance in apple trees grafted on rootstocks from G., CG, and M series. Hortic. Environ. Biotechnol. 2020, 61, 685–692. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Kwon, S.I.; Kim, J.H. Effects of the exposed length of dwarf rootstock M.9 on growth and yield of ‘Seohong’, ‘Summer Dream’ and ‘Honggeum’ apples. Protected Hort. Plant. Fac. 2016, 25, 168–172. [Google Scholar] [CrossRef]
- Korea Seed and Variety Service (KSVS). 2017. Available online: www.korea.kr/common/download.do?fileId=184846063&tblKey=GMN (accessed on 17 September 2021).
- Kim, N.Y.; Hwang, H.D.; Kim, J.H.; Kwon, B.M.; Kim, D.; Park, S.Y. Efficient production of virus-free apple plantlets using the temporary immersion bioreactor system. Hortic. Environ. Biotechnol. 2020, 61, 779–785. [Google Scholar] [CrossRef]
- Vahdati, K.; Aliniaeifard, S. Investigation of physiological components involved in low water conservation capacity of in vitro walnut plants. Sci. Hortic. 2017, 224, 1–7. [Google Scholar] [CrossRef]
- Asayesh, Z.M.; Vahdati, K.; Aliniaeifard, S.; Askari, N. 2017. Enhancement of ex vitro acclimation of walnut plantlets through modification of stomatal characteristics in vitro. Sci. Hortic. 2017, 220, 114–121. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Food and Rural Affairs (MAFRA). Protected Horticultural Industrialization of Apple Virus Free Stocks; Chungbuk National University Industry–University Incorporation: Cheongju, Korea, 2020; 11-1543000-000918-01. (In Korean) [Google Scholar]
- Blievernicht, A.; Irrgang, S.; Zander, M.; Ulrichs, C. Sphagnum biomass—The next generation of growing media. Peat. Int. 2013, 1, 32–35. [Google Scholar]
- Larcher, F.; Scariot, V. Assessment of partial peat substitutes for the production of Camellia japonica. HortScience 2009, 44, 312–316. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Kim, K.H. Physical properties of the horticultural substrate according to mixing ratio of peatmoss, perlite and vermiculite. Korean. J. Soil Sci. Fert. 2011, 44, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Kamrani, M.H.; Chegeni, A.R.; Hosseinniya, H. Effects of different growing media on yield and growth parameters of potato minitubers (Solanum tuberosum L.). Commun. Soil Sci. Plant. Anal. 2019, 50, 1838–1853. [Google Scholar] [CrossRef]
- Voropaeva, V.; Karpachev, V.; Varlamov, V.; Figovsky, O. Influence of improved (nano) systems on cultivated corn growth, development and yield. Int. Lett. Chem. Phys. Astron. 2014, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Asaduzzaman, M.; Kobayashi, Y.; Mondal, M.F.; Ban, T.; Matsubara, H.; Adachi, F.; Asao, T. Growing carrots hydroponically using perlite substrates. Sci. Horict. 2013, 159, 113–121. [Google Scholar] [CrossRef]
- Ronchi, C.P.; DaMatta, F.M.; Batista, K.D.; Moraes, G.A.B.K.; Loureiro, M.E.; Ducatti, C. Growth and photosynthetic down-regulation in Coffea arabica in response to restricted root volume. Funct. Plant. Biol. 2006, 33, 1013–1023. [Google Scholar] [CrossRef]
- Sendi, H.; Mohamed, M.T.M.; Anwar, M.P.; Saud, H.M. Spent mushroom waste as a media replacement for peat moss in Kai-Lan (Brassica oleracea var. Alboglabra) production. Sci. World J. 2013. [Google Scholar] [CrossRef] [Green Version]
- Oberpaur, C.; Puebla, V.; Vaccarezza, F.; Arevalo, M.E. Preliminary substrate mixtures including peat moss (Sphagnum magellanicum) for vegetable crop nurseries. Cien. Investig. Agr. 2010, 37, 123–132. [Google Scholar] [CrossRef]
- Lee, C.H.; Shin, H.S. Adsorption study of humin from peat moss with Cd(II), Cu(II) and Pb(II) in aqueous solution. J. Korean Soc. Environ. Eng. 2004, 26, 1079–1085. [Google Scholar]
- Vahdati, K.; Leslie, C.; Zamani, Z.; McGranahan, G. Rooting and acclimatization of in vitro-grown shoots from mature trees of three Persian walnut cultivars. HortScience 2004, 39, 324–327. [Google Scholar] [CrossRef]
- Martín, M.; Noarbe, D.M.; Serrot, P.H.; Sabater, B. The rise of the photosynthetic rate when light intensity increases is delayed in ndh gene-defective tobacco at high but not at low CO2 concentrations. Front. Plant. Sci. 2015, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Urban, J.; Ingwers, M.; McGuire, M.A.; Teskey, R.O. Stomatal conductance increases with rising temperature. Plant. Signal. Behav. 2017, 12, e1356534. [Google Scholar] [CrossRef] [Green Version]
- Dinh, T.H.; Takaragawa, H.; Watanabe, K.; Nakabaru, M.; Kawamitsu, Y. Leaf photosynthesis response to change of soil moisture content in sugarcane. Sugar Tech. 2019, 21, 949–958. [Google Scholar] [CrossRef]
- Yamori, W.; Kusumi, K.; Iba, K.; Terashima, I. Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice. Plant. Cell Environ. 2020, 43, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Sale, F.A. Evaluation of watering regimes and different pot sizes in the growth of Parkia biglobosa (jacq) benth seedlings under nursery condition. Eur. Sci. J. 2015, 11, 313–325. [Google Scholar]
- Vaknin, Y.; Dudai, N.; Murkhovsky, L.; Gelfandbein, L.; Fischer, R.; Degani, A. Effects of pot size on leaf production and essential oil content and composition of Eucalyptus citriodora hook. (lemon-scented gum). J. Herb. Spice. Med. Plant. 2009, 15, 164–176. [Google Scholar] [CrossRef]
- Dambreville, A.; Griolet, M.; Rolland, G.; Dauzat, M.; Bédiée, A.; Balsera, C.; Muller, B.; Vile, D.; Granier, C. Phenotyping oilseed rape growth-related traits and their responses to water deficit: The disturbing pot size effect. Func. Plant. Biol. 2017, 44, 35–45. [Google Scholar] [CrossRef]
- Paltineanu, C.; Nicolae, S.; Tanasescu, N.; Chitu, E.; Ancu, S. Investigating root density of plum and apple trees grafted on low-vigor rootstocks to improve orchard management. Erwerbs-Obstbau 2017, 59, 29–37. [Google Scholar] [CrossRef]
- Poorter, H.; Bühler, J.; Dusschoten, D.V.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Func. Plant. Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Kroon, H.D.; Smits, A.J.M. Combined effects of partial root drying and patchy fertilizer placement on nutrient acquisition and growth of oilseed rape. Plant. Soil. 2007, 295, 207–216. [Google Scholar] [CrossRef]
- Durand, M.; Mainson, D.; Porcheron, B.; Maurousset, L.; Lemoine, R.; Pourtau, N. Carbon source–sink relationship in Arabidopsis thaliana: The role of sucrose transporters. Planta 2018, 247, 587–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Zhu, L.; Wang, S.; Gu, W.; Huang, D.; Xu, W.; Jiang, A.; Li, S. Nitrate uptake kinetics of grapevine under root restriction. Sci. Hortic. 2007, 111, 358–364. [Google Scholar] [CrossRef]
- White, A.C.; Rogers, A.; Rees, M.; Osborne, C.P. How can we make plants grow faster? A source–sink perspective on growth rate. J. Exp. Bot. 2016, 67, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Puangbut, D.; Jogloy, S.; Vorasoot, N. Association of photosynthetic traits with water use efficiency and SPAD chlorophyll meter reading of Jerusalem artichoke under drought conditions. Agric. Water Manag. 2017, 188, 29–35. [Google Scholar] [CrossRef]
- Chung, G.J.; Lee, J.H.; Oh, M.M. Growth and Acclimation of In Vitro-Propagated M9 Apple Rootstock Plantlets under Various Visible Light Spectrums. Agronomy 2020, 10, 1017. [Google Scholar] [CrossRef]
- Kandel, B.P. Spad value varies with age and leaf of maize plant and its relationship with grain yield. BMC Res. Notes 2020, 13, 1–4. [Google Scholar] [CrossRef]
- Shimomoto, K.; Takayama, K.; Takahashi, N.; Nishina, H.; Inaba, K.; Isoyama, Y.; Oh, S.C. Real-time monitoring of photosynthesis and transpiration of a fully-grown tomato plant in greenhouse. Environ. Control Biol. 2020, 58, 65–70. [Google Scholar] [CrossRef]




| Treatment | Stem Diameter from Ground (mm) | Root Length (cm) | Fresh Weight (g/plant) | Dry Weight (g/plant) | |||
|---|---|---|---|---|---|---|---|
| 1 cm | 30 cm | Shoot | Root | Shoot | Root | ||
| S1 | 6.1 ± 0.81 za y | 4.5 ± 1.21a | 20.5 ± 0.71b | 25.9 ± 2.19b | 9.2 ± 0.18b | 9.4 ± 0.54b | 2.0 ± 0.73a |
| S2 | 5.7 ± 1.21a | 4.2 ± 1.62a | 20.6 ± 0.21b | 30.1 ± 3.41ab | 9.2 ± 0.07b | 11.2 ± 0.71ab | 2.0 ± 0.58a |
| S3 | 6.0 ± 1.33a | 4.7 ± 1.73a | 22.7 ± 0.30a | 31.9 ± 2.31a | 10.5 ± 0.17a | 12.1 ± 0.29a | 2.3 ± 0.21a |
| Treatment | SPAD (Value) | Photosynthesis (µmol·CO2·m–2·s–1) | Conductance (mol·H2O·m–2·s–1) | Transpiration (mol·H2O·m–2·s–1) |
|---|---|---|---|---|
| S1 | 38.9 ± 3.10 za y | 10.21 ± 1.21a | 0.37 ± 0.08a | 4.93 ± 0.32a |
| S2 | 34.8 ± 4.27a | 11.02 ± 2.21a | 0.38 ± 0.11a | 4.93 ± 0.17a |
| S3 | 37.5 ± 3.41a | 10.19 ± 1.89a | 0.36 ± 0.12a | 4.84 ± 1.81a |
| Treatment | Stem Diameter from Ground (mm) | Root Length (cm) | Fresh Weight (g/plant) | Dry Weight (g/plant) | |||
|---|---|---|---|---|---|---|---|
| 1 cm | 30 cm | Shoot | Root | Shoot | Root | ||
| C1 | 6.8 ± 0.40 za y | 4.4 ± 0.30a | 29.6 ± 2.12a | 31.4 ± 1.18a | 12.9 ± 0.46a | 11.5 ± 0.27a | 2.4 ± 0.74a |
| C2 | 6.1 ± 0.10a | 4.5 ± 0.20a | 20.5 ± 3.24b | 25.9 ± 1.46b | 9.2 ± 0.36b | 9.4 ± 0.42b | 2.0 ± 0.31a |
| C3 | 5.1 ± 0.30b | 3.5 ± 0.20b | 23.1 ± 2.33b | 22.8 ± 3.24b | 9.2 ± 0.47b | 8.6 ± 0.31b | 1.9 ± 0.22a |
| Treatment | SPAD (Value) | Photosynthesis (µmol·CO2·m–2·s–1) | Conductance (mol·H2O·m–2·s–1) | Transpiration (mol·H2O·m–2·s–1) |
|---|---|---|---|---|
| C1 | 42.0 ± 4.79 za y | 8.52 ± 1.86ab | 0.35 ± 0.09a | 4.80 ± 0.31a |
| C2 | 38.9 ± 3.96a | 10.21 ± 1.12a | 0.37 ± 0.03a | 4.93 ± 0.09a |
| C3 | 38.3 ± 3.21a | 11.50 ± 2.21a | 0.37 ± 0.06a | 4.97 ± 1.21a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.K.; Shawon, M.R.A.; An, J.H.; Yun, Y.J.; Park, S.J.; Na, J.K.; Choi, K.Y. Influence of Substrate Composition and Container Size on the Growth of Tissue Culture Propagated Apple Rootstock Plants. Agronomy 2021, 11, 2450. https://doi.org/10.3390/agronomy11122450
Kim JK, Shawon MRA, An JH, Yun YJ, Park SJ, Na JK, Choi KY. Influence of Substrate Composition and Container Size on the Growth of Tissue Culture Propagated Apple Rootstock Plants. Agronomy. 2021; 11(12):2450. https://doi.org/10.3390/agronomy11122450
Chicago/Turabian StyleKim, Jae Kyung, Md. Rayhan Ahmed Shawon, Jin Hee An, Yeo Jong Yun, Soo Jeong Park, Jong Kuk Na, and Ki Young Choi. 2021. "Influence of Substrate Composition and Container Size on the Growth of Tissue Culture Propagated Apple Rootstock Plants" Agronomy 11, no. 12: 2450. https://doi.org/10.3390/agronomy11122450
APA StyleKim, J. K., Shawon, M. R. A., An, J. H., Yun, Y. J., Park, S. J., Na, J. K., & Choi, K. Y. (2021). Influence of Substrate Composition and Container Size on the Growth of Tissue Culture Propagated Apple Rootstock Plants. Agronomy, 11(12), 2450. https://doi.org/10.3390/agronomy11122450





