The Influence of Industrial Waste on the Magnetic Properties of Salt-Affected Soils from Two Soda Ash Manufacturing Sites
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Work
2.3. Laboratory Analysis
2.4. Data Analysis
- i.
- Salt percentage in soil (Pss) [29]:
- ii.
- Frequency-dependent magnetic susceptibility χfd calculated as a relative and absolute change of magnetic susceptibility obtained for low and high frequencies [31]:
- iii.
- Contamination factor (CF):
- iv.
- Pollution Load Index (PLI):
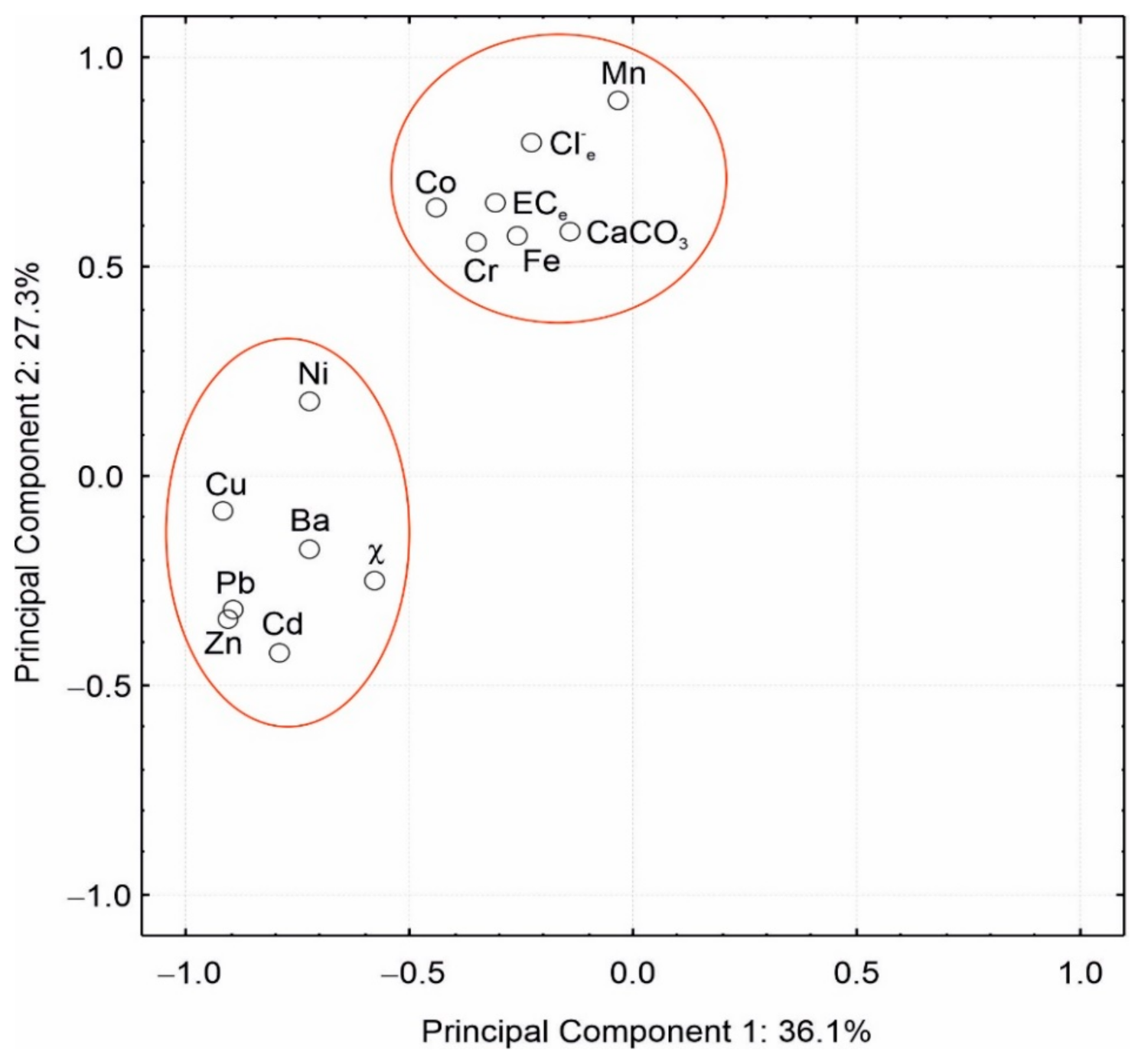
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strzyszcz, Z. Magnetic susceptibility of soils in the areas influenced by industrial emissions. In Soil Monitoring; Schulin, R., Sesaules, A., Webster, R., Steiger, B.V., Eds.; Birkhäuser Verlag: Basel, Switzerland, 1993; pp. 255–269. [Google Scholar]
- Lu, S.; Yu, X.; Chen, Y. Magnetic properties, microstructure and mineralogical phases of technogenic magnetic particles (TMPs) in urban soils: Their source identification and environmental implications. Sci. Total Environ. 2016, 543, 239–247. [Google Scholar] [CrossRef]
- Magiera, T.; Jabłońska, M.; Strzyszcz, Z.; Rachwal, M. Morphological and mineralogical forms of technogenic magnetic particles in industrial dusts. Atmos. Environ. 2011, 45, 4281–4290. [Google Scholar] [CrossRef]
- Vasiliev, A.; Gorokhova, S.; Razinsky, M. Technogenic Magnetic Particles in Soils and Ecological—Geochemical Assessment of the Soil Cover of an Industrial City in the Ural, Russia. Geosciences 2020, 10, 443. [Google Scholar] [CrossRef]
- Szuszkiewicz, M.; Łukasik, A.; Magiera, T.; Mendakiewicz, M. Combination of geo- pedo- and technogenic magnetic and geochemical signals in soil profiles—Diversification and its interpretation: A new approach. Environ. Pollut. 2016, 214, 464–477. [Google Scholar] [CrossRef]
- Maher, B. Characterisation of soils by mineral magnetic measurements. Phys. Earth Planet. Inter. 1986, 42, 76–92. [Google Scholar] [CrossRef]
- Hulisz, P.; Michalski, A.; Dąbrowski, M.; Kusza, G.; Łęczyński, L. Human-induced changes in the soil cover at the mouth of the Vistula River Cross-Cut (northern Poland). Soil Sci. Annu. 2015, 66, 67–74. [Google Scholar] [CrossRef][Green Version]
- Thompson, R.; Oldfield, F. Environmental Magnetism; Springer: London, UK, 1986; p. 228. [Google Scholar] [CrossRef]
- Magiera, T.; Szuszkiewicz, M.M.; Michczyński, A.; Chróst, L.; Szuszkiewicz, M. Peat bogs as archives of local ore mining and smelting activities over the centuries: A case study of Miasteczko Śląskie (Upper Silesia, Poland). CATENA 2021, 198, 105063. [Google Scholar] [CrossRef]
- Gołuchowska, B.; Strzyszcz, Z.; Kusza, G. Magnetic susceptibility and heavy metal content in dust from the lime plant and the cement plant in Opole voivodeship. Arch. Environ. Prot. 2012, 38, 71–80. [Google Scholar] [CrossRef]
- Beckwith, P.; Ellis, J.; Revitt, D.; Oldfield, F. Heavy metal and magnetic relationships for urban source sediments. Phys. Earth Planet. Inter. 1986, 42, 67–75. [Google Scholar] [CrossRef]
- Rachwał, M.; Wawer, M.; Magiera, T.; Steinnes, E. Integration of soil magnetometry and geochemistry for assessment of human health risk from metallurgical slag dumps. Environ. Sci. Pollut. Res. 2017, 24, 26410–26423. [Google Scholar] [CrossRef]
- Grimley, D.; Lynn, A.; Brown, C.; Blair, N. Magnetic fly ash as a chronological marker in post-settlement alluvial and lacustrine sediment: Examples from North Carolina and Illinois. Minerals 2021, 11, 476. [Google Scholar] [CrossRef]
- Peralta, N.R.; Costa, J.L.; Balzarini, M.; Angelini, H. Delineation of management zones with measurements of soil apparent electrical conductivity in the southeastern pampas. Can. J. Soil Sci. 2013, 93, 205–218. [Google Scholar] [CrossRef]
- Jabłońska, M.; Rachwał, M.; Wawer, M.; Kądziołka-Gaweł, M.; Teper, E.; Krzykawski, T.; Smołka-Danielowska, D. Mineralogical and chemical specificity of dusts originating from iron and non-ferrous metallurgy in the light of their magnetic susceptibility. Minerals 2021, 11, 216. [Google Scholar] [CrossRef]
- Grison, H.; Petrovsky, E.; Kapicka, A.; Stejskalova, S. Magnetic and chemical parameters of andic soils and their relation to selected pedogenesis factors. CATENA 2016, 139, 179–190. [Google Scholar] [CrossRef]
- Virto, I.; Imaz, M.J.; Fernández-Ugalde, O.; Gartzia-Bengoetxea, N.; Enrique, A.; Bescansa, P. Soil degradation and soil quality in Western Europe: Current situation and future perspectives. Sustainability 2014, 7, 313–365. [Google Scholar] [CrossRef]
- European Environment Agency. Contamination from Local Sources. Available online: https://www.eea.europa.eu/themes/soil/soil-threats (accessed on 12 September 2021).
- Piernik, A.; Hulisz, P.; Rokicka, A. Micropattern of halophytic vegetation on technogenic soils affected by the soda industry. Soil Sci. Plant Nutr. 2015, 61, 98–112. [Google Scholar] [CrossRef]
- Hulisz, P.; Pindral, S.; Kobierski, M.; Charzyński, P. Technogenic layers in organic soils as a result of the impact of the soda industry. Eurasian Soil Sci. 2018, 51, 1133–1141. [Google Scholar] [CrossRef]
- Hatje, V.; Payne, T.; Hill, D.; McOrist, G.; Birch, G.; Szymczak, R. Kinetics of trace element uptake and release by particles in estuarine waters: Effects of pH, salinity, and particle loading. Environ. Int. 2003, 29, 619–629. [Google Scholar] [CrossRef]
- Lutts, S.; Lefèvre, I. How can we take advantage of halophyte properties to cope with heavy metal toxicity in salt-affected areas? Ann. Bot. 2015, 115, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak, A.; Jaworska, H.; Dąbkowska-Naskręt, H.; Rydlewska, M. Effect of salinity on the mobility of trace metals in soils near a soda chemical factory. J. Elem. 2020, 25, 501–512. [Google Scholar] [CrossRef]
- Wang, X.-B.; Yan, X.; Li, X.-Y. Environmental risk for application of ammonia-soda white mud in soils in China. J. Integr. Agric. 2020, 19, 601–611. [Google Scholar] [CrossRef]
- Abramski, K.; Sobolewski, J. Environment protection against waste of soda production collected in waste ponds. Gospod. Wodna 1977, 4, 107–110. (In Polish) [Google Scholar]
- Hulisz, P.; Krawiec, A.; Pindral, S.; Mendyk, Ł.; Pawlikowska, K. The impact of environmental conditions on water salinity in the area of the city of Inowrocław (north-central Poland). Bull. Geogr. Phys. Geogr. Ser. 2017, 13, 5–15. [Google Scholar] [CrossRef][Green Version]
- Gołub, A.; Piekutin, J. Pollution of sedimentary ponds at an industrial plant in Janikowo (Poland). Water 2020, 12, 536. [Google Scholar] [CrossRef]
- Piernik, A. Inland halophilous vegetation as indicator of soil salinity. Basic Appl. Ecol. 2003, 4, 525–536. [Google Scholar] [CrossRef]
- Van Reeuwijk, L. Technical Paper 09: Procedures for Soil Analysis, 6th ed.; ISRIC: Wageningen, The Netherlands; FAO: Rome, Italy, 2002; p. 119. ISBN 90-6672-044-1. [Google Scholar]
- Hermanowicz, W.; Dojlido, J.; Dożańska, W.; Koziorowski, B.; Zerbe, J. Fizyczno-Chemiczne Analizy Wody i Ścieków; Wydawnictwo Arkady: Warsaw, Poland, 1999; p. 556. (In Polish) [Google Scholar]
- Dearing, J. Environmental Magnetic Susceptibility: Using the Bartington MS2 System, 2nd ed.; Chi Publishing: Keniloworth, UK, 1999; p. 43. [Google Scholar]
- Czarnowska, K. Total content of heavy metals in parent rocks as reference background levels of soil. Soil Sci. Annu. 1996, 47, 43–50. (In Polish) [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press LLC: Boca Raton, FL, USA, 2001; p. 397. ISBN 0-8493-1575-1. [Google Scholar]
- Müller, G. The heavy metal pollution of the sediments of Neckars and its tributary: A stocktaking. Chem. Ztg. 1981, 105, 157–164. [Google Scholar]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol. Meeresunters. 1980, 33, 566–575. [Google Scholar] [CrossRef]
- IUSS Working Group WRB 2015. World Reference Base for Soil Resources 2014, Update 2015, International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; Food and Agriculture Organization: Rome, Italy, 2014. [Google Scholar]
- Czerwiński, Z.; Pracz, J.; Piątek, A. Wpływ Odpadów z Janikowskich Zakładów Sodowych na Tereny Rolnicze. Rocz. Glebozn. 1984, 35, 87–105. (In Polish) [Google Scholar]
- Solntseva, N.P. Trends in soil evolution under technogenic impacts. Eurasian Soil Sci. 2002, 35, 6–16. [Google Scholar]
- Allison, L.E.; Bernstein, L.; Bower, C.A.; Brown, J.W.; Fireman, M.; Hatcher, J.T.; Hayward, H.E.; Pearson, G.A.; Reeve, R.C.; Richards, A.; et al. Diagnosis and Improvement of Saline and Alkali Soils; Agriculture Handbook 60; USDA: Washington, WA, USA, 1954; p. 160. [Google Scholar]
- Dearing, J.A.; Dann, R.J.L.; Hay, K.; Lees, J.A.; Loveland, P.J.; Maher, B.; O’Grady, K. Frequency-dependent susceptibility measurements of environmental materials. Geophys. J. Int. 1996, 124, 228–240. [Google Scholar] [CrossRef]
- Hay, K.; Dearing, J.; Baban, S.; Loveland, P. A preliminary attempt to identify atmospherically-derived pollution particles in English topsoils from magnetic susceptibility measurements. Phys. Chem. Earth 1997, 22, 207–210. [Google Scholar] [CrossRef]
- Ministry of Environment of the Republic of Poland. Regulation of the Minister of Environment dated 1 September 2016 on assessment procedures for the land surface pollution. J. In Laws 2016; 1395. Available online: http://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20160001395 (accessed on 15 November 2021).
- Sutkowska, K.; Teper, L.; Stania, M. Tracing potential soil contamination in the historical Solvay soda ash plant area, Jaworzno, Southern Poland. Environ. Monit. Assess. 2015, 187, 704. [Google Scholar] [CrossRef] [PubMed]
- Wiatrowska, K.; Komisarek, J.; Marcinek, J.; Kozłowski, M. Evaluation of the soil quality in the vicinity of Inowrocław soda plants. Arch. Environ. Prot. 2018, 44, 58–67. [Google Scholar] [CrossRef]
- Strzyszcz, Z. Hazards to arable soils and forest sites caused by immissions of ferromagnetic minerals. Arch. Environ. Prot. 2004, 30, 101–112. [Google Scholar]
- Dube, A.; Zbytniewski, R.; Kowalkowski, T.; Cukrowska, E.; Buszewski, B. Adsorption and migration of heavy metals in soil. Pol. J. Environ. Stud. 2001, 10, 1–10. [Google Scholar]
- Weng, L.; Temminghoff, E.J.M.; Lofts, S.; Tipping, E.; van Riemsdijk, W.H. Complexation with dissolved organic matter and solubility control of heavy metals in a sandy soil. Environ. Sci. Technol. 2002, 36, 4804–4810. [Google Scholar] [CrossRef]
- Sherene, T. Mobility and transport of heavy metals in polluted soil environment. Biol. Forum—Int. J. 2010, 2, 112–121. [Google Scholar]
- Kadkhodaie, A.; Kelich, S.; Baghbani, A. Effects of salinity levels on heavy metals (Cd, Pb and Ni) adsorption by sunflower and sudangrass plants. Bull. Environ. Pharmacol. Life Sci. 2012, 1, 47–53. [Google Scholar]
- Acosta, J.A.; Jansen, B.; Kalbitz, K.; Faz, A.; Martínez-Martínez, S. Salinity increases mobility of heavy metals in soils. Chemosphere 2011, 85, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Caçador, I.; Vale, C.; Catarino, F. Seasonal variation of Zn, Pb, Cu and Cd concentrations in the root-sediment system of Spartina maritima and Halimione portulacoides from Tagus estuary salt marshes. Mar. Environ. Res. 2000, 49, 279–290. [Google Scholar] [CrossRef]
- Weis, P.; Windham, L.; Burke, D.; Weis, J. Release into the environment of metals by two vascular salt marsh plants. Mar. Environ. Res. 2002, 54, 325–329. [Google Scholar] [CrossRef]
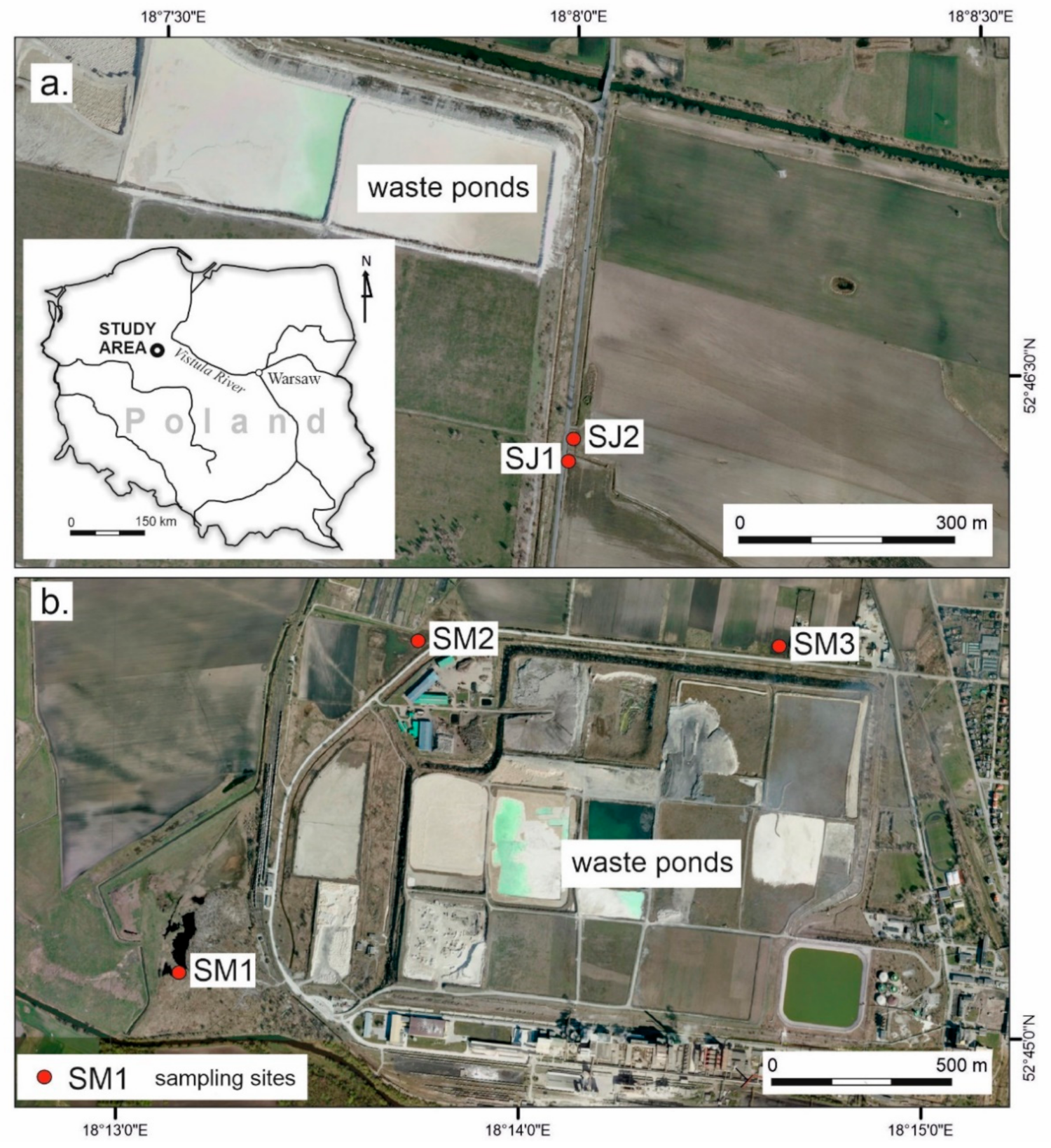


| Site | Coordinates | Location |
|---|---|---|
| SM1 | 52°45′5.671″ N 18°13′9.433″ E | edge of the old peat excavation pond |
| SM2 | 52°45′37.732″ N 18°13′50.672″ E | saline wetland |
| SM3 | 52°45′35.972″ N 18°14′36.626″ E | arable field |
| SJ1 | 52°46′25.490″ N 18°7′59.445″ E | arable field |
| SJ2 | 52°46′26.883″ N 18°7′59.576″ E | edge of the drainage ditch |
| SM1 | SM2 | SM3 | SJ1 | SJ2 | |
|---|---|---|---|---|---|
| Cutover Peatland (Pond) | Drainage Ditch | Arable Field (Ground Water) | Drainage Ditch | Drainage Ditch | |
| pH | 10.2 | 7.9 | 6.6 | 7.4 | 7.5 |
| EC (dS·m−1) | 31.0 | 32.2 | 58.0 | 111 | 123 |
| Cl− (g·dm−3) | 8.1 | 13.2 | 28.9 | 54.2 | 62.0 |
| Na+ (g·dm−3) | 6.1 | 3.0 | 5.9 | 11.0 | 22.0 |
| K+ (g·dm−3) | 0.3 | 0.1 | 0.2 | 0.2 | 0.8 |
| Ca2+ (g·dm−3) | 0.1 | 5.9 | 11.9 | 20.3 | 14.8 |
| Mg2+ (g·dm−3) | 22.8 | 18.5 | 24.2 | 88.4 | 0.1 |
| Mn (mg·dm−3) | 0.1 | 0.1 | 1.0 | 6.8 | <0.1 |
| Fe (mg·dm−3) | 2.1 | 0.4 | 0.2 | 0.2 | 0.2 |
| Co (mg·dm−3) | 0.2 | <0.1 | <0.1 | <0.1 | 0.0 |
| Ni (mg·dm−3) | 0.2 | <0.1 | <0.1 | <0.1 | 0.1 |
| Cu (mg·dm−3) | 0.1 | <0.1 | <0.1 | 0.1 | 0.1 |
| Zn (mg·dm−3) | 0.2 | <0.1 | <0.1 | 0.2 | 0.1 |
| Cd (mg·dm−3) | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Ba (mg·dm−3) | 0.8 | 2.2 | 5.8 | 3.6 | 1.1 |
| Pb (mg·dm−3) | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Cr (mg·dm−3) | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Study Site | Parameter | ϕ > 2 mm | TOC (%) | CaCO3 (%) | pH-H2O | ECe (dS·m−1) | Cle− | SP | Pss |
|---|---|---|---|---|---|---|---|---|---|
| (%) | (g·dm−3) | (%) | (%) | ||||||
| SM (n = 11) | Min | 0 | 1.5 | 2.4 | 7.4 | 9.05 | 3.3 | 27.2 | 0.2 |
| Max | 11 | 15.6 | 26.6 | 10.4 | 64.5 | 32.9 | 41.0 | 1.3 | |
| Mean | 2 a | 6.8 a | 10.7 a | 8.3 a | 33.7 a | 16.0 a | 33.3 a | 0.7 a | |
| SD | 3 | 5.5 | 8.2 | 1.3 | 19.1 | 9.7 | 4.9 | 0.4 | |
| CV (%) | 138 | 81 | 76 | 16 | 57 | 61 | 15 | 60 | |
| SJ (n = 15) | Min | 0 | 0.4 | 3.6 | 7.3 | 20.5 | 16.1 | 31.6 | 0.5 |
| Max | 56 | 8.7 | 54.5 | 7.8 | 110 | 69.0 | 57.0 | 3.6 | |
| Mean | 17 b | 3.9 a | 27.0 b | 7.6 a | 55.6 a | 40.0 b | 47.6 b | 1.7 b | |
| SD | 21 | 2.4 | 16.9 | 0.2 | 30 | 16.7 | 7.5 | 1.0 | |
| CV (%) | 123 | 61 | 63 | 3 | 54 | 42 | 16 | 57 |
| Study Site | Parameter | χ (×10−8 m3·kg−1) | χfd (%) | Mn | Fe | Co | Ni | Cu | Zn | Cd | Ba | Pb | Cr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg·kg−1) | |||||||||||||
| SM (n = 11) | Min | 9.0 | 0.3 | 55.8 | 3900 | 1.5 | 3.2 | 6.4 | 13.2 | 0.1 | 66.5 | 11.7 | 6.1 |
| Max | 205 | 8.1 | 213 | 15240 | 4.3 | 8.2 | 22.8 | 114 | 0.8 | 292 | 104 | 10.6 | |
| Mean | 78.9 a | 2.8 a | 130a | 6900 a | 2.4 a | 5.4 a | 11.9 b | 44.0 a | 0.3 b | 135 a | 38.8 b | 7.5 a | |
| SD | 71.4 | 2.4 | 54.1 | 3970 | 1.0 | 1.9 | 4.9 | 34.4 | 0.3 | 77.8 | 29.3 | 1.4 | |
| CV (%) | 91 | 85 | 42 | 58 | 42 | 35 | 41 | 78 | 76 | 60 | 76 | 19 | |
| SJ (n = 15) | Min | 24.7 | 0.0 | 158 | 4210 | 1.5 | 2.5 | 4.7 | 4.6 | 0.03 | 29.6 | 2.5 | 2.5 |
| Max | 86.6 | 4.3 | 1150 | 36740 | 6.1 | 9.5 | 15.9 | 33.6 | 0.27 | 282 | 31.9 | 21.8 | |
| Mean | 52.2 a | 2.2 a | 594 b | 12,810 b | 3.1 a | 5.5 a | 9.2 a | 16.7 a | 0.1 a | 97.4 a | 14.9 a | 8.7 a | |
| SD | 19.8 | 1.1 | 385 | 9050 | 1.4 | 2.6 | 3.5 | 10.1 | 0.1 | 59.8 | 9.1 | 4.9 | |
| CV (%) | 38 | 53 | 65 | 71 | 45 | 47 | 38 | 61 | 48 | 60 | 61 | 56 | |
| χ | Mn | Fe | Co | Ni | Cu | Zn | Cd | Ba | Pb | Cr | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SM (n = 11) | |||||||||||
| χ | 1.00 | ||||||||||
| Mn | −0.03 | 1.00 | |||||||||
| Fe | 0.55 | −0.01 | 1.00 | ||||||||
| Co | 0.85 * | 0.18 | 0.79 * | 1.00 | |||||||
| Ni | 0.90 * | 0.10 | 0.75 * | 0.92 * | 1.00 | ||||||
| Cu | 0.82 * | 0.26 | 0.76 * | 0.87 * | 0.94 * | 1.00 | |||||
| Zn | 0.64 * | 0.00 | 0.90 * | 0.82 * | 0.76 * | 0.76 * | 1.00 | ||||
| Cd | 0.41 | −0.06 | 0.90 * | 0.70 * | 0.55 | 0.55 | 0.91 * | 1.00 | |||
| Ba | 0.75 * | 0.12 | 0.88 * | 0.88 * | 0.79 * | 0.82 * | 0.95 * | 0.84 * | 1.00 | ||
| Pb | 0.64 * | −0.03 | 0.93 * | 0.81 * | 0.74 * | 0.77 * | 0.96 * | 0.89 * | 0.95 * | 1.00 | |
| Cr | 0.04 | 0.08 | 0.78 * | 0.37 | 0.31 | 0.37 | 0.61 * | 0.79 * | 0.52 | 0.67 * | 1.00 |
| SJ (n = 15) | |||||||||||
| χ | 1.00 | ||||||||||
| Mn | 0.62 * | 1.00 | |||||||||
| Fe | 0.33 | 0.55 | 1.00 | ||||||||
| Co | 0.59 | 0.82 * | 0.60 | 1.00 | |||||||
| Ni | 0.78 * | 0.79 * | 0.22 | 0.57 | 1.00 | ||||||
| Cu | 0.68 * | 0.94 * | 0.50 | 0.79 * | 0.89 * | 1.00 | |||||
| Zn | 0.46 | 0.49 | 0.02 | 0.06 | 0.80 * | 0.62 * | 1.00 | ||||
| Cd | −0.07 | −0.02 | −0.50 | −0.26 | 0.32 | 0.10 | 0.60 | 1.00 | |||
| Ba | 0.39 | 0.56 | 0.77 * | 0.56 | 0.20 | 0.51 | −0.05 | −0.44 | 1.00 | ||
| Pb | 0.52 | 0.48 | 0.13 | 0.08 | 0.81 * | 0.61 * | 0.93 * | 0.44 | −0.01 | 1.00 | |
| Cr | 0.50 | 0.48 | 0.12 | 0.17 | 0.84 * | 0.62 * | 0.90 * | 0.55 | −0.09 | 0.94 * | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łuczak, K.; Pindral, S.; Michalski, A.; Kusza, G.; Ślęzak, E.; Hulisz, P. The Influence of Industrial Waste on the Magnetic Properties of Salt-Affected Soils from Two Soda Ash Manufacturing Sites. Agronomy 2021, 11, 2419. https://doi.org/10.3390/agronomy11122419
Łuczak K, Pindral S, Michalski A, Kusza G, Ślęzak E, Hulisz P. The Influence of Industrial Waste on the Magnetic Properties of Salt-Affected Soils from Two Soda Ash Manufacturing Sites. Agronomy. 2021; 11(12):2419. https://doi.org/10.3390/agronomy11122419
Chicago/Turabian StyleŁuczak, Katarzyna, Sylwia Pindral, Adam Michalski, Grzegorz Kusza, Ewelina Ślęzak, and Piotr Hulisz. 2021. "The Influence of Industrial Waste on the Magnetic Properties of Salt-Affected Soils from Two Soda Ash Manufacturing Sites" Agronomy 11, no. 12: 2419. https://doi.org/10.3390/agronomy11122419
APA StyleŁuczak, K., Pindral, S., Michalski, A., Kusza, G., Ślęzak, E., & Hulisz, P. (2021). The Influence of Industrial Waste on the Magnetic Properties of Salt-Affected Soils from Two Soda Ash Manufacturing Sites. Agronomy, 11(12), 2419. https://doi.org/10.3390/agronomy11122419







