1. Introduction
Soil organic matter depletion over time is one of the critical problems encountered in agricultural lands. Application of organic fertilizers would be the best option to overcome this issue. The applied barn manure, compost, or green manure decomposes very quickly and needs to be applied in every two years. In this case, the required amount of organic fertilizer cannot always be provided. Biochar (B), which can survive in the soil for many years after application depending on the soil conditions, appears as a good alternative [
1]. It was first encountered in the Amazon basin. The use of biochar gained importance with the discovery of Terra Pretra, which is a dark colored, high carbon and organic matter content and a highly fertile soil [
2]. It is thought that biochar obtained by physical and chemical differentiation of plant-based wastes at high temperatures can reduce the washing of nutrients and increase fertilizer use efficiency [
3]. Since the carbon compounds in the produced biochar are resistant to decomposition, they therefore tend to stay in the soil for a long time [
1].
Biochar has a highly porous structure and its macropores provide ventilation to the soil where it is applied, and it creates a living space for microorganisms [
4]. Low amounts of chemical substances in biochar help plant growth by acting as hormones. At the same time, with its physical and chemical properties, biochar supports the growth of rhizobacteria and fungi that help plant growth [
5]. As a result of biochar application in agricultural areas, organic carbon in the soil decomposes more slowly, increasing the total amount of organic matter in the soil. Consequently, it has been stated by many researchers that the application of different biochar materials may have a slowing effect on the global climate. In addition, it has been reported in many published studies that the application of biochar to the soil can reduce greenhouse gas like nitrous oxide (N
2O) emission [
6]. Biochars obtained from different materials used in the soil prevent the washing out of nutrients, increase the KDK, buffer soil pH, and increase the water-holding capacity of the soil [
1,
7,
8,
9]. The increase in soil pH with biochar application limits the mobility of heavy metals [
10,
11].
It has been reported that the effects of plant growth-promoting rhizobacteria (PGPRs) on the development of plants are generally in the form of encouraging the production of phytohormones, promoting the increase in enzymatic activities, increasing nutrient intake, and increasing other mechanisms [
12]. They are also used as biological agents against diseases and pests by increasing plant systemic resistance [
13]. Some researchers have also suggested that the aliphatic carbon compounds found in the humus of the soil are degradation products of extracellular polysaccharide, protein, and chitin, which are released or broken down from microbial cells, and hydrophobic long-chain fatty acids synthesized by microbial cells, and they have stated that these substances could persist in the soil for several hundred years without degradation [
14]. Thus, it has been suggested that an active PGPR community can also contribute to long-term C accumulation [
15]. PGPRs promote resistance to many plant diseases and increase yield significantly by bringing the phosphorus and nitrogen in the soil to the available form that the plant can take [
16]. Previous studies have been conducted on the yield-increasing effect of root bacteria, which stimulate plant growth in many countries of the world, and it has been reported that they directly or indirectly enhance the growth of plants [
17,
18,
19].
By considering the importance of biochar and PGPRs for the agricultural system, the present study aimed to determine the effects of increasing doses of biochar applications on the growth criteria of einkorn wheat grown in PGPR-infused and uninoculated environments and also on some physical properties of the soil and the biofortification of wheat.
3. Results
The averages and variance analysis results of the effects of biochar and PGPR applications on soil properties, development criteria, macroelements, microelements, and heavy metal contents are shown in
Table 2,
Table 3,
Table 4,
Table 5 and
Table 6. The important data for BiocharxPGPR (BxP) interaction are given in
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5 and
Figure 6.
As it can be seen in
Table 2, biochar (B) application had a significant effect on soil pH, salt, lime, and soil organic matter (SOM) contents at the 1% level. In comparison, PGPR applications only affected the pH at the 5% level. Only the effect of BxP interaction on soil pH was determined to be significant. Biochar applications had an effect of 5% and 1% on all growth parameters except the root length and tillering. PGPR applications had an effect of 5% and 1% on all growth parameters except the plant fresh weight and root length. While BxP interaction had an effect only on the root fresh weight and root–stem ratio at a level of 1%, it was found that it did not significantly affect the other growth parameters (
Table 3 and
Table 4).
As can be seen in
Table 5, application of B affected K, Ca, Mg, and Na contents at the 5% and 1% level, while inoculation of PGPR affected P, K, Ca, and Mg contents at the 1% level. BxP interaction only affected phosphorus and sodium contents at the level of 5%. Biochar affected Fe, Cu, Zn, and Ni contents of Einkorneinkorn wheat at 1% level. Its effect on Mn and Pb contents was not determined.
It was determined that PGPR applications had a significant effect on Fe (1%), Cu (1%), Zn (5%), and Ni (1%) contents except for Mn and Pb. While the interaction of BxPhad an effect only on Cu content at the level of 5%, it was determined that it did not affect others (
Table 6).
While the average pH of 7.35 was observed under BioC
0 (0% biochar; control) condition, it decreased with the application of biochar and the minimum pH of 7.11 was determined in BioC
3 (10% biochar) application. PGPR application remarkably increased the soil pH. While the average soil pH without PGPR was determined as 7.19, and with PGPR measured as 7.24 (
Table 7). The changes observed in soil pH in BxP interactions occurred as a slight increase in pH with PGPR applications. The pH was determined as 7.26 in BioC
0 without PGPR, and the value was 7.44 with PGPR. A decrease in soil pH was observed with the addition of biochar to the environment, but it was also determined that PGPR applications partially limited this decrease. Without PGPR(-), BioC
1 (2.5% biochar) and BioC
2 (5.0% biochar) showed the soil pH of 7.23 and 7.16, while with PGPR, the values were 7.25 and 7.18, respectively (
Figure 1).
The application of biochar decreased the lime content in the soil. However, the highest lime content (18.95%) was recorded at BioC
0 condition, and it decreased to 17.40% with BioC
3 (10.0% Biochar) application. Biochar at 10% decreased the lime content by 8.18%. The application of PGPR did not cause a significant decrease in lime content. While non-PGPR (PGPR-) soils were determined as the application of BioC
1, BioC
2, and BioC
3 without PGPR exhibited the lime content of 18.85, 17.59, and 17.29%, and with PGPR, the values were 19.26, 18.21, and 17.50%, respectively (
Table 7).
The electrical conductivity (EC) of soil increased with increasing biochar levels. The minimum EC of 342 µS m
−1 was recorded at BioC
0, and the EC increased to 576, 785, and 933 µS m
−1 in BioC
1, BioC
2, and BioC
3, respectively. The EC increased by 68.4, 129.5, and 172.8% over control at BioC
1, BioC
2, and BioC
3, respectively. Applying PGPR to growing media, a partial decrease in EC was observed especially in BioC
0 and BioC
1 treatments. The EC of control (BioC
0) soil without PGPR was 383 µS m
−1; it decreased to 302 µS m
−1 with PGPR application. Similarly, the treatments BioC
1 and BioC
3 showed the EC of 633 and 917 µS m
−1 without application of PGPR(-), whereas the EC values were 520 and 948 µS m
−1 with the application PGPR(+) under the same treatment, respectively (
Table 7).
Soil organic matter (SOM) content of soils increased with increasing biochar, while the average SOM content of BioC
0 applications was 0.99%, it increased to 2.46, 3.80, and 7.48% in BioC
1, BioC
2, and BioC
3 applications, respectively. These increases were respectively 148.5%, 283.8%, and 655.6% (
Table 7).
It was determined that the plant height (PH) significantly decreased with biochar application compared to control (BioC
0). However, the tallest plant of 52.50 cm was recorded in the control condition, and it decreased to 46.0 cm with BioC
3 application. The rate of reduction was 14.1%. Similarly, PGPR significantly impacted the PH, and PGPR treated plants showed shorter plants (47.42 cm) than non-treated plants (50.33 cm). The average plant height decreased 6.1% due to the application of PGPR (
Table 8).
Plant fresh weight (PFW), plant dry weight (PDW), root fresh weight (RFW), and root dry weight (RDW) increased with the increase in biochar applications to the growing medium. Control (BioC
0) application was determined as 1.894 g, 0.291 g, 1.397 g, and 0.221 g, respectively. The highest values were determined as 2.403 g, 0.425 g, 1.904 g, and 0.293 g at 5% (BioC
2) application, respectively. With the application of 10% biochar (BioC
3), a decrease in these values was determined. While PGPR applications had an increasing effect on root fresh weight and dry weight, they caused a decrease in plant fresh weight. Non-PGPR applied plants PFW, PDW, RFW, and RDW were determined to be 2.220 g, 0.353 g, 1.324 g, and 0.177 g, respectively, while in plants with PGPR were determined to be 1.988 g, 0.357 g, 1.688 g, and 0.289 g, respectively (
Table 8).
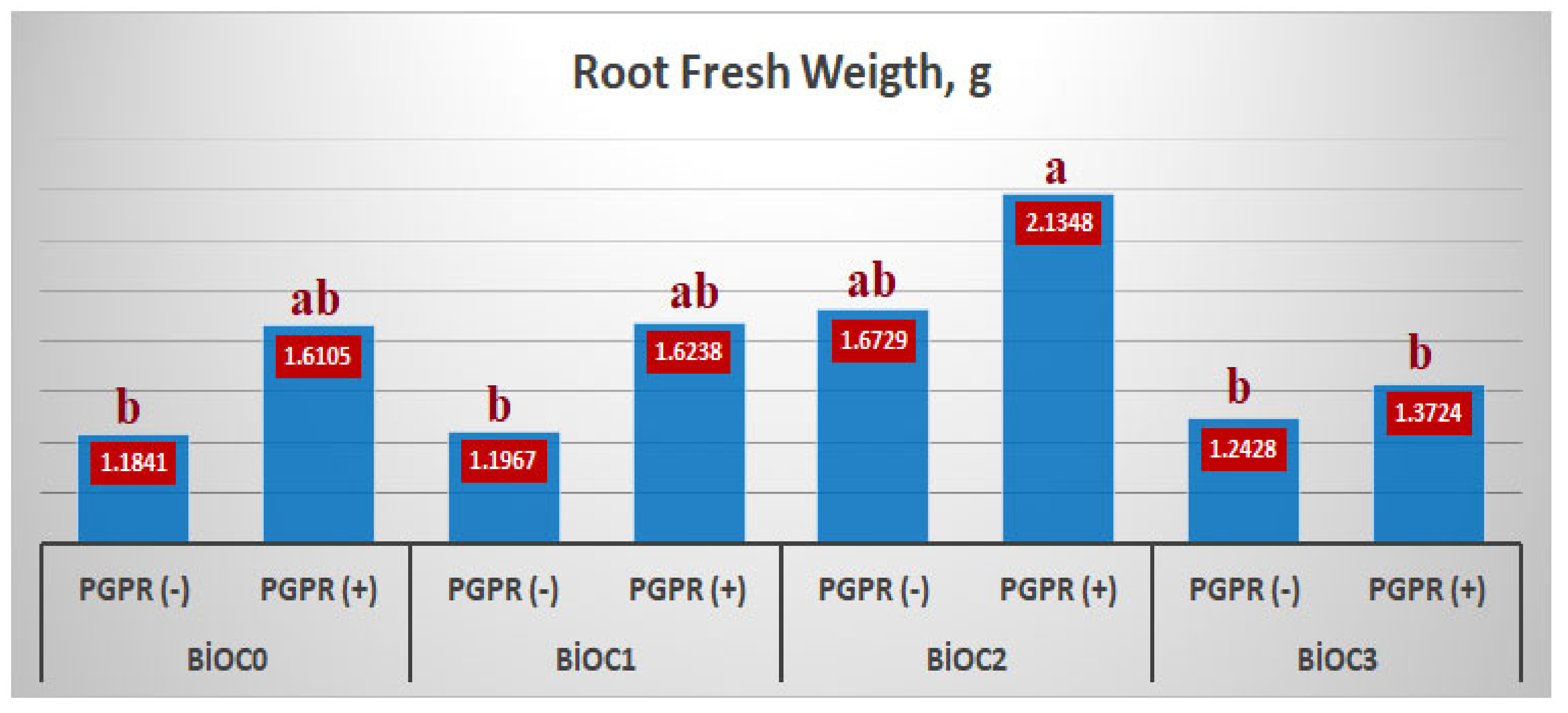
The effect of BxP interaction was significant only on RFW. Maximum root fresh weight was measured as 2.1348 g in the BioC
2×PGPR(+) application. This increased by 32.6% compared to BioC
0xPGPR(+) application and 80.3% compared to BioC
0xPGPR(-) application. BioC
3xPGPR(+) application caused a sharp decrease in RFW according to BioC
0xPGPR(+), BioC
1xPGPR(+), and BioC
2xPGPR(+) applications (
Figure 2).
As it can be seen in
Table 8, tillering of Einkorneinkorn wheat has increased with biochar applications. The number of tillers was 2.69 number in BioC
0, increased with biochar application doses and the highest value was determined as 4.32 number in BioC
3 application. This increase was 60.6% (
Table 8).
While the germination rate of Einkorneinkorn wheat was 3.27 days
−1 in BioC
0 application, it was determined as 4.04 days
−1 in biochar BioC
3 application; a 23.4% increase was achieved. PGPR applications decreased the germination rate. The germination rate was 3.74 days in plants without PGPR, and was determined as 3.58 days with PGPR applications (
Table 8).
Root–shoot ratio (R/S) decreased with biochar application. R/S ratio of 78.3% in BioC
0 decreased to 51.5% with BioC
3 application. This decrease was 52%. The root–shoot ratio has increased with PGPR applications. The root–shoot ratio was determined as 54.3% in pots without PGPR and 80.9% in pots with PGPR applied. This increase was 48.9% (
Table 8).
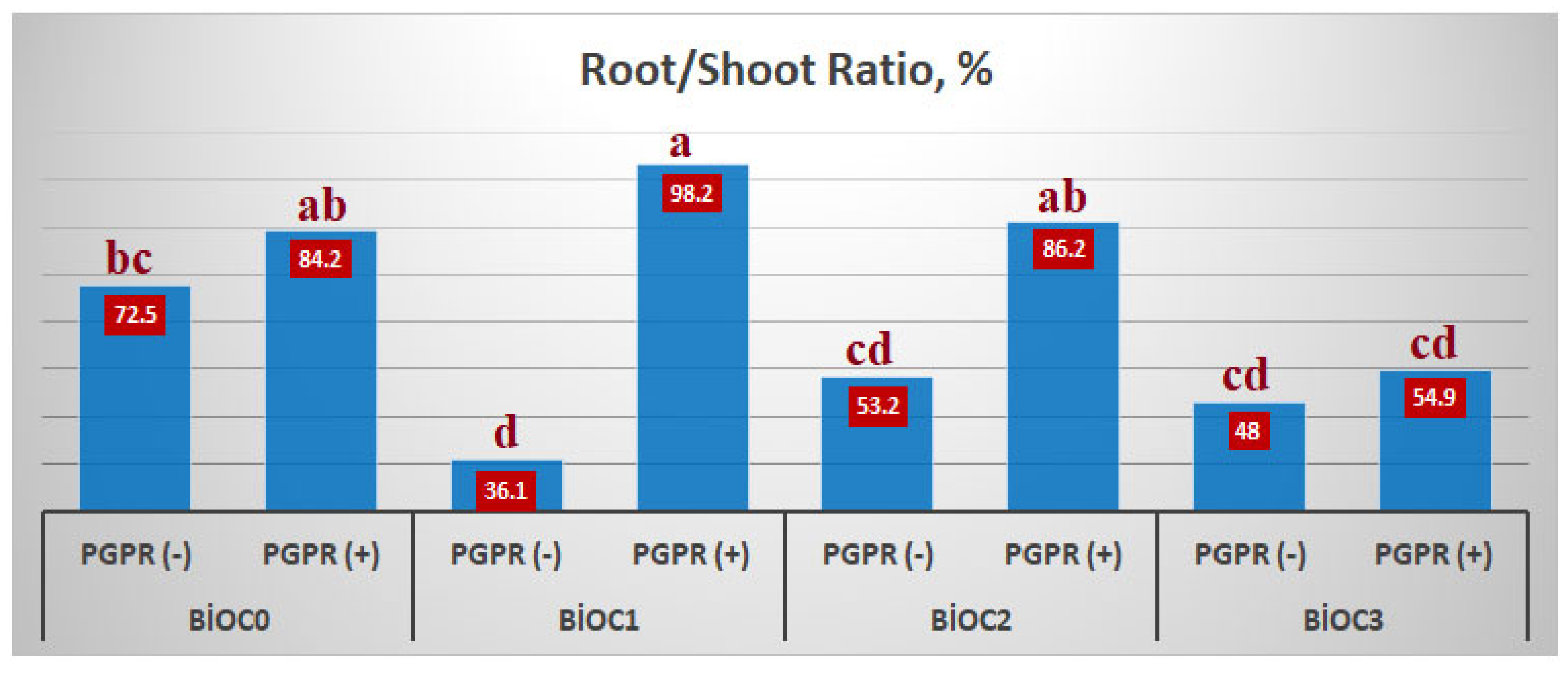
The BxP interaction was found to be statistically significant. The highest R/S ratio was determined in BioC
2xPGPR(+) application, the lowest R/S ratio was determined in BioC
1xPGPR(-) application, 98.2% and 36.1%, respectively (
Figure 3).
Biochar applications on the macroelement contents of einkorn wheat caused an increase in K, Ca, and Na, but decreased P and Mg contents. The highest potassium and sodium contents were determined as 2.450%; 0.2489% in BioC
3 application; 2.363% in BioC
2 application in calcium; and 0.515% and 0.499% in BioC
0 application for phosphorus and magnesium, respectively (
Table 9).
While PGPR application increased potassium, calcium and magnesium contents of einkorn wheat, it decreased in P and Na. The K, Ca, P, Mg, and Na contents of the plants without PGPR were determined as 2.090%, 1.97%, 0.4930%, 0.4204%, and 0.2304%, respectively, while the plants treated with PGPR were 2.496%, 2.368%, 0.4452%, 0.4687%, and 0.2183%, respectively. (
Table 9).
While increasing biochar applications increased einkorn wheat’s K, Ca, and Na contents, it decreased the P and Mg contents. The highest K and Na in einkorn wheat were determined as 2.450% and 0.2489% in BioC
3 application, respectively, while it was determined as 2.363% in BioC
3 application in Ca. The highest values of phosphorus and magnesium were determined as 0.499% and 0.515% in BioC
0 application, respectively. Although PGPR applications increased einkorn wheat’s K, Ca, and Mg contents, it decreased the P and Na contents. Einkorn wheat K, Ca, and Mg contents reached the highest values with PGPR applications, 2.496%, 2.368%, and 0.468%, respectively. Einkorn wheat’s phosphorus and sodium contents gave the highest values in samples without PGPR, at 0.493% and 0.2304%, respectively (
Table 9).
Although BxP interaction caused significant changes in potassium, calcium, and magnesium contents of einkorn wheat, it was not found statistically significant. BxP interaction had a significant effect only on einkorn wheat’s P and Na contents (
Table 5).
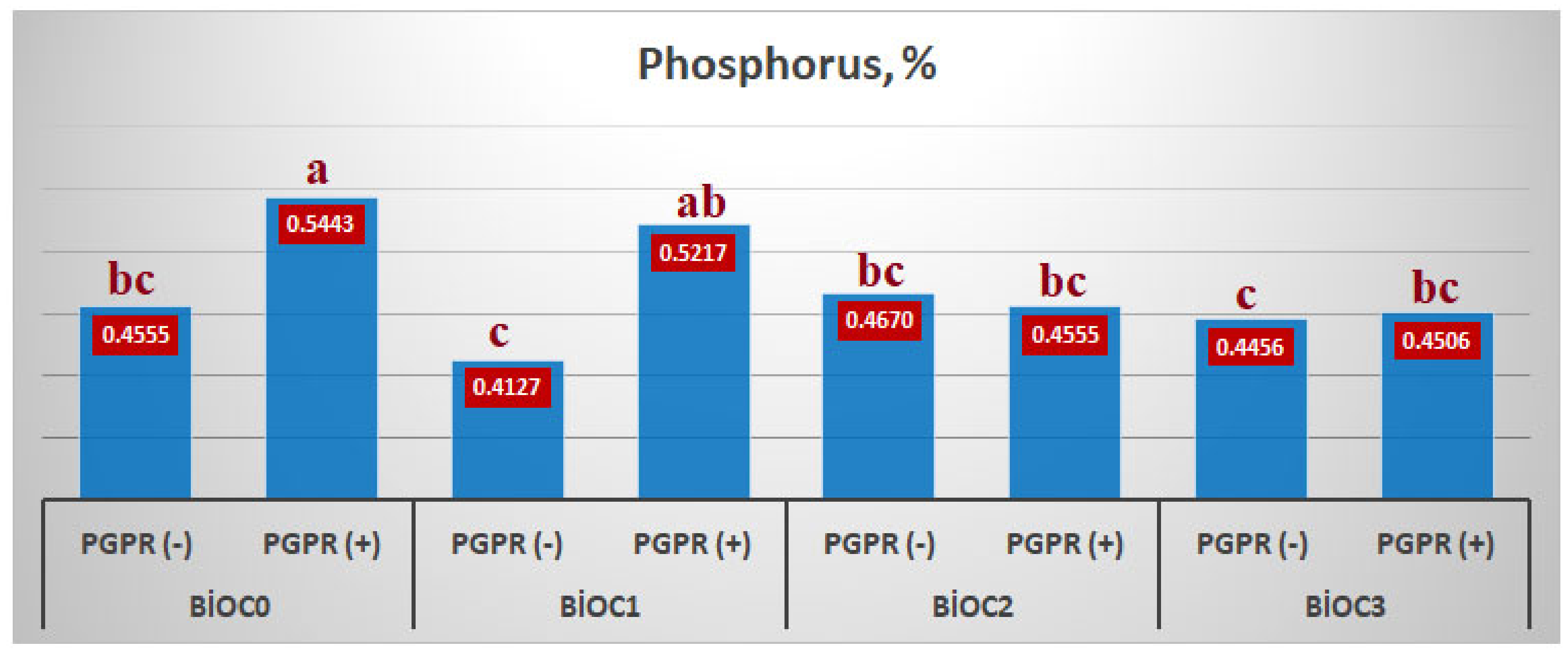
When the effect of BxP interaction on the phosphorus content of einkorn wheat was examined, the highest phosphorus content of einkorn wheat was obtained with BioC
0xPGPR(+) application with 0.5443%. As the application of biochar increases, the effect of PGPRs on phosphorus uptake decreases. As shown in
Figure 1, with the application of 2.5% biochar to the environment, the effect of PGPRs on phosphorus uptake was partially reduced. Whereas, when 5% and 10% biochar is applied to the environment, the effect of PGPRs has completely decreased (
Figure 4).
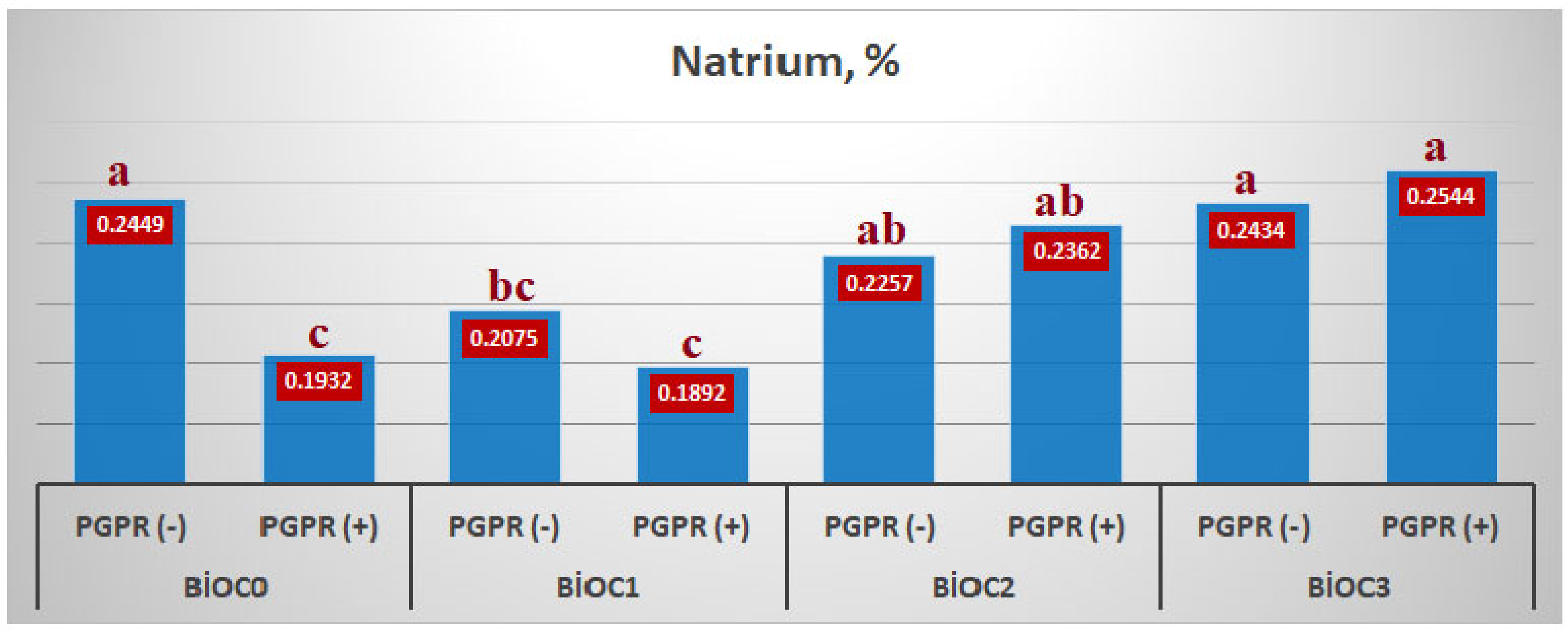
The effect of BxP interaction on the sodium content of einkorn wheat differed whether biochar was applied or not in the environment. In BioC
0 and BioC
1 applications, sodium intake decreased when PGPR was given to the growing medium, while when PGPR was inoculated in BioC
2 and BioC
3 applications, the sodium content of einkorn wheat increased. The lowest sodium content in BioC
1xPGPR(+) application and the highest in BioC
3xPGPR(+) application were determined as 0.1892% and 0.2544%, respectively (
Figure 5).
As shown in
Table 9, the Fe, Cu, Mn, and Zn contents of einkorn wheat increased with the increase in biochar applications in the growing medium. The highest Fe content of einkorn wheat was determined as 491 mg kg
−1 in BioC
2 application. BioC
3 application in Cu, Zn, and Mn was determined as 20.62 mg kg
−1, 240 mg kg
−1, and 152 mg kg
−1, respectively.
When PGPR was applied to einkorn wheat, the content of Fe and Mn increased, while Zn and Cu contents decreased. Fe and Mn content of einkorn wheat without PGPR was 338 mg kg
−1 and 139 mg kg
−1, respectively, while it increased to 441 mg kg
−1 and 144 mg kg
−1 with PGPR application. While zinc and copper contents were 198 mg kg
−1 and 17.15 mg kg
−1 in einkorn wheat samples without PGPR, it decreased to 178 mg kg
−1 and 14.24 mg kg
−1, respectively, in PGPR applied einkorn wheat (
Table 9).
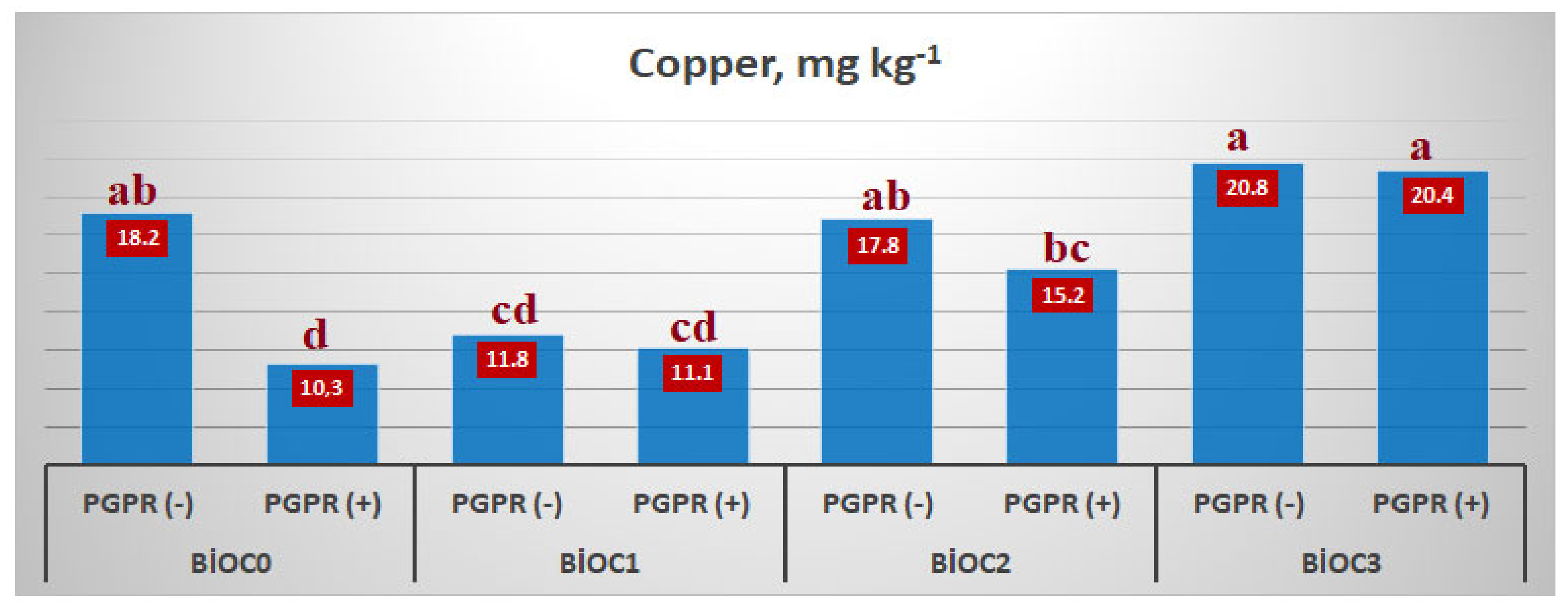
The interaction of BxP only had a significant effect on copper content (
Table 6). The inoculation of einkorn wheat with PGPR caused a decrease in the copper content (10.3 mg kg
−1). The most significant reduction was detected in the BioC
0 application. With the application of biochar to the growing medium, the decrease caused by PGPR was reduced. This was especially evident in BioC
3 application (
Figure 6).
Biochar applications caused an increase in the lead content of einkorn wheat. In control, the lead content application doses of 16.75 mg kg
−1 increased and the highest value was obtained in BioC
1 and BioC
3 applications with 17.63 mg kg
−1 and 17.47 mg kg
−1. However, this increase was not found to be statistically significant. Similarly, although PGPR application caused an increase in lead content, it was determined that this increase was not statistically significant. (
Table 6 and
Table 9).
Nickel content increased with biochar application doses compared to control plants and the highest value was reached in BioC
3 application. The nickel content which was 1.733 mg kg
−1 in the control was determined as 2.033 mg kg
−1, 2.218 mg kg
−1, and 2.450 mg kg
−1 in BioC
1, BioC
2, and BioC
3 applications, respectively. It has been determined that the nickel content of the plant has increased with PGPR applications. The nickel content of 1.950 mg kg
−1 in plants without PGPR was determined as 2.267 mg kg
−1 in plants treated with PGPR (
Table 9).
4. Discussion
The diversity of microorganisms is important for crop growing media, and it varies considerably. Very few species positively affect the plant growth or nutrient content, and the rest are either neutral or harmless [
24]. Among these organisms, the beneficial ones for plants have been studied for many years [
19,
25]. The beneficial organisms, especially those that play a significant role as N-fixer, P-solubilizing. or IAA synthesizer, stand out from the others [
26,
27].
Bacillus subtilis and
Paenibacillus azotofixans organisms used in our study stand out with their phosphate dissolving and nitrogen-fixing properties. Biochar differs from many carbon-sourced organic wastes that can stay in the soil for a long time [
1]; it reduces the removal of nutrients from the root zone by washing, and has a high water-holding capacity [
28]. It has been reported that biochar and PGPR applications cause significant changes in plant growth [
15] and nutrient content [
29]. In a study investigating the effect of biochar applications on PGPR activity, it was reported that biochar applications did not have any negative effects on PGPR [
30,
31].
During this study, a decrease in soil pH was observed with biochar applications, which was 3.4% (
Table 7). Dai et al. [
32] reported in their study that there was 0.5–1.0-unit improvement in soil pH with biochar applications. These improvements in soil pH are due to the oxygen-containing functional groups, carbonates, and silicates available in biochar [
33]. It has been determined that there is a significant increase in soil salinity with the increase in the levels of biochar application. This increase in our study was 172.8% in biochar applied plot compared to control pots (
Table 7). However, this increase remained well below the limit values (<4 dS m
−1) reported for soil salinity.
Similarly, it has been reported that biochar applications increase soil EC, pH, Na, and K contents [
34]. This situation is caused by cations such as K, Ca, Mg, and Na, which are contained in biochar at a high rate [
35,
36,
37,
38]. An increase in organic matter content of soils (655.6%) was observed with biochar application doses compared to the control soil (
Table 7). Da Silvia et al. [
39] reported a linear increase in soil organic carbon content with increasing levels of biochar. It is probably because biochar obtained due to pyrolysis has a high molecular stability to pyrogenic carbon and its oxidation is inhibited [
40]. The half-life of oxidation-resistant carbon-containing materials is estimated to be between 50 and 100 years, depending on particle size and soil conditions [
41].
It is reported that biochar applications with many plant species positively affect plant growth and yield traits [
42,
43,
44]. Our study determined that the plant height decreased (12.38%) with the increase in biochar application. It has been determined that there is a decrease in plant height (5.78%) in PGPR application in the environment (
Table 8). This is probably attributed to the limitation of growth due to increased ambient salinity (
Figure 2). Similarly, Hamzah and Shuhaimi [
45] reported decreased plant height with increasing biochar application in their study. The plant’s fresh and dry weights increased up to 5% biochar application and after that decreased. According to the control, BioC
2 application increased 26.9% and 45.9% in the fresh and dry weights of the plant, respectively. In the case of PGPR application to the environment, the plant fresh weight was lower with the application of PGPR; on the contrary, the plant dry weight increased slightly with the application of PGPR. The highest plant dry weight was obtained in BioC
2xPGPR(+) application and increased by 61.4% compared to the control (BioC
0xPGPR(-)) (
Figure 4). This is especially because PGPRs produce IAA that provides the nutrient content in plants [
46,
47,
48,
49,
50]. Zafar-ul-Hye et al. [
51] reported in their study that combined application of PGPR and biochar showed the highest values of grain, stalk, and biological yield.
The fresh and dry roots were increased up to the BioC
2 dose, while a decrease was determined in the BioC
3 dose. The increase in root fresh and dry weights obtained in 10% application dose of biochar compared to the control was 36.2 and 11.3%, respectively. It was determined that the root fresh and dry weight of PGPR applications increased by 27.5 and 63.4%, respectively, compared to the plants without PGPR (
Table 8). The highest values of the root fresh and dry weight were obtained in BioC
2xPGPR(+), and the increases obtained compared to the control were determined as 80.3% and 112.3%, respectively (
Figure 4). Mohawesh et al. [
52] and Hashmi et al. [
53] reported that biochar increases the fresh and dry weight of both the plant and the root up to a certain dose and decreases in the subsequent doses. It is due to the positive effect of biochar applications on the soil [
54] and the improvements in the plant’s nutrient intake [
55]. In a study, it has been reported that biochar and PGPR together significantly increase the grain and stem yield of rice [
56].
Tillers and germination ratio significantly increased due to the application of biochar. The highest values of tillers and germination ratio were recorded at BioC
3 treatment. R/S ratio decreased with biochar applications and changed by 52.0% compared to control. While PGPR applications caused a decrease in tillers and germination ratio, they caused an increase in the R/S ratio. PGPR applications encouraged root growth (48.9%) compared to plants without PGPR. This was confirmed by the increase in root fresh and dry weights (
Table 8). Lakitan et al. [
57] and Thavanesan and Seran [
58] reported that biochar positively affected tillering and other efficiency criteria. Studies have reported that PGPR applications have an enhancing effect on tillering [
47,
59]. It was determined that the root–stem ratio of the plant decreased with the application of biochar. Biochar application encouraged the body ratio more. The most significant reduction compared to the control was obtained in BioC
3 application with 52.0%. These results showed us that biochar applications are effective on root and supra-root accents, but they encourage a more radical accent development. Similar results have been reported by Olmo et al. [
60], da Silva et al. [
61] and Zhu et al. [
62]. Plants treated with PGPR had a higher root–stem ratio than those without PGPR. In terms of root–stem ratio, a difference of 48.9% was determined between plants not applied PGPR and plants treated with PGPR (
Table 8). This is because PGPRs stimulate phytohormone and secondary metabolite production in the plant and increase nutrient intake [
63,
64,
65,
66].
The 10% BioC
3 increased the K and Na contents of macroelements by 19.1% and 13.6%, respectively, compared to the control, while it caused a decrease in Ca, P, and Mg contents by 12.4%, 13.5%, and 25.9%, respectively (
Table 9). Biochar application increases the growth of plants, cell morphology, production of hormones such as jasmonic acid, auxin, and stoconin, and increases the production of secondary metabolites [
67]. It affects the physiology of plants [
61]. The encouragement may have caused it. In addition, biochar applications are expected to increase the soluble sugar content of the root and leaf [
62], and as a result of the soluble sugar content being associated with photosynthesis, more plant growth and development is achieved [
68].
Compared to plants that did not apply PGPR, the K, Ca, and Mg contents of plants treated with PGPR were increased by 19.4, 19.9, and 11.4%, respectively, while the P and Na contents decreased by 10.8 and 5.6%, respectively. When the effect of BxP interaction is examined, the highest Na and P contents (0.254 and 0.544%, respectively) were recorded at BioC
3xPGPR(+) and BioC
0xPGPR(+) application. However, compared to the control, the Na and P contents increased by 3.9 and 19.5%, respectively (
Table 9;
Figure 5 and
Figure 6). In general, PGPR applications have been reported to increase the amount of other nutrients, especially N and P, in the plant [
47,
60,
69,
70,
71]. Our study determined that PGPR applications caused a decrease in P and Na contents in the biochar applied environment. This situation is probably related to the high surface and intrinsic load area that biochar slowly releases essential nutrients [
72,
73,
74]. It is thought that the loss of O-alkyl carbon in biochars obtained at high temperatures is low, and as a result, it is due to the accumulation of aromatic carbons that provide high P sorption [
75]. At the end of our study, it is thought that the increase in K and Ca content of plant samples is due to the increase in the extractable macroelement content of soils with biochar applications [
76]. The decrease in Mg content may be due to plant roots’ low Mg uptake ability and the competition of Mg
+ with K
+ and NH
4+ during passive absorption [
77]. It has been reported that increases in N, P, and K contents have been achieved in studies where biochar and PGPR applications are performed together [
51,
56].
Iron (Fe) and zinc (Zn) contents increased with biochar applications compared to control. Although some decrease was observed in the Fe content at 10% application dose of biochar, it was determined that the Fe content was above the control group applications. Although copper (Cu) and manganese (Mn) contents showed a decrease in the BioC
1 application dose compared to the control, it increased BioC
2 and BioC
3 compared to the control. The highest values were determined at the BioC
3 application dose and an increase of 44.7 and 4.1% was obtained, respectively, compared to the control (
Table 9). It has been reported that biochar applications have an effect in the form of an increase or decrease in the microelement content of the plant [
78]. Increasing intake of some elements due to biochar applications and decreasing some of them are related to the surface chemistry of the applied biochar [
76]. Biochar’s high CEC and various functional groups (OH−, C–H, –C=O, and C=C) may reduce the bioavailability of metalloids to plants with certain nutrients [
76,
79,
80]. With this feature of biochar, it can be used in the treatment of wastewater [
81] and for the protection of the environment and the remediation of soils [
79].
While PGPR applications increased Fe and Mn content, they caused a decrease in Zn and Cu content. The Fe and Mn contents increased by 30.5 and 3.6%, respectively, and the Zn and Cu contents decreased by 11.2 and 20.4%, respectively (
Table 9). PGPRs provide plants with greater nutrient uptake and disease resistance by producing compounds that stimulate plant growth, alter plant root morphology, and increase root surface area [
82,
83,
84,
85]. PGPRs provide acidification of the environment by increasing the amount of CO
2 in the root area as well as increasing the availability of nutrients by secreting some organic acids [
86]. As a matter of fact, PGPR applications have been reported to increase the nutrient content of the plant [
87,
88,
89].
When the effect of BxP interaction on microelement contents was examined, it was determined that it only had a significant effect on the Cu content. The lowest Cu content was generally determined in BioC
0xPGPR(+) applications. The highest value in Cu was determined in BioC
3xPGPR(-) applications, and it increased by 101.9% compared to BioC
0xPGPR(+) applications (
Figure 6).
Siderophore production of the plant increases with PGPR application and thus, the uptake of some microelements especially increases Fe content [
90,
91]. In our study, a decrease in copper content was observed with the application of PGPR. Similarly, Win et al. [
92] reported that biochar and PGPR application decreased the S, Mn, and Zn contents in rice genotypes and increased the Fe content. This could be due to the increased salinity of the growing medium and the use of Cu in the environment for their own needs, and thus compete with the plant.
Apart from the essential elements for plant development, some elements are also called useful elements, one of which is nickel [
77]. It was determined that the increase observed in nickel (Ni) content with biochar applications was statistically significant. Compared to control (1.733 mg kg
−1) plants, the highest Ni content (2.450 mg kg
−1) was obtained in BioC
3 application and an increase of 41.4% was obtained compared to the control. A significant decrease in the pH of the soil with the application of biochar (
Table 7) may have caused to increase in the Ni content in wheat. Hameeda et al. [
93] reported an increase in the Ni content of the plant with the application of biochar in their study. Contrary to these findings, it has been reported that Ni uptake is reduced with biochar applications, both in Ni-contaminated environments and in environments without Ni contamination [
94].
Lead (Pb) is an element that is highly toxic to living things. This element has a destructive effect on plant growth, root elongation, seed germination, cell division, and transpiration [
95,
96,
97,
98]. Although biochar application increased lead content compared to control, this increase was not found to be statistically significant. A 4.3% increase was obtained in BioC
3 (17.470 mg kg
−1) application compared to control (16.750 mg kg
−1) (
Table 9). The decrease in the intake of some heavy metals due to biochar applications is associated with the change in the physicochemical properties of the soil with the surface chemistry and biochar applications [
2,
99,
100].
Similarly, Sayyadıan et al. [
101] reported that biochar application from different origins decreased the extractable Ni, Pb, and Cd contents of the soil and consequently decreased the Ni, Pb, and Cd contents of both root and root parts of maize [
102]. They also reported that biochar application to the environment contaminated with Pb, Cd, and Cr caused a decrease in the Cd, Pb, and Cr contents of both the soil and the corn plant. In our study, the increase in Pb content, albeit insignificantly, may be due to the decrease in soil pH with the application of biochar (
Table 7).
Root organisms promoting plant growth are used in the phytoremediation of heavy metals to improve the yield and nutrient content of plants [
9,
103]. PGPR application caused an increase in both Pb and Ni content compared to plants where PGPR was not applied. Among these elements, only the increase in Ni content was found to be statistically significant (
Table 6). PGPR applications increased Ni and Ni content by 1.9 and 16.3%, respectively. Ma et al. [
104] reported that PGPB applications cause an increase in the Ni content of the plant. Again, Gullap et al. [
105] reported that PGPR application increase the heavy metal content of both soil and plants. Generally, the bioavailability of heavy metals decreased with the increase in soil pH with biochar application [
106,
107]. Similarly, Zigang et al. [
108] reported that biochar and
Bacillus thuringiensis HC-2 applications reduced the Cd and Pb uptake by increasing soil pH.