Adaptability Mechanisms of Japonica Rice Based on the Comparative Temperature Conditions of Harbin and Qiqihar, Heilongjiang Province of Northeast China
Abstract
:1. Introduction
2. Material and Methods
2.1. Description of Study Area
2.2. Study Plan and Data Source
2.2.1. Crop Data
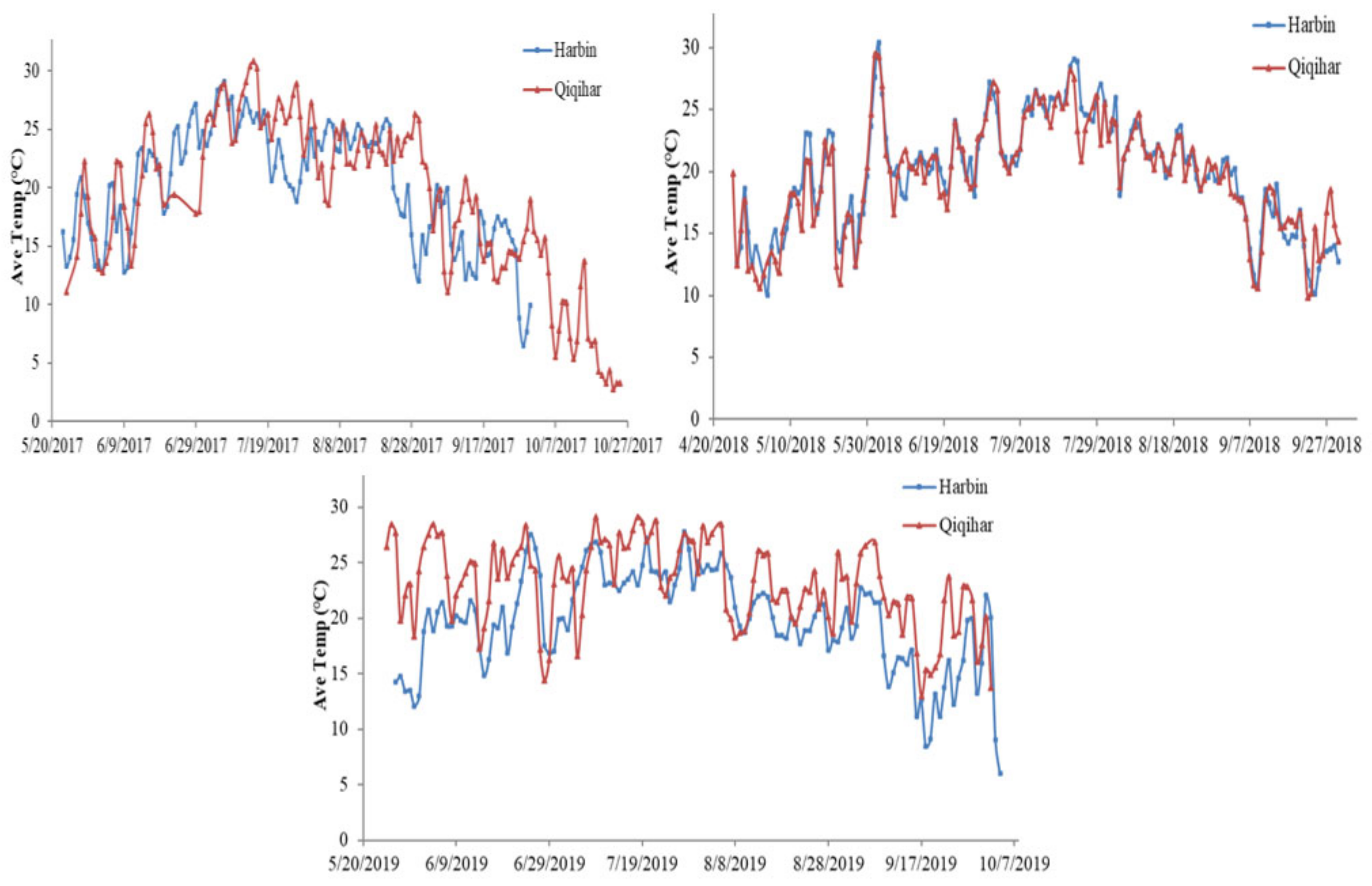
2.2.2. Meteorological Data
2.2.3. Statistical Analysis
3. Results
3.1. Yield Components Data
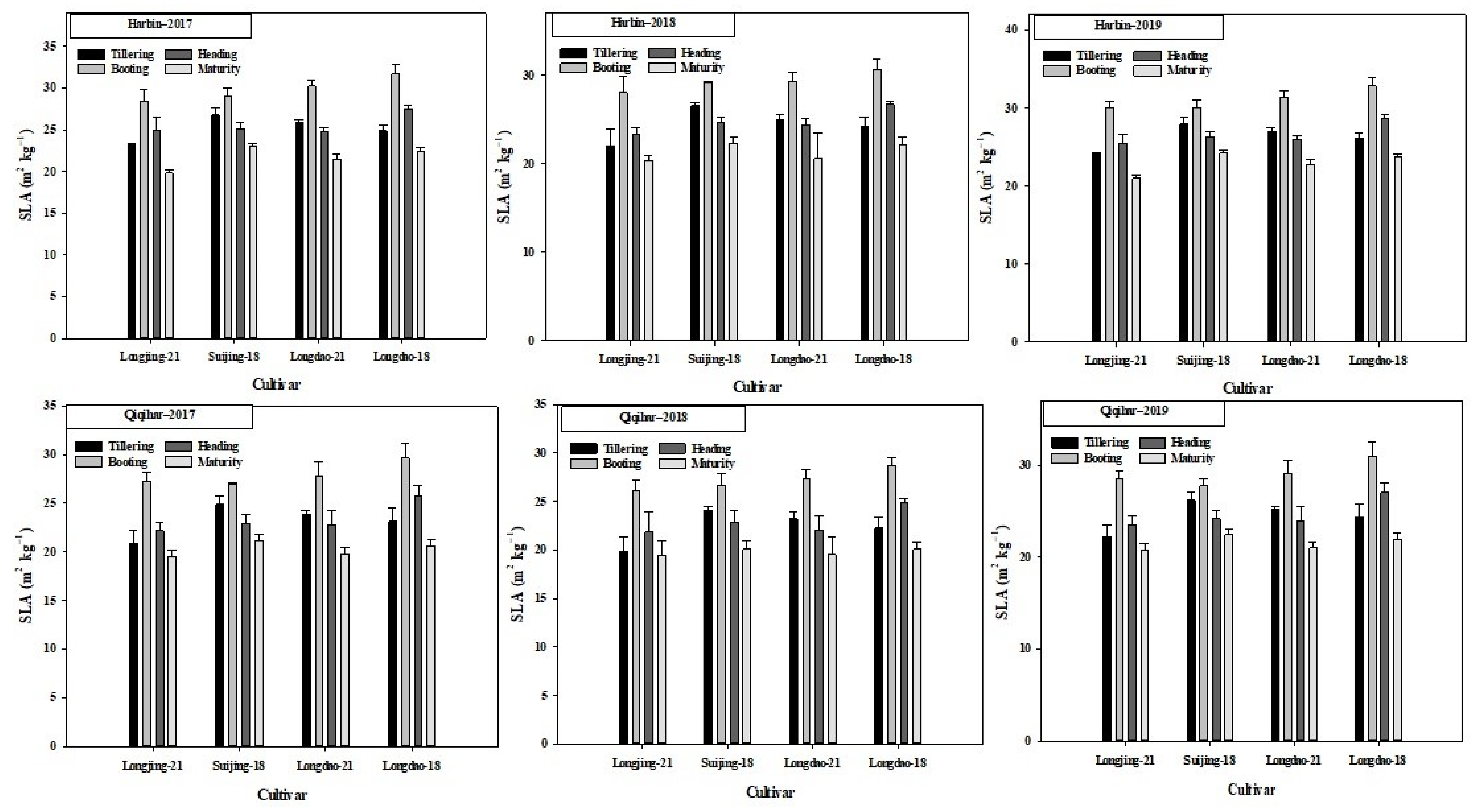
3.2. Crop Growth Rate (CGR) and Specific Leaf Area (SLA)
3.3. Variation in Time of Day of Anthesis and Duration
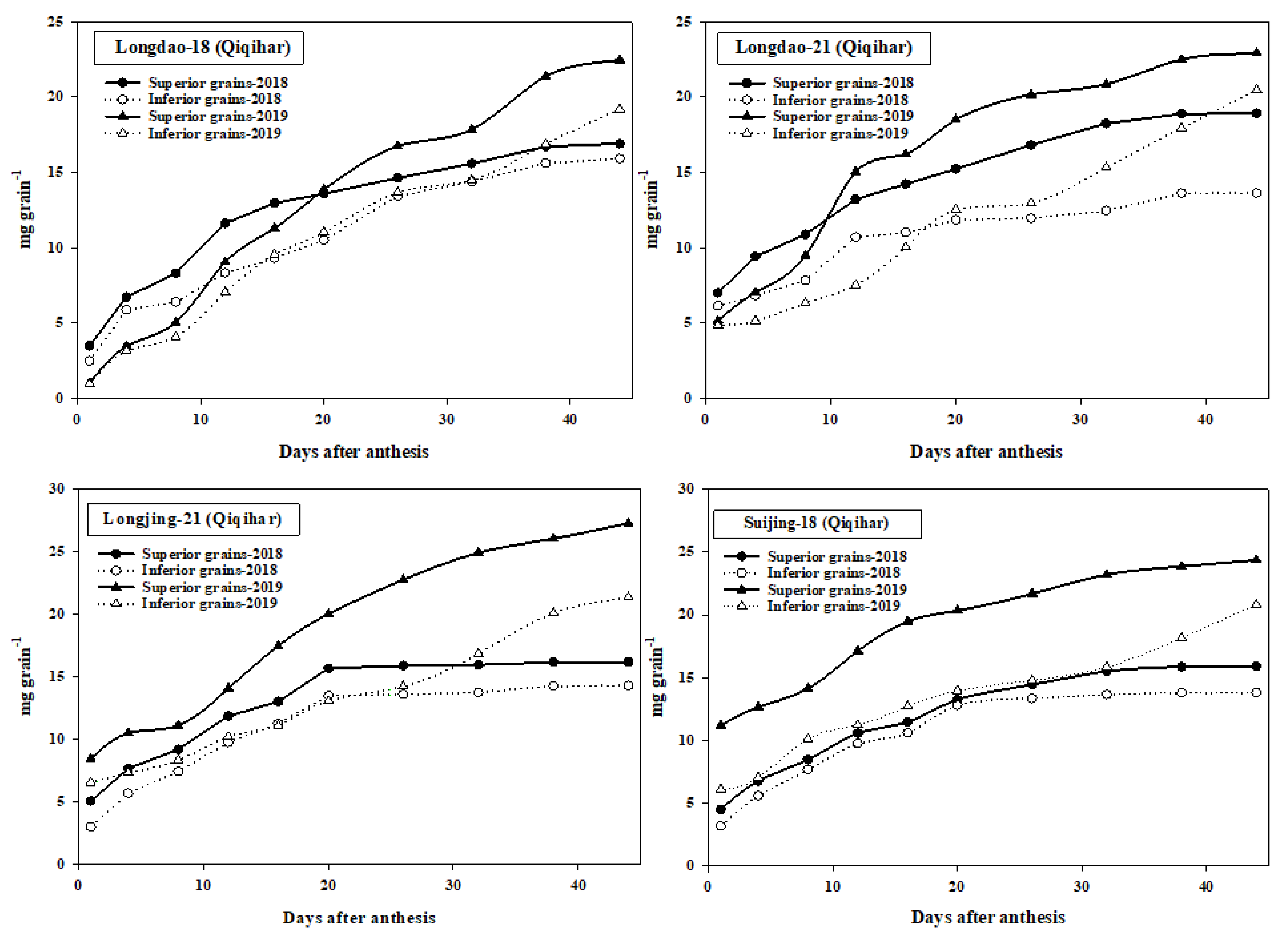
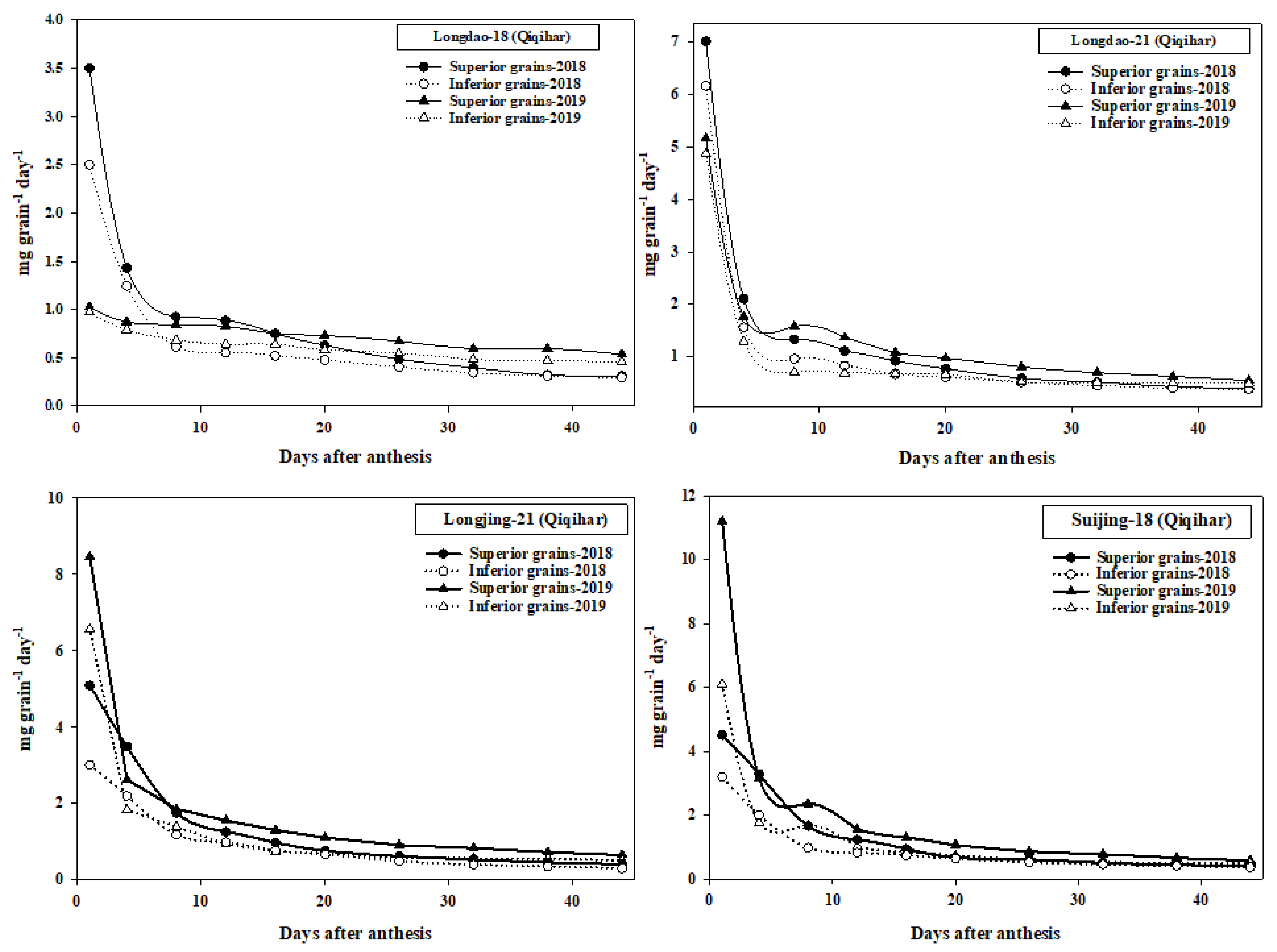
3.4. Grain-Filling Data
3.5. Quality Assessment
3.6. The Relationship between Climatic Variables and Japonica Rice Growth and Yield
4. Discussion
4.1. Yield and Yield Components
4.2. Variation in Time of Day of Anthesis (Hasr)
4.3. Japonica Rice Quality of Superior and Inferior Grains
4.4. Grain-Filling of Superior and Inferior Grains
4.5. Prevailed Environmental Components and Different Growth Phases
4.6. Impacts of Environmental Factors on Specific Leaf Area and Crop Growth Rate
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kaufmann, R.K.; Zhou, L.; Tucker, C.J.; Slayback, D.; Shabanov, N.V.; Myneni, R.B. Ipcc Ar5. Report 2002, 107, 1535. [Google Scholar] [CrossRef]
- Piao, S.; Ciais, P.; Huang, Y.; Shen, Z.; Peng, S.; Li, J.; Fang, J. The impacts of climate change on water resources and agriculture in China. Nature 2010, 467, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, D.; Gao, J.; Deng, W. Land Use/Cover Changes, the Environment and Water Resources in Northeast China. Environ. Manage 2005, 36, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Samol, P.; Umponstira, C.; Klomjek, P.; Thongsanit, P. Responses of Rice Yield and Grain Quality to High Temperature in Open-Top Chamber to Predict Impact of Future Global Warming in Thailand. Aust. J. Crop. Sci. 2015, 9, 886–894. [Google Scholar]
- Masutomi, Y.; Takahashi, K.; Harasawa, H.; Matsuoka, Y. Impact Assessment of Climate Change on Rice Production in Asia in Comprehensive Consideration of Process/Parameter Uncertainty in General Circulation Models. Agric. Ecosyst. Environ. 2009, 131, 281–291. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising Temperatures Reduce Global Wheat Production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. Further Mycotoxin Effects from Climate Change. Food Res. Int. 2011, 44, 2555–2566. [Google Scholar] [CrossRef] [Green Version]
- Wassmann, R.; Jagadish, S.V.K.; Heuer, S.; Ismail, A.; Redona, E.; Serraj, R.; Singh, R.K.; Howell, G.; Pathak, H.; Sumfleth, K. Chapter 2 Climate Change Affecting Rice Production. The Physiological and Agronomic Basis for Possible Adaptation Strategies, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; Volume 101. [Google Scholar] [CrossRef]
- Pramanick, B.; Brahmachari, K.; Kar, S.; Mahapatra, B.S. Can Foliar Application of Seaweed Sap Improve the Quality of Rice Grown under Rice–Potato–Greengram Crop Sequence with Better Efficiency of the System? J. Appl. Phycol. 2020, 32, 3377–3386. [Google Scholar] [CrossRef]
- Li, Z.; Tang, H.; Yang, P.; Wu, W.; Chen, Z.; Zhou, Q.; Zhang, L.; Zou, J. Spatio-Temporal Responses of Cropland Phenophases to Climate Change in Northeast China. J. Geogr. Sci. 2012, 22, 29–45. [Google Scholar] [CrossRef]
- Wang, E.; Martre, P.; Zhao, Z.; Ewert, F.; Maiorano, A.; Rötter, R.P.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. The Uncertainty of Crop Yield Projections Is Reduced by Improved Temperature Response Functions. Nat. Plants 2017, 3, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Andronova, N.G.; Schlesinger, M.E. Causes of Global Temperature Changes during the 19th and 20th Centuries. Geophys. Res. Lett. 2000, 27, 2137–2140. [Google Scholar] [CrossRef]
- Crowley, T.J. Causes of Climate Change over the Past 1000 Years. Science 2000, 289, 270–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.; Eltahir, E.A.B. North China Plain Threatened by Deadly Heatwaves Due to Climate Change and Irrigation. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, T.; Wu, W.B.; Zhou, Q.B.; Yu, Q.Y.; Verburg, P.H.; Yang, P.; Lu, Z.J.; Tang, H.J. Spatio-Temporal Changes in the Rice Planting Area and Their Relationship to Climate Change in Northeast China: A Model-Based Analysis. J. Integr. Agric. 2014, 13, 1575–1585. [Google Scholar] [CrossRef]
- Peng, S.; Tang, Q.; Zou, Y. Current Status and Challenges of Rice Production in China. Plant Prod. Sci. 2009, 12, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Tong, C.; Hall, C.A.S.; Wang, H. Land Use Change in Rice, Wheat and Maize Production in China (1961–1998). Agric. Ecosyst. Environ. 2003, 95, 523–536. [Google Scholar] [CrossRef]
- Kedra, M. Regional Response to Global Warming: Water Temperature Trends in Semi-Natural Mountain River Systems. Water 2020, 12, 283. [Google Scholar] [CrossRef] [Green Version]
- Muller, A.; Jawtusch, J.; Gattinger, A. Mitigating Greenhouse Gases in Agriculture; Brot für die Welt: 2011; Volume 88. Available online: https://orgprints.org/id/eprint/19989/1/gatti.pdf (accessed on 2 October 2021).
- Reay, D.; Sabine, C.; Smith, P.; Hymus, G. Intergovernmental Panel on Climate Change; Fourth Assessment Report Geneva, Switzerland: Inter-Gov-Ernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, W.; You, L.; Zhu, T.; van Vliet, J.; Verburg, P.H.; Liu, Z.; Li, Z.; Yang, P.; Zhou, Q.; et al. Assessing the Harvested Area Gap in China. Agric. Syst. 2017, 153, 212–220. [Google Scholar] [CrossRef]
- Sakamoto, T.; Van Nguyen, N.; Ohno, H.; Ishitsuka, N.; Yokozawa, M. Spatio-Temporal Distribution of Rice Phenology and Cropping Systems in the Mekong Delta with Special Reference to the Seasonal Water Flow of the Mekong and Bassac Rivers. Remote Sens. Environ. 2006, 100, 1–16. [Google Scholar] [CrossRef]
- Li, T.; Hasegawa, T.; Yin, X.; Zhu, Y.; Boote, K.; Adam, M.; Bregaglio, S.; Buis, S.; Confalonieri, R.; Fumoto, T.; et al. Uncertainties in Predicting Rice Yield by Current Crop Models under a Wide Range of Climatic Conditions. Glob. Chang. Biol. 2015, 21, 1328–1341. [Google Scholar] [CrossRef]
- Dong, J.; Xiao, X.; Zhang, G.; Menarguez, M.A.; Choi, C.Y.; Qin, Y.; Luo, P.; Zhang, Y.; Moore, B. Northward Expansion of Paddy Rice in Northeastern Asia during 2000–2014. In Geophysical Research Letters; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2016; pp. 3754–3761. [Google Scholar] [CrossRef] [Green Version]
- Kontgis, C.; Schneider, A.; Ozdogan, M. Mapping Rice Paddy Extent and Intensification in the Vietnamese Mekong River Delta with Dense Time Stacks of Landsat Data. Remote Sens. Environ. 2015, 169, 255–269. [Google Scholar] [CrossRef]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate Trends and Global Crop Production since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Wang, E.; Zhu, Y.; Tang, L. Contrasting Effects of Warming and Autonomous Breeding on Single-Rice Productivity in China. Agric. Ecosyst. Environ. 2012, 149, 20–29. [Google Scholar] [CrossRef]
- Tao, F.; Zhang, Z.; Shi, W.; Liu, Y.; Xiao, D.; Zhang, S.; Zhu, Z.; Wang, M.; Liu, F. Single Rice Growth Period Was Prolonged by Cultivars Shifts, but Yield Was Damaged by Climate Change during 1981-2009 in China, and Late Rice Was Just Opposite. Glob. Chang. Biol. 2013, 19, 3200–3209. [Google Scholar] [CrossRef] [PubMed]
- Iizumi, T.; Ramankutty, N. How Do Weather and Climate Influence Cropping Area and Intensity? In Global Food Security; Elsevier B.V.: Amsterdam, The Netherlands, 2015; pp. 46–50. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Liu, Y. Climate Warming and Land Use Change in Heilongjiang Province, Northeast China. Appl. Geogr. 2011, 31, 476–482. [Google Scholar] [CrossRef]
- Baker, J.T.; Allen, L.H.J. 1 Temperature Treatments as Daytime J Nighttime Bulb Air Temperature. J. Agric. Meteorol. 1993, 48, 575–582. [Google Scholar] [CrossRef]
- Rasul, G.; Chaudhry, Q.Z.; Mahmood, A.; Hyder, K.W. Effect of Temperature Rise on Crop Growth & Productivity. Pakistan J. Meteorol. 2011, 8, 53–62. [Google Scholar]
- Sheehy, J.E.; Mitchell, P.L.; Ferrer, A.B. Decline in Rice Grain Yields with Temperature: Models and Correlations Can Give Different Estimates. Field Crop. Res. 2006, 98, 151–156. [Google Scholar] [CrossRef]
- Krishnan, P.; Swain, D.K.; Chandra Bhaskar, B.; Nayak, S.K.; Dash, R.N. Impact of Elevated CO2 and Temperature on Rice Yield and Methods of Adaptation as Evaluated by Crop Simulation Studies. Agric. Ecosyst. Environ. 2007, 122, 233–242. [Google Scholar] [CrossRef]
- De Souza, N.M.; Marschalek, R.; Sangoi, L.; Weber, F.S. Spikelet Sterility in Rice Genotypes Affected by Temperature at Microsporogenesis. Rev. Bras. Eng. Agric. Ambient. 2017, 21, 817–821. [Google Scholar] [CrossRef] [Green Version]
- Counce, P.A.; Keisling, T.C.; Mitchell, A.J. A Uniform, Objective, and Adaptive System for Expressing Rice Development. Crop Sci. 2000, 40, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Samejima, H.; Kikuta, M.; Katura, K.; Menge, D.; Gichuhi, E.; Wainaina, C.; Kimani, J.; Inukai, Y.; Yamauchi, A.; Makihara, D. A Method for Evaluating Cold Tolerance in Rice during Reproductive Growth Stages under Natural Low-Temperature Conditions in Tropical Highlands in Kenya. Plant Prod. Sci. 2020, 23, 466–476. [Google Scholar] [CrossRef]
- Farrell, T.C.; Fox, K.M.; Williams, R.L.; Fukai, S. Genotypic Variation for Cold Tolerance during Reproductive Development in Rice: Screening with Cold Air and Cold Water. Field Crop. Res. 2006, 98, 178–194. [Google Scholar] [CrossRef]
- Mamun, E.; Alfred, S.; Cantrill, L.; Overall, R.; Sutton, B. Effects of Chilling on Male Gametophyte Development in Rice. Cell Biol. Int. 2006, 30, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.; Lin, S.; Jones, D.; Rutger, J. Cool Night Temperatures Cause Sterility in Rice. Calif. Agric. 1974, 28, 12–14. [Google Scholar]
- Zeng, Y.; Zhang, Y.; Xiang, J.; Uphoff, N.T.; Pan, X.; Zhu, D. Effects of Low Temperature Stress on Spikelet-Related Parameters during Anthesis in Indica–Japonica Hybrid Rice. Front. Plant Sci. 2017, 8, 1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Oort, P.A.J.; Saito, K.; Zwart, S.J.; Shrestha, S. A Simple Model for Simulating Heat Induced Sterility in Rice as a Function of Flowering Time and Transpirational Cooling. Field Crop. Res. 2014, 156, 303–312. [Google Scholar] [CrossRef]
- Wassmann, R.; Jagadish, S.V.K.; Sumfleth, K.; Pathak, H.; Howell, G.; Ismail, A.; Heuer, S. Regional Vulnerability of Climate Change Impacts on Asian Rice Production and Scope for Adaptation. Adv. Agron. 2009, 102, 91–133. [Google Scholar]
- Maruyama, D.; Hamamura, Y.; Takeuchi, H.; Susaki, D.; Nishimaki, M.; Kurihara, D.; Kasahara, R.D.; Higashiyama, T. Independent Control by Each Female Gamete Prevents the Attraction of Multiple Pollen Tubes. Dev. Cell 2013, 25, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Zhu, Y.; Hannaway, D.; Meng, Y.; Liu, L.; Chen, L.; Cao, W. RiceGrow: A Rice Growth and Productivity Model. NJAS Wageningen J. Life Sci. 2009, 57, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Peng, S.B. Yield Potential and Nitrogen Use Efficiency of China’s Super Rice. J. Integr. Agric. 2017, 16, 1000–1008. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, J. Grain-Filling Problem in “super” Rice. J. Exp. Bot. 2010, 61, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Ju, C.X.; Wang, Z.Q.; Zhang, H.; Liu, L.J.; Yang, J.C.; Zhang, J.H. Grain Yield and Water Use Efficiency of Super Rice under Soil Water Deficit and Alternate Wetting and Drying Irrigation. J. Integr. Agric. 2017, 16, 1028–1043. [Google Scholar] [CrossRef]
- Tian, X.H.; Matsui, T.; Li, S.H.; Lin, J.C. High Temperature Stress on Rice Anthesis: Research Progress and Prospects. Chin. J. Appl. Ecol. 2007, 18, 2632–2636. [Google Scholar]
- Krishnan, P.; Ramakrishnan, B.; Reddy, K.R.; Reddy, V.R. High-Temperature Effects on Rice Growth, Yield, and Grain Quality, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 111. [Google Scholar] [CrossRef]
- Oh-e, I.; Saitoh, K.; Kuroda, T. Effects of High Temperature on Growth, Yield and Dry-Matter Production of Rice Grown in the Paddy Field. Plant Prod. Sci. 2007, 10, 412–422. [Google Scholar] [CrossRef]
- Kobata, T.; Uemuki, N. High Temperatures during the Grain-Filling Period Do Not Reduce the Potential Grain Dry Matter Increase of Rice. Agron. J. 2004, 96, 406. [Google Scholar] [CrossRef]
- Fábián, A.; Sáfrán, E.; Szabó-Eitel, G.; Barnabás, B.; Jäger, K. Stigma Functionality and Fertility Are Reduced by Heat and Drought Co-Stress in Wheat. Front. Plant Sci. 2019, 10, 244. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The Effect of Drought and Heat Stress on Reproductive Processes in Cereals. Plant. Cell Environ. 2007, 31, 11–38. [Google Scholar] [CrossRef]
- Alghabari, F.; Ihsan, M.Z. Effects of Drought Stress on Growth, Grain Filling Duration, Yield and Quality Attributes of Barley (Hordeum vulgare L.). Bangladesh J. Bot. 2018, 47, 421–428. [Google Scholar] [CrossRef]
- Mohanty, S.; Wassmann, R.; Nelson, A.; Moya, P.; Jagadish, S. Rice and Climate Change: Significance for Food Security and Vulnerability. IRRI Discuss. Pap. Ser. 2013, 49, 1–14. [Google Scholar]
- Challinor, A.J.; Wheeler, T.R.; Craufurd, P.Q.; Ferro, C.A.T.; Stephenson, D.B. Adaptation of Crops to Climate Change through Genotypic Responses to Mean and Extreme Temperatures. Agric. Ecosyst. Environ. 2007, 119, 190–204. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Cao, X.; Luo, Y. Growth analysis on the process of grain filling in rice. Acta Agron. Sin. 1988, 14, 182–193. [Google Scholar]
- Meehl, G.A.; Stocker, T.F.; Collins, W.D.; Friedlingstein, P.; Gaye, T.; Gregory, J.M.; Kitoh, A.; Knutti, R.; Murphy, J.M.; Noda, A.; et al. Global Climate Projections; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Rang, Z.W.; Jagadish, S.V.K.; Zhou, Q.M.; Craufurd, P.Q.; Heuer, S. Effect of High Temperature and Water Stress on Pollen Germination and Spikelet Fertility in Rice. Environ. Exp. Bot. 2010, 70, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Kocmánková, E.; Trnka, M.; Juroch, J.; Dubrovský, M.; Semerádová, D.; Možný, M.; Žalud, Z. Impact of Climate Change on the Occurrence and Activity of Harmful Organisms. Plant Protect. Sci. 2009, 45, S48. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, S.; Asch, F.; Dingkuhn, M.; Becker, M. Cropping Calendar Options for Rice–Wheat Production Systems at High-Altitudes. Field Crop. Res. 2011, 121, 158–167. [Google Scholar] [CrossRef]
- Acuña, T.L.B.; Lafitte, H.R.; Wade, L.J. Genotype × Environment Interactions for Grain Yield of Upland Rice Backcross Lines in Diverse Hydrological Environments. Field Crop. Res. 2008, 108, 117–125. [Google Scholar] [CrossRef]
- Peng, S.; Bouman, B.; Visperas, R.M.; Castañeda, A.; Nie, L.; Park, H.K. Comparison between Aerobic and Flooded Rice in the Tropics: Agronomic Performance in an Eight-Season Experiment. Field Crop. Res. 2006, 2–3, 252–259. [Google Scholar] [CrossRef]
- Bajracharya, J.; Rana, R.B.; Gauchan, D.; Sthapit, B.R.; Jarvis, D.I.; Witcombe, J.R. Rice Landrace Diversity in Nepal. Socio-Economic and Ecological Factors Determining Rice Landrace Diversity in Three Agro-Ecozones of Nepal Based on Farm Surveys. Genet. Resour. Crop Evol. 2010, 57, 1013–1022. [Google Scholar] [CrossRef] [Green Version]
- Ao, H.; Peng, S.; Zou, Y.; Tang, Q.; Visperas, R.M. Reduction of Unproductive Tillers Did Not Increase the Grain Yield of Irrigated Rice. Field Crop. Res. 2010, 116, 108–115. [Google Scholar] [CrossRef]
- Moradpour, S.; Koohi, R.; Babaei, M.; Khorshidi, M.G. Effect of Planting Date and Planting Density on Rice Yield and Growth Analysis (Fajr Variety). Int. J. Agric. Crop Sci. 2013, 5, 267. [Google Scholar]
- Zhu, J.; Zhou, Y.; Liu, Y.; Wang, Z.; Tang, Z.; Yi, C.; Tang, S.; Gu, M.; Liang, G. Fine Mapping of a Major QTL Controlling Panicle Number in Rice. Mol. Breed. 2011, 27, 171–180. [Google Scholar] [CrossRef]
- Akinwale, M.G.; Gregorio, G.; Nwilene, F.; Akinyele, B.O.; Ogunbayo, S.A.; Odiyi, A.C. Heritability and Correlation Coefficient Analysis for Yield and Its Components in Rice (Oryza sativa L.). Afr. J. Plant Sci. 2011, 5, 207–212. [Google Scholar]
- Kovi, M.R.; Bai, X.; Mao, D.; Xing, Y. Impact of Seasonal Changes on Spikelets per Panicle, Panicle Length and Plant Height in Rice (Oryza sativa L.). Euphytica 2011, 179, 319–331. [Google Scholar] [CrossRef]
- Matsui, T.; Omasa, K.; Horie, T. Comparison between Anthers of Two Rice (Oryza saliva L.) Cultivars with Tolerance to High Temperatures at Flowering or Susceptibility. Plant Prod. Sci. 2001, 4, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Jagadish, S.V.K.; Craufurd, P.Q.; Wheeler, T.R. Phenotyping Parents of Mapping Populations of Rice for Heat Tolerance during Anthesis. Crop Sci. 2008, 48, 1140. [Google Scholar] [CrossRef]
- Kobayasi, K.; Matsui, T.; Yoshimoto, M.; Hasegawa, T. Effects of Temperature, Solar Radiation, and Vapor-Pressure Deficit on Flower Opening Time in Rice. Plant Prod. Sci. 2010, 13, 21–28. [Google Scholar] [CrossRef]
- Jagadish, S.; Craufurd, P.; Wheeler, T. High Temperature Stress and Spikelet Fertility in Rice (Oryza sativa L.). J. Exp. Bot. 2007, 58, 1627–1635. [Google Scholar] [CrossRef] [Green Version]
- Cooper, N.T.W.; Siebenmorgen, T.J.; Counce, P.A. Effects of Nighttime Temperature During Kernel Development on Rice Physicochemical Properties. Cereal Chem. J. 2008, 85, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Lisle, A.J.; Martin, M.; Fitzgerald, M.A. Chalky and Translucent Rice Grains Differ in Starch Composition and Structure and Cooking Properties. Cereal Chem. J. 2000, 77, 627–632. [Google Scholar] [CrossRef]
- Ambardekar, A.A.; Siebenmorgen, T.J.; Counce, P.A.; Lanning, S.B.; Mauromoustakos, A. Impact of Field-Scale Nighttime Air Temperatures during Kernel Development on Rice Milling Quality. Field Crop. Res. 2011, 122, 179–185. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J. Grain Filling of Cereals under Soil Drying. New Phytol. 2006, 169, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, J.; Wang, Z.; Liu, K.; Wang, P. Post-Anthesis Development of Inferior and Superior Spikelets in Rice in Relation to Abscisic Acid and Ethylene. J. Exp. Bot. 2006, 57, 149–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.J.; Li, C.Y.; Lin, S.K.; Yang, F.H.; Huang, J.J.; Liu, Y.H.; Lur, H.S. Influence of High Temperature during Grain Filling on the Accumulation of Storage Proteins and Grain Quality in Rice (Oryza sativa L.). J. Agric. Food Chem. 2010, 58, 10545–10552. [Google Scholar] [CrossRef] [PubMed]
- Asaoka, M.; Okuno, K.; Fuwa, H. Effect of Environmental Temperature at the Milky Stage on Amylose Content and Fine Structure of Amylopectin of Waxy and Nonwaxy Endosperm Starches of Rice (Oryza sativa L.). Agric. Biol. Chem. 1985, 49, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.; Maekawa, M.; Tetlow, I.J. Effects of Low Temperature on Grain Filling, Amylose Content, and Activity of Starch Biosynthesis Enzymes in Endosperm of Basmati Rice. Aust. J. Agric. Res. 2008, 59, 599. [Google Scholar] [CrossRef]
- Counce, P.A.; Bryant, R.J.; Bergman, C.J.; Bautista, R.C.; Wang, Y.-J.; Siebenmorgen, T.J.; Moldenhauer, K.A.K.; Meullenet, J.-F.C. Rice Milling Quality, Grain Dimensions, and Starch Branching as Affected by High Night Temperatures. Cereal Chem. J. 2005, 82, 645–648. [Google Scholar] [CrossRef] [Green Version]
- Ali, F.; Waters, D.L.E.; Ovenden, B.; Bundock, P.; Raymond, C.A.; Rose, T.J. Australian Rice Varieties Vary in Grain Yield Response to Heat Stress during Reproductive and Grain Filling Stages. J. Agron. Crop Sci. 2019, 205, 179–187. [Google Scholar] [CrossRef]
- Tsukaguchi, T.; Iida, Y. Effects of Assimilate Supply and High Temperature during Grain-Filling Period on the Occurrence of Various Types of Chalky Kernels in Rice Plants (Oryza sativa L.). Plant Prod. Sci. 2008, 11, 203–210. [Google Scholar] [CrossRef]
- Yoshida, S.; Hara, T.; Hara, T. Effects of Air Temperature and Light on Grain Filling of an Indica and a Japonica Rice (Oryza sativa L.) under Controlled Environmental Conditions. Soil Sci. Plant Nutr. 1977, 23, 93–107. [Google Scholar] [CrossRef]
- Shah, F.; Huang, J.; Cui, K.; Nie, L.; Shah, T.; Chen, C.; Wang, K. Impact of High-Temperature Stress on Rice Plant and Its Traits Related to Tolerance. J. Agric. Sci. 2011, 149, 545–556. [Google Scholar] [CrossRef]
- Xie, X.; Li, B.; Li, Y.; Shen, S. High Temperature Harm at Flowering in Yangtze River Basin in Recent 55 Years. Jiangsu J. Agric. Sci. 2009, 25, 28–32. [Google Scholar]
- Yang, L.; Wang, Y.; Kobayashi, K.; Zhu, J.; Huang, J.; Yang, H.; Wang, Y.; Dong, G.; Liu, G.; Han, Y.; et al. Seasonal Changes in the Effects of Free-Air CO 2 Enrichment (FACE) on Growth, Morphology and Physiology of Rice Root at Three Levels of Nitrogen Fertilization. Glob. Chang. Biol. 2008, 14, 1844–1853. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z.; Zhang, T.; Fahad, S.; Cui, K.; Nie, L.; Peng, S.; Huang, J. The Effect of Season-Long Temperature Increases on Rice Cultivars Grown in the Central and Southern Regions of China. Front. Plant Sci. 2017, 8, 1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, S.; Shiratsuchi, H.; Takahashi, J.I.; Fujita, K. Effect of High Temperature on Grain Ripening in Rice Plants—Analysis of the Effects of High Night and High Day Temperatures Applied to the Panicle and Other Parts of the Plant. Jpn. J. Crop Sci. 2004, 73, 77–83. [Google Scholar] [CrossRef]
- Tardieu, F. Plant Response to Environmental Conditions: Assessing Potential Production, Water Demand, and Negative Effects of Water Deficit. Front. Physiol. 2013, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghaee, A.; Moradi, F.; Zare-Maivan, H.; Zarinkamar, F.; Irandoost, H.P.; Sharifi, P. Physiological responses of two rice (Oryza sativa L.) genotypes to chilling stress at seedling stage. African J. Biotechnol. 2011, 10, 7617–7621. [Google Scholar]
- Nagai, T.; Makino, A. Differences Between Rice and Wheat in Temperature Responses of Photosynthesis and Plant Growth. Plant Cell Physiol. 2009, 50, 744–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitman, A.J.; Narisma, G.T.; Pielke, R.A.; Holbrook, N.J. Impact of Land Cover Change on the Climate of Southwest Western Australia. J. Geophys. Res. 2004, 109, D18109. [Google Scholar] [CrossRef] [Green Version]
- Graf, A.; Schlereth, A.; Stitt, M.; Smith, A.M. Circadian Control of Carbohydrate Availability for Growth in Arabidopsis Plants at Night. Proc. Natl. Acad. Sci. USA 2010, 107, 9458–9463. [Google Scholar] [CrossRef] [Green Version]
- Peraudeau, S.; Roques, S.O.; Quiñones, C.; Fabre, D.; Van Rie, J.; Ouwerkerk, P.B.F.; Jagadish, K.S.V.; Dingkuhn, M.; Lafarge, T. Increase in Night Temperature in Rice Enhances Respiration Rate without Significant Impact on Biomass Accumulation. Field Crop. Res. 2015, 171, 67–78. [Google Scholar] [CrossRef]
- Stuerz, S.; Sow, A.; Muller, B.; Manneh, B.; Asch, F. Leaf Area Development in Response to Meristem Temperature and Irrigation System in Lowland Rice. Field Crop. Res. 2014, 163, 74–80. [Google Scholar] [CrossRef]
- Stuerz, S.; Sow, A.; Muller, B.; Manneh, B.; Asch, F. Canopy Microclimate and Gas-Exchange in Response to Irrigation System in Lowland Rice in the Sahel. Field Crop. Res. 2014, 163, 64–73. [Google Scholar] [CrossRef]
- Hirai, G.I.; Okmura, T.; Takeuchi, S.; Tanaka, O.; Chujo, H. Studies on the Effect of the Relative Humidity of the Atmosphere on the Growth and Physiology of Rice Plants: Effects of Relative Humidity during the Light and Dark Periods on the Growth. Plant Prod. Sci. 2000, 3, 129–133. [Google Scholar] [CrossRef]
- Kuwagata, T.; Ishikawa-Sakurai, J.; Hayashi, H.; Nagasuga, K.; Fukushi, K.; Ahamed, A.; Takasugi, K.; Katsuhara, M.; Murai-Hatano, M. Influence of Low Air Humidity and Low Root Temperature on Water Uptake, Growth and Aquaporin Expression in Rice Plants. Plant Cell Physiol. 2012, 53, 1418–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peraudeau, S.; Lafarge, T.; Roques, S.; Quiñones, C.O.; Clement-Vidal, A.; Ouwerkerk, P.B.F.; Van Rie, J.; Fabre, D.; Jagadish, K.S.V.; Dingkuhn, M. Effect of Carbohydrates and Night Temperature on Night Respiration in Rice. J. Exp. Bot. 2015, 66, 3931–3944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunoj, V.S.J.; Shroyer, K.J.; Jagadish, S.V.K.; Prasad, P.V.V. Diurnal Temperature Amplitude Alters Physiological and Growth Response of Maize (Zea mays L.) during the Vegetative Stage. Environ. Exp. Bot. 2016, 130, 113–121. [Google Scholar] [CrossRef]



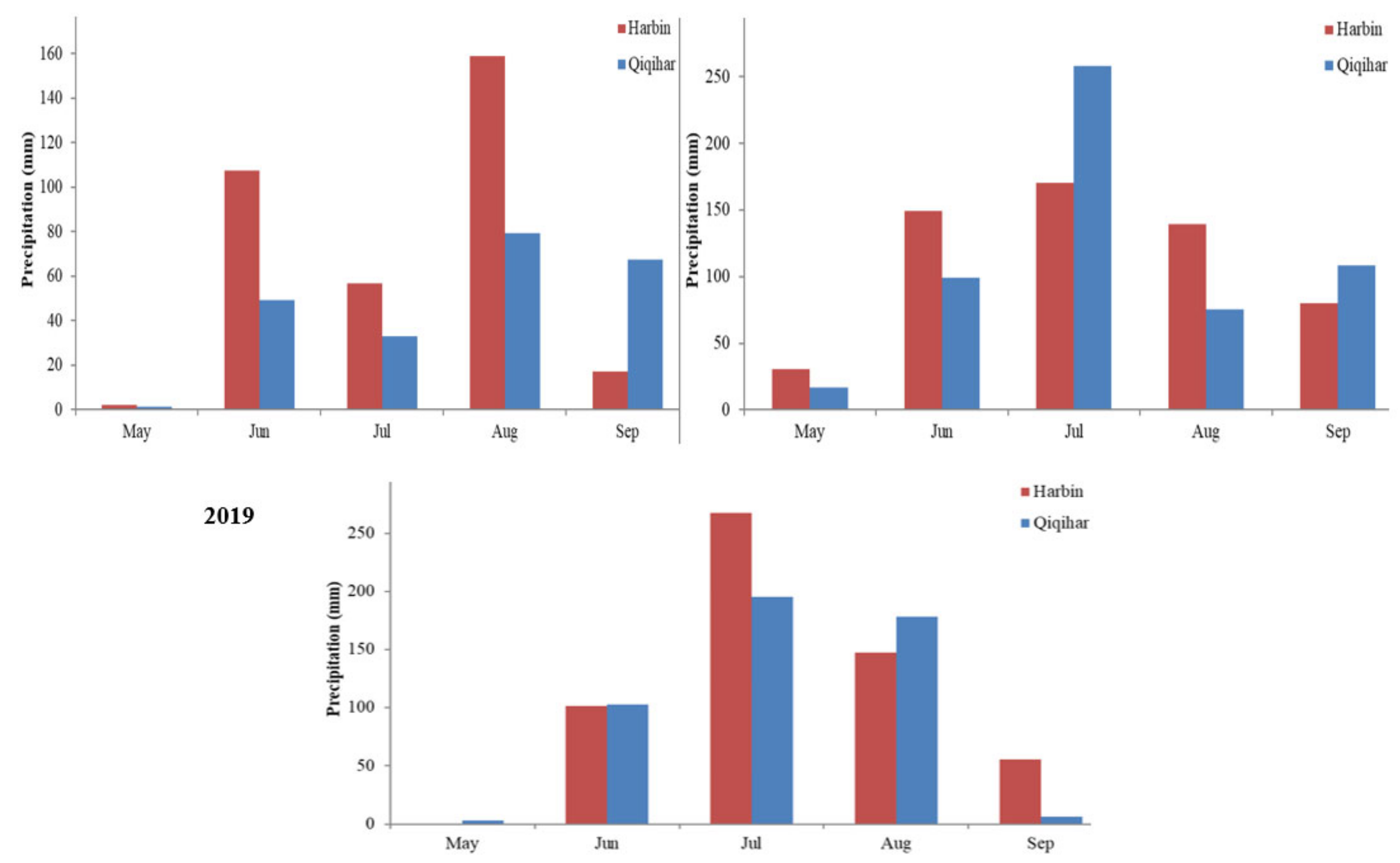

| Site | Cultivar | Year | Plant Height (cm) mv ± sd * a ** | Prod. Tillers/Hill mv ± sd | Spike Weight (g) mv ± sd | Grains/Panicle mv ± sd | 1000-Grain Weight (g) mv ± sd | Spike Length (cm) mv ± sd | Grain Yield (kg/ha) mv ± sd |
|---|---|---|---|---|---|---|---|---|---|
| Harbin | Longdao-18 | 2017 | 103.1 ± 3.1 a | 13 ± 2 b | 57.4 ± 3.7 a | 151 ± 15 a | 23.5 ± 0.4 c | 21.5 ± 0.7 a | 9367 ± 369 a |
| 2018 | 105.6 ± 3.5 a | 14 ± 2 b | 59.1 ± 9.4 a | 161 ± 15 a | 25.3 ± 0.7 b | 20.8 ± 0.7 b | 9500 ± 400 a | ||
| 2019 | 100.5 ± 4.0 a | 13 ± 1 b | 55.9 ± 5.5 a | 146 ± 11 a | 22.5 ± 0.4 c | 21.6 ± 1.6 a | 7705 ± 297 a | ||
| Longdao-21 | 2017 | 93.4 ± 4.7 c | 12 ± 1 b | 60.8 ± 7.3 a | 144 ± 23 a | 23.7 ± 1.1 c | 20.0 ± 1.5 b | 9017 ± 283 a | |
| 2018 | 95.8 ± 4.2 c | 14 ± 1 bc | 63.4 ± 4.2 a | 151 ± 23 a | 26.0 ± 0.6 ab | 22.6 ± 1.5 a | 9166 ± 289 a | ||
| 2019 | 95.5 ± 1.6 a | 12 ± 2 bc | 58.6 ± 9.8 a | 144 ± 13 a | 22.7 ± 1.1 c | 22.4 ± 0.5 a | 7167 ± 133 a | ||
| Longjing-21 | 2017 | 95.1 ± 1.8 bc | 11 ± 1 c | 46.9 ± 5.4 b | 106 ±14 b | 26.9 ± 0.8 a | 16.3 ± 0.7 c | 7050 ± 296 c | |
| 2018 | 96.7 ± 2.5 bc | 12 ± 1 c | 49.9 ± 5.7 b | 146 ± 15 a | 27.0 ± 0.6 a | 17.4 ± 0.7 c | 7166 ± 305 c | ||
| 2019 | 96.9 ± 2.9 a | 11 ± 2 c | 42.0 ± 6.2 b | 101 ± 18 b | 25.9 ± 0.8 a | 16.2 ± 0.5 c | 6962 ± 117 a | ||
| Suijing-18 | 2017 | 98.1 ± 1.6 b | 15 ± 1 a | 53.1 ± 4.9 ab | 141 ± 7 a | 25.2 ± 0.4 b | 17.4 ± 0.6 c | 8200 ± 176 b | |
| 2018 | 100.0 ± 1.3 b | 17 ± 1 a | 56.1 ± 7.0 ab | 149 ± 8 a | 26.1 ± 0.7 ab | 18.5 ± 0.6 c | 8333 ± 208 b | ||
| 2019 | 97.2 ± 2.5 a | 15 ± 1 a | 49.6 ± 1.8 ab | 136 ± 13 a | 24.1 ± 0.2 b | 18.6 ± 0.9 b | 7309 ± 98 a | ||
| Qiqihar | Longdao-18 | 2017 | 111.7 ± 0.4 a | 11 ± 1 a | 34.2 ± 9.1 b | 154 ± 4 a | 24.1 ± 0.6 a | 20.5 ± 1.0 a | 12,267 ± 453 a |
| 2018 | 113.5 ± 0.5 a | 12 ± 1 a | 36.9 ± 12.3 b | 158 ± 4.0 a | 25.2 ± 0.3 a | 21.3 ± 1.0 a | 13,267 ± 351 a | ||
| 2019 | 93.9 ± 2.9 a | 11 ± 1 a | 30.6 ± 8.1 c | 155 ± 9 a | 22.2 ± 1.0 a | 19.6 ± 0.4 ab | 7350 ± 300 c | ||
| Longdao-21 | 2017 | 99.2 ± 0.6 b | 11 ± 1 ab | 40.3 ± 5.1 ab | 153 ± 7 a | 24.6 ± 0.1 a | 19.8 ± 0.8 ab | 11,133 ± 305 a | |
| 2018 | 100.7 ± 0.7 b | 12 ± 2 a | 43.5 ± 7.9 ab | 150 ± 7 ab | 25.7 ± 0.2 a | 20.1 ± 0.8 a | 13,133 ± 350 a | ||
| 2019 | 83.1 ± 1.9 c | 12 ± 1 a | 38.4 ± 7.8 b | 144 ± 11 b | 22.6 ± 2.9 a | 21.0 ± 0.7 a | 9217 ± 75 b | ||
| Longjing-21 | 2017 | 94.6 ± 0.5 c | 10 ± 1 ab | 51.8 ± 3.7 a | 133 ± 4 b | 24.2 ± 0.3 a | 19.0 ± 1.4 ab | 9733 ± 208 b | |
| 2018 | 96.5 ± 0.5 c | 11 ± 1 a | 53.1 ± 11.8 a | 132 ± 3 c | 25.3 ± 0.4 a | 20.9 ± 1.4 a | 10,500 ± 225 b | ||
| 2019 | 86.7 ± 0.5 bc | 13 ± 2 a | 58.4 ± 6.8 a | 138 ± 7 bc | 22.9 ± 2.1 a | 18.0 ± 0.9 ab | 11,383 ± 120 a | ||
| Suijing-18 | 2017 | 94.1 ± 1.7 c | 10 ± 1 b | 35.8 ± 7.9 b | 142 ± 9 ab | 24.0 ± 0.2 a | 18.1 ± 0.3 b | 9634 ± 57 b | |
| 2018 | 94.5 ± 1.2 d | 13 ± 1 a | 37.2 ± 11.7 b | 143 ± 8 bc | 25.1 ± 0.3 a | 19.2 ± 0.3 a | 9700 ± 100 c | ||
| 2019 | 92.4 ± 2.2 ab | 12 ± 2 a | 30.2 ± 4.3 c | 134 ± 13 c | 18.5 ± 2.0 b | 17.6 ± 1.2 b | 7048 ± 150 d |
| Region | Year | SL * (cm) | PT/P ** | G/P *** | SS 0 (%) | 1000-GW 1 (g) | GY 2 (kg/ha) | |
|---|---|---|---|---|---|---|---|---|
| Harbin | MV | 2017 | 20.07 | 11.00 | 121.00 | 0.93 | 21.53 | 9367.73 |
| 2018 | 21.17 | 13.00 | 124.00 | 0.95 | 22.09 | 9500.00 | ||
| 2019 | 18.6 | 14.00 | 118.00 | 0.90 | 20.10 | 7313.09 | ||
| CV (%) | 2017 | 11.18 | 6.80 | 11.79 | 2.53 | 4.32 | 7.52 | |
| 2018 | 11.37 | 7.01 | 12.13 | 2.75 | 4.59 | 7.83 | ||
| 2019 | 10.81 | 6.23 | 11.94 | 2.61 | 4.11 | 7.08 | ||
| Qiqihar | MV | 2017 | 18.84 | 12.00 | 139.00 | 0.86 | 24.90 | 10,528.75 |
| 2018 | 19.16 | 13.00 | 137.00 | 1.11 | 25.10 | 10,978.12 | ||
| 2019 | 18.19 | 11.00 | 145.00 | 1.19 | 21.2 | 9217.00 | ||
| CV (%) | 2017 | 17.39 | 12.89 | 9.23 | 7.64 | 5.78 | 5.28 | |
| 2018 | 17.62 | 13.03 | 9.52 | 8.12 | 6.21 | 5.73 | ||
| 2019 | 16.28 | 13.08 | 8.98 | 7.92 | 5.89 | 5.51 |
| Site | Cultivar | Time of Day of Anthesis (hasr) | Duration (h) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean Onset mv ± sd * a ** | Mean Max mv ± sd | Mean End mv ± sd | Mean mv ± sd | ||||||
| 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | 2018 | 2019 | ||
| Harbin | Longdao-18 | 5.9 ± 0.1 a | 5.3 ± 0.1 a | 6.6 ± 0.1 a | 6.3 ± 0.1 a | 9.0 ± 0.1 a | 9.0 ± 0.1 a | 3.1 ± 0.1 ab | 3.7 ± 0.2 ab |
| Longdao-21 | 5.7 ± 0.1 a | 4.8 ± 0.1 c | 6.7 ± 0.2 a | 5.9 ± 0.1 b | 9.0 ± 0.1 a | 8.7 ± 0.1 b | 3.1 ± 0.1 a | 4.2 ± 0.4 a | |
| Longjing-21 | 5.8 ± 0.1 a | 5.1 ± 0.1 b | 6.6 ± 0.3 a | 6.2 ± 0.1 a | 9.0 ± 0.1 a | 8.7 ± 0.1 b | 3.1 ± 0.1 ab | 3.9 ± 0.1 ab | |
| Suijing-18 | 5.8 ± 0.1 a | 5.1 ± 0.1 b | 6.7 ± 0.1 a | 6.3 ± 0.1 a | 8.8 ± 0.1 a | 8.7 ± 0.1 b | 2.9 ± 0.1 b | 3.6 ± 0.2 b | |
| Qiqihar | Longdao-18 | 5.0 ± 0.1 b | 6.5 ± 0.2 a | 6.1 ± 0.2 b | 7.4 ± 0.1 c | 8.7 ± 0.1 b | 8.9 ± 0.1 b | 3.6 ± 0.1 ab | 2.4 ± 0.2 ab |
| Longdao-21 | 5.1 ± 0.2 b | 6.4 ± 0.1 a | 6.1 ± 0.1 b | 7.7 ± 0.1 a | 8.9 ± 0.1 a | 9.1 ± 0.1 a | 3.8 ± 0.1 a | 2.7 ± 0.1 a | |
| Longjing-21 | 5.4 ± 0.1 a | 6.2 ± 0.1 a | 6.7 ± 0.1 a | 7.5 ± 0.1 b | 8.7 ± 0.1 b | 9.1 ± 0.1 a | 3.3 ± 0.1 b | 2.9 ± 0.1 a | |
| Suijing-18 | 5.1 ± 0.1 b | 6.4 ± 0.1 a | 6.6 ± 0.3 a | 7.7 ± 0.1 a | 8.7 ± 0.1 ab | 9.1 ± 0.1 a | 3.7 ± 0.1 ab | 2.7 ± 0.1 ab | |
| Cultivars | Region | Year | Tavg (°C) | Tmax (°C) | Tmin (°C) | CO2 (ppm) | Rad. Accum. (MJ/m2) | RH (%) | Soil Temp. (5 cm) (°C) | Soil Temp. (10 cm) (°C) |
|---|---|---|---|---|---|---|---|---|---|---|
| Suijing-18 | Harbin | 2018 | 23.58 | 28.80 | 18.08 | 396.63 | 12.48 | 87.61 | 23.80 | 23.35 |
| 2019 | 24.97 | 27.13 | 16.65 | 384.11 | 11.93 | 82.13 | 21.42 | 20.17 | ||
| Qiqihar | 2018 | 23.26 | 28.36 | 18.89 | 374.36 | 10.53 | 85.59 | 23.99 | 22.53 | |
| 2019 | 23.57 | 28.14 | 18.51 | 388.28 | 12.91 | 84.37 | 23.60 | 22.48 | ||
| Longjing-21 | Harbin | 2018 | 23.12 | 28.31 | 17.65 | 408.87 | 12.07 | 85.78 | 21.87 | 22.51 |
| 2019 | 24.51 | 27.82 | 17.31 | 374.58 | 12.08 | 81.43 | 21.75 | 20.04 | ||
| Qiqihar | 2018 | 22.73 | 27.78 | 18.63 | 399.35 | 11.06 | 83.42 | 23.47 | 21.97 | |
| 2019 | 23.10 | 27.79 | 17.74 | 402.18 | 13.31 | 82.94 | 23.41 | 21.93 | ||
| Londao-21 | Harbin | 2018 | 22.91 | 27.98 | 18.39 | 407.64 | 12.83 | 84.39 | 24.78 | 21.98 |
| 2019 | 24.18 | 26.79 | 16.23 | 386.75 | 12.32 | 81.95 | 20.93 | 20.21 | ||
| Qiqihar | 2018 | 23.56 | 28.49 | 17.95 | 396.63 | 10.82 | 84.38 | 24.06 | 22.17 | |
| 2019 | 22.92 | 27.86 | 17.97 | 406.36 | 13.11 | 83.11 | 22.90 | 22.01 | ||
| Longdao-18 | Harbin | 2018 | 23.01 | 28.23 | 17.91 | 402.53 | 12.30 | 86.75 | 22.47 | 23.63 |
| 2019 | 24.40 | 26.92 | 17.10 | 392.76 | 12.58 | 80.24 | 22.01 | 19.63 | ||
| Qiqihar | 2018 | 23.13 | 27.92 | 19.05 | 401.65 | 10.35 | 82.88 | 23.28 | 22.31 | |
| 2019 | 23.08 | 28.03 | 17.18 | 407.13 | 12.76 | 81.42 | 23.14 | 22.37 |
| Cultivars | Region | Year | Tavg (°C) | Tmax (°C) | Tmin (°C) | CO2 (ppm) | RH (%) | Soil Temp. (5 cm) (°C) | Soil Temp. (10 cm) (°C) | Rad. Accum. (MJ/m2) |
|---|---|---|---|---|---|---|---|---|---|---|
| Suijing-18 | Harbin | 2018 | 20.1 | 26.1 | 15.0 | 407.6 | 82.8 | 21.7 | 20.2 | 17.6 |
| 2019 | 19.2 | 25.4 | 14.2 | 386.2 | 80.1 | 22.2 | 20.7 | 17.9 | ||
| Qiqihar | 2018 | 18.3 | 24.8 | 12.6 | 416.1 | 80.9 | 19.7 | 18.3 | 16.7 | |
| 2019 | 19.1 | 25.4 | 13.5 | 409.7 | 78.1 | 21.0 | 20.3 | 16.9 | ||
| Longjing-21 | Harbin | 2018 | 20.3 | 26.0 | 15.9 | 402.5 | 82.7 | 21.8 | 20.5 | 17.7 |
| 2019 | 21.1 | 27.8 | 16.3 | 385.5 | 80.4 | 22.7 | 21.4 | 17.9 | ||
| Qiqihar | 2018 | 18.7 | 24.4 | 13.6 | 401.5 | 81.4 | 19.9 | 18.7 | 16.6 | |
| 2019 | 18.9 | 25.7 | 14.4 | 403.9 | 79.1 | 20.4 | 19.3 | 16.3 | ||
| Londao-21 | Harbin | 2018 | 19.9 | 25.8 | 14.0 | 398.3 | 83.3 | 21.2 | 19.4 | 16.8 |
| 2019 | 20.8 | 26.1 | 15.1 | 396.4 | 81.9 | 21.7 | 20.2 | 16.0 | ||
| Qiqihar | 2018 | 16.9 | 22.5 | 11.5 | 402.5 | 77.3 | 18.6 | 17.1 | 16.8 | |
| 2019 | 17.5 | 23.6 | 13.1 | 438.3 | 79.1 | 20.0 | 18.6 | 17.1 | ||
| Longdao-18 | Harbin | 2018 | 20.1 | 25.2 | 14.9 | 379.9 | 83.3 | 21.4 | 20.6 | 17.0 |
| 2019 | 21.5 | 26.2 | 16.3 | 396.8 | 81.4 | 22.1 | 19.6 | 17.5 | ||
| Qiqihar | 2018 | 17.5 | 23.9 | 12.5 | 401.3 | 79.8 | 19.2 | 17.2 | 16.5 | |
| 2019 | 18.1 | 24.3 | 14.8 | 417.2 | 80.6 | 21.0 | 19.7 | 17.1 |
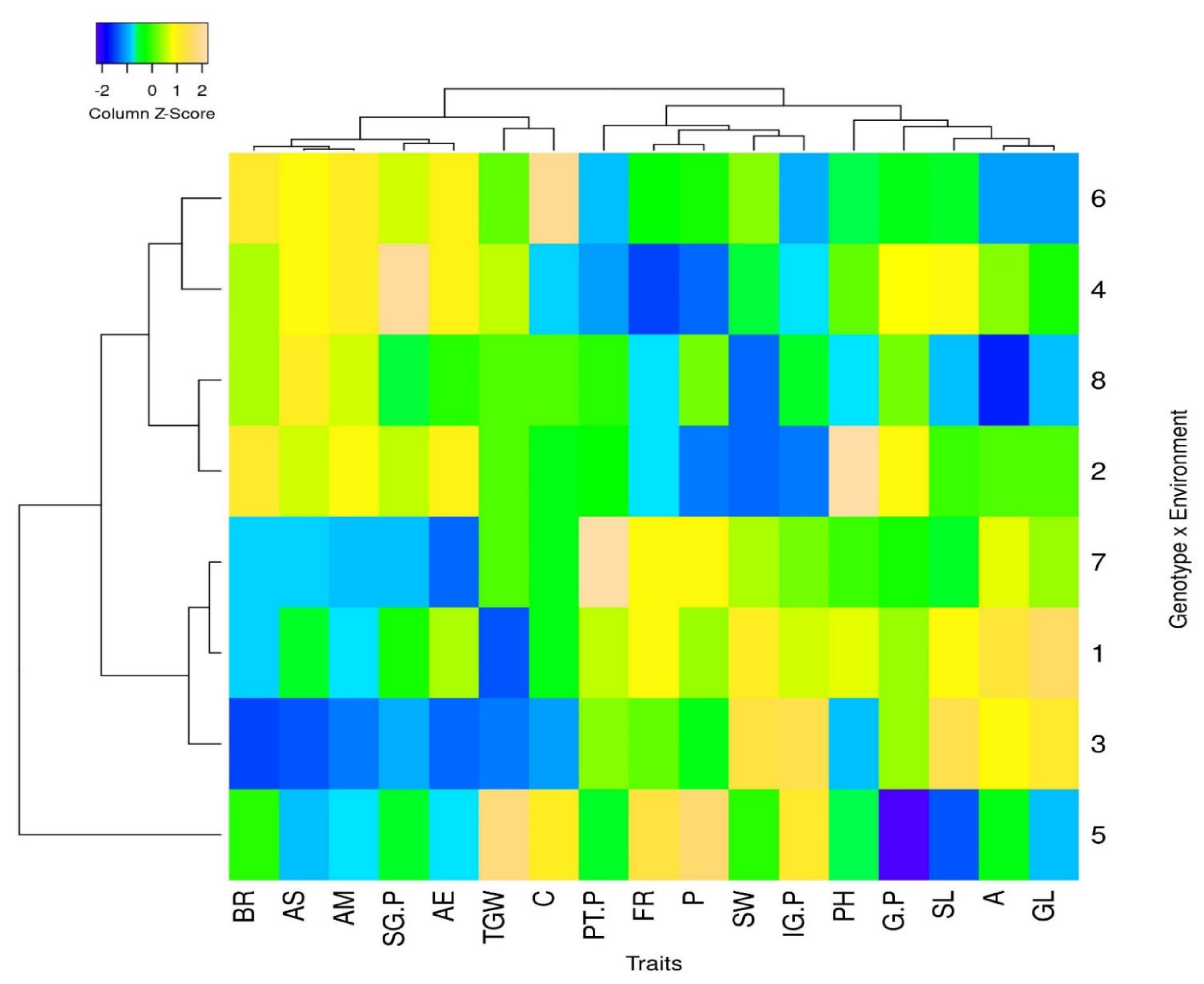
| Region | Cultivar | Year | Protein (%) mv ± sd * a ** | Amylose (%) mv ± sd | BR (%) mv ± sd | FR (%) mv ± sd | L-W Ratio mv ± sd | GL (mm) mv ± sd | GW (mm) mv ± sd | Chalkiness mv ± sd |
|---|---|---|---|---|---|---|---|---|---|---|
| Harbin | Longdao-18 | 2018 | 7.92 ± 0.5 c | 18.91 ± 0.1 a | 77.63 ± 2.7 a | 67.90 ± 2.9 a | 2.01 ± 0.07 a | 5.12 ± 0.07 a | 2.51 ± 0.1 b | 1.01 ± 0.07 bc |
| 2019 | 7.21 ± 0.6 c | 17.90 ± 0.3 a | 75.71 ± 2.1 a | 68.71 ± 2.2 a | 2.20 ± 0.01 a | 5.73 ± 0.05 ab | 2.71 ± 0.01 b | 1.20 ± 0.12 b | ||
| Longdao-21 | 2018 | 7.83 ± 0.7 c | 18.35 ± 0.5 ab | 76.24 ± 5.6 a | 65.97 ± 4.2 a | 2.02 ± 0.01 a | 4.84 ± 0.03 b | 2.32 ± 0.02 c | 0.52 ± 0.18 c | |
| 2019 | 7.10 ± 0.2 c | 17.53 ± 0.3 ab | 74.42 ± 5.2 a | 66.53 ± 5.1 a | 2.11 ± 0.01 b | 5.51 ± 0.02 a | 2.62 ± 0.03 c | 0.71 ± 0.11 c | ||
| Longjing-21 | 2018 | 9.34 ± 0.5 a | 16.82 ± 0.8 c | 78.95 ± 4.1 a | 68.71 ± 3.8 a | 1.83 ± 0.03 b | 4.62 ± 0.09 c | 2.72 ± 0.03 a | 2.09 ± 0.4 a | |
| 2019 | 9.15 ± 0.5 a | 15.81 ± 0.7 c | 76.12 ± 4.7 a | 69.03 ± 3.1 a | 1.51 ± 0.01 c | 4.41 ± 0.04 b | 2.91 ± 0.02 a | 2.31 ± 0.10 a | ||
| Suijing-21 | 2018 | 8.76 ± 0.6 b | 17.77 ± 0.5 b | 77.67 ± 3.3 a | 68.36 ± 2.0 a | 1.93 ± 0.03 b | 4.82 ± 0.08 b | 2.71 ± 0.04 a | 1.10 ± 0.61 bc | |
| 2019 | 8.37 ± 0.1 b | 17.23 ± 0.3 b | 75.36 ± 3.4 a | 68.75 ± 2.8 a | 2.01 ± 0.1 b | 5.13 ± 0.09 ab | 2.83 ± 0.02 a | 1.21 ± 0.21 b | ||
| Qiqihar | Longdao-18 | 2018 | 6.85 ± 0.5 c | 17.92 ± 0.1 a | 79.62 ± 2.1 a | 65.78 ± 2.5 a | 1.91 ± 0.02 a | 4.30 ± 0.25 ab | 2.31 ± 0.02 b | 0.81 ± 0.21 c |
| 2019 | 6.31 ± 0.5 c | 16.30 ± 0.4 ab | 78.31 ± 2.8 a | 64.71 ± 2.7 a | 1.10 ± 0.02 b | 4.93 ± 0.28 a | 2.90 ± 0.01 a | 1.21 ± 0.03 b | ||
| Longdao-21 | 2018 | 6.67 ± 0.33 c | 17.57 ± 0.4 ab | 78.13 ± 6.1 a | 63.34 ± 3.1 a | 1.92 ± 0.04 a | 4.31 ± 0.1 ab | 2.31 ± 0.01 b | 0.82 ± 0.28 c | |
| 2019 | 6.23 ± 0.3 c | 16.65 ± 0.7 a | 77.54 ± 6.1 a | 62.39 ± 3.1 a | 1.82 ± 0.04 a | 4.80 ± 0.11 a | 2.63 ± 0.01 b | 0.91 ± 0.09 b | ||
| Longjing-21 | 2018 | 7.81 ± 0.53 a | 15.51 ± 0.9 c | 79.49 ± 4.3 a | 66.11 ± 3.9 a | 1.80 ± 0.02 ab | 4.30 ± 0.06 ab | 2.53 ± 0.07 a | 2.31 ± 0.43 a | |
| 2019 | 7.33 ± 0.3 b | 14.83 ± 0.2 c | 78.87 ± 4.3 a | 65.10 ± 3.9 a | 1.71 ± 0.02 ab | 4.32 ± 0.09 b | 2.60 ± 0.08 b | 2.91 ± 0.07 a | ||
| Suijing-21 | 2018 | 7.23 ± 0.6 b | 16.82 ± 0.3 b | 78.96 ± 4.3 a | 65.32 ± 2.1 a | 1.73 ± 0.03 b | 4.43 ± 0.09 a | 2.43 ± 0.06 a | 1.21 ± 0.41 b | |
| 2019 | 7.70 ± 0.6 a | 14.10 ± 0.6 c | 77.63 ± 4.3 a | 64.39 ± 2.1 a | 1.93 ± 0.03 a | 4.41 ± 0.10 b | 2.52 ± 0.09 b | 1.53 ± 0.11 b |
| Site | Cultivar | Year | Sowing | Transplanting | Emergence | Tillering | Booting | Heading | Grain-Filling | Maturity |
|---|---|---|---|---|---|---|---|---|---|---|
| Harbin | Longdao-21 | 2017 | 4/18 | 5/24 | 6/04 | 6/20 | 7/27 | 8/10 | 8/16 | 9/18 |
| 2018 | 4/17 | 5/18 | 5/30 | 6/11 | 7/20 | 7/28 | 8/03 | 9/20 | ||
| 2019 | 4/17 | 5/16 | 5/28 | 6/13 | 7/25 | 8/01 | 8/04 | 9/23 | ||
| Longdao-18 | 2017 | 4/18 | 5/24 | 6/04 | 6/19 | 7/22 | 8/06 | 8/12 | 9/17 | |
| 2018 | 4/17 | 5/18 | 5/30 | 6/11 | 7/18 | 7/23 | 7/31 | 9/20 | ||
| 2019 | 4/17 | 5/16 | 5/28 | 6/13 | 7/24 | 7/31 | 8/06 | 9/25 | ||
| Longjing-21 | 2017 | 4/18 | 5/24 | 6/04 | 6/18 | 7/17 | 8/04 | 8/08 | 9/14 | |
| 2018 | 4/17 | 5/18 | 5/30 | 6/11 | 7/12 | 7/22 | 7/28 | 9/15 | ||
| 2019 | 4/17 | 5/16 | 5/28 | 6/13 | 7/19 | 7/24 | 7/29 | 9/20 | ||
| Suijing-18 | 2017 | 4/18 | 5/24 | 6/04 | 6/18 | 7/16 | 8/04 | 8/12 | 9/15 | |
| 2018 | 4/17 | 5/18 | 5/30 | 6/11 | 7/15 | 7/23 | 8/01 | 9/15 | ||
| 2019 | 4/17 | 5/16 | 5/28 | 6/13 | 7/18 | 7/23 | 7/28 | 9/20 | ||
| Qiqihar | Longdao-21 | 2017 | 4/17 | 5/27 | 6/05 | 6/27 | 8/2 | 8/19 | 8/24 | 9/29 |
| 2018 | 4/17 | 5/31 | 6/09 | 6/21 | 7/23 | 8/05 | 8/12 | 10/03 | ||
| 2019 | 4/17 | 5/29 | 6/07 | 6/21 | 7/23 | 7/29 | 8/05 | 9/25 | ||
| Longdao-18 | 2017 | 4/17 | 5/27 | 6/05 | 6/27 | 7/30 | 8/19 | 8/24 | 9/26 | |
| 2018 | 4/17 | 5/31 | 6/09 | 6/21 | 7/23 | 8/05 | 8/12 | 10/03 | ||
| 2019 | 4/17 | 5/29 | 6/07 | 6/21 | 7/23 | 7/29 | 8/05 | 9/25 | ||
| Longjing-21 | 2017 | 4/17 | 5/27 | 6/05 | 6/23 | 7/29 | 8/13 | 8/20 | 9/22 | |
| 2018 | 4/17 | 5/31 | 6/09 | 6/21 | 7/19 | 7/29 | 8/07 | 9/27 | ||
| 2019 | 4/17 | 5/29 | 6/07 | 6/21 | 7/19 | 7/23 | 7/29 | 9/17 | ||
| Suijing-18 | 2017 | 4/17 | 5/27 | 6/05 | 6/23 | 7/29 | 8/13 | 8/20 | 9/24 | |
| 2018 | 4/17 | 5/31 | 6/09 | 6/21 | 7/19 | 7/29 | 8/07 | 9/27 | ||
| 2019 | 4/17 | 5/29 | 6/07 | 6/21 | 7/19 | 7/23 | 7/29 | 9/17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahbaz Farooq, M.; Gyilbag, A.; Virk, A.L.; Xu, Y. Adaptability Mechanisms of Japonica Rice Based on the Comparative Temperature Conditions of Harbin and Qiqihar, Heilongjiang Province of Northeast China. Agronomy 2021, 11, 2367. https://doi.org/10.3390/agronomy11112367
Shahbaz Farooq M, Gyilbag A, Virk AL, Xu Y. Adaptability Mechanisms of Japonica Rice Based on the Comparative Temperature Conditions of Harbin and Qiqihar, Heilongjiang Province of Northeast China. Agronomy. 2021; 11(11):2367. https://doi.org/10.3390/agronomy11112367
Chicago/Turabian StyleShahbaz Farooq, Muhammad, Amatus Gyilbag, Ahmad Latif Virk, and Yinlong Xu. 2021. "Adaptability Mechanisms of Japonica Rice Based on the Comparative Temperature Conditions of Harbin and Qiqihar, Heilongjiang Province of Northeast China" Agronomy 11, no. 11: 2367. https://doi.org/10.3390/agronomy11112367
APA StyleShahbaz Farooq, M., Gyilbag, A., Virk, A. L., & Xu, Y. (2021). Adaptability Mechanisms of Japonica Rice Based on the Comparative Temperature Conditions of Harbin and Qiqihar, Heilongjiang Province of Northeast China. Agronomy, 11(11), 2367. https://doi.org/10.3390/agronomy11112367






