Cd and Zn Concentrations in Soil and Silage Maize following the Addition of P Fertilizer
Abstract
:1. Introduction
2. Materials and Methods
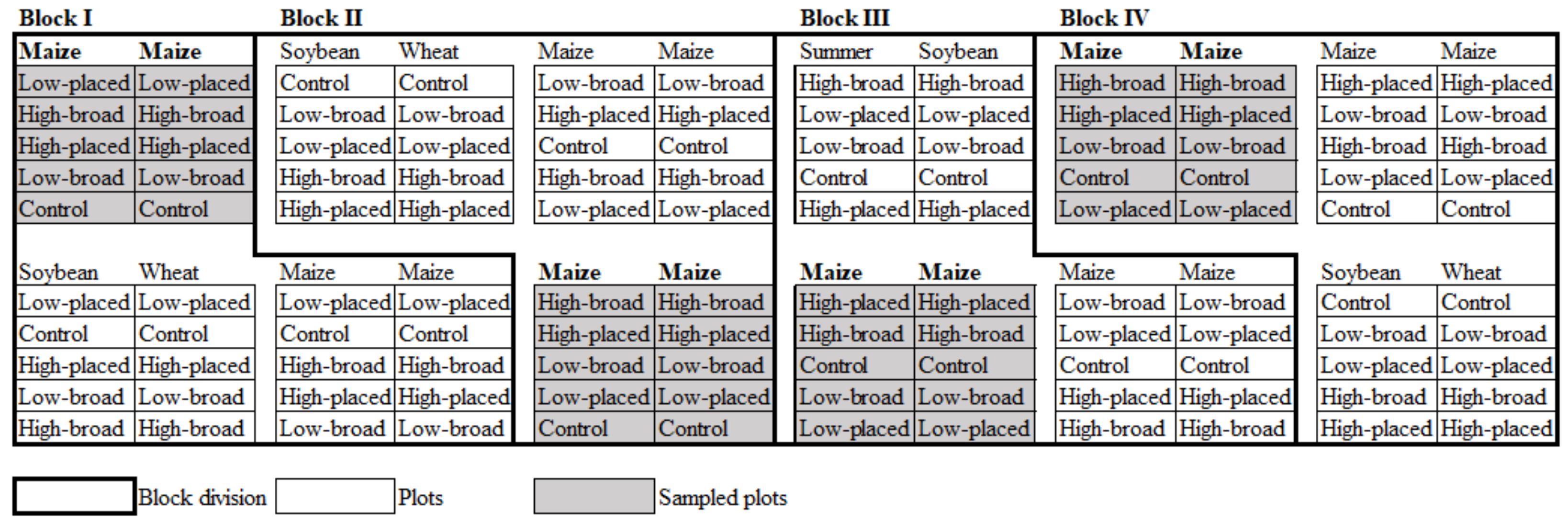
2.1. Field Design
2.2. Sample Collection and Pretreatment
2.3. Extraction Methods and Analysis
2.4. Statistics
3. Results
3.1. Soil pH
3.2. Total Metal Concentration in Soil
3.3. Exchangeable Metal Fraction in Soil
3.4. Total Metal Concentration in Silage Maize
3.5. Pearson Correlations and Linear Regressions
4. Discussion
4.1. Soil pH
4.2. Total Metal Concentration in Soil
4.3. Exchangeable Metal Fraction
4.4. Total Metal Concentration in Silage Maize
4.5. Pearson Correlations and Linear Regressions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niño-Savala, A.G.; Zhuang, Z.; Ma, X.; Fangmeier, A.; Li, H.; Tang, A.; Liu, X. Cadmium pollution from phosphate fertilizers in arable soils and crops: An overview. Front. Agric. Sci. Eng. 2019, 6, 419. [Google Scholar] [CrossRef] [Green Version]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Balafrej, H.; Bogusz, D.; Triqui, Z.-E.A.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc Hyperaccumulation in Plants: A Review. Plants 2020, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Nieder, R.; Benbi, D.K.; Reichl, F.X. (Eds.) Microelements and Their Role in Human Health. In Soil Components and Human Health; Springer: Dordrecht, The Netherlands, 2018; pp. 317–374. [Google Scholar] [CrossRef]
- Hill, G.M.; Shannon, M.C. Copper and Zinc Nutritional Issues for Agricultural Animal Production. Biol. Trace Elem. Res. 2019, 188, 148–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Crops. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 12 March 2021).
- Statistisches Bundesamt. Statistisches Jahrbuch Deutschland 2019, 1st ed.; Statistisches Bundesamt: Wiesbaden, Germany, 2019; ISBN 9783824610860.
- Deutsches Maiskomitee, e.v. Maize—A Key Crop for the Implementation of the Arable Farming Strategy 2035, Bonn. 2020. Available online: www.maiskomitee.de (accessed on 12 March 2021).
- Rosemarin, A.; Ekane, N. The governance gap surrounding phosphorus. Nutr. Cycl. Agroecosyst. 2016, 104, 265–279. [Google Scholar] [CrossRef]
- Bigalke, M.; Ulrich, A.; Rehmus, A.; Keller, A. Accumulation of cadmium and uranium in arable soils in Switzerland. Environ. Pollut. 2017, 221, 85–93. [Google Scholar] [CrossRef]
- Guan, D.X.; Sun, F.S.; Yu, G.H.; Polizzotto, M.L.; Liu, Y.G. Total and available metal concentrations in soils from six long-term fertilization sites across China. Environ. Sci. Pollut. Res. Int. 2018, 25, 31666–31678. [Google Scholar] [CrossRef]
- Kirkham, M.B. Cadmium in plants on polluted soils: Effects of soil factors, hyperaccumulation, and amendments. Geoderma 2006, 137, 19–32. [Google Scholar] [CrossRef]
- Schipper, L.A.; Sparling, G.P.; Fisk, L.M.; Dodd, M.B.; Power, I.L.; Littler, R.A. Rates of accumulation of cadmium and uranium in a New Zealand hill farm soil as a result of long-term use of phosphate fertilizer. Agric. Ecosyst. Environ. 2011, 144, 95–101. [Google Scholar] [CrossRef]
- Drissi, S.; Houssa, A.; Bamouh, A.; Coquant, J.-M.; Benbella, M. Effect of Zinc-Phosphorus Interaction on Corn Silage Grown on Sandy Soil. Agriculture 2015, 5, 1047–1059. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.R. Zinc in Crop Production and Interaction with Phosphorus. Aust. J. Basic Appl. Sci. 2011, 5, 1503–1509. [Google Scholar]
- Liu, B.; Mo, C.-H.; Zhang, Y. Using cadmium bioavailability to simultaneously predict its accumulation in crop grains and the bioaccessibility in soils. Sci. Total Environ. 2019, 665, 246–252. [Google Scholar] [CrossRef]
- Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen. Cadmium-Monitoring zu Böden und Nahrungspflanzen. 2015. Available online: https://docplayer.org/13584243-Cadmium-monitoring-zu-boeden-und-nahrungspflanzen.html (accessed on 10 November 2021).
- LWK Niedersachsen. Anbauempfehlungen für Schwermetallbelastete Böden zur Gewährleistung der Lebensmittel- und Futtermittelqualität, Oldenburg, Germany. 2015. Available online: https://www.lwk-niedersachsen.de/index.cfm/portal/6/nav/196/article/16643.html (accessed on 11 November 2021).
- DIN ISO 11466:1997-06 Bodenbeschaffenheit - Extraktion in Königswasser Löslicher Spuren Elemente. 1997. Available online: https://www.beuth.de/de/norm/din-iso-11466/2965404 (accessed on 15 January 2021).
- Traub, H.; Scharf, H. NH4NO3 extractable trace element contents of soil samples prepared for proficiency testing—A stability study. Fresenius’ J. Anal. Chem. 2001, 370, 270–274. [Google Scholar] [CrossRef]
- DIN ISO 19730 Bodenbeschaffenheit–Extraktion von Spurenelementen aus Böden mit Ammoniumnitratlösung (ISO 19730:2008). 2009. Available online: https://www.beuth.de/de/norm/din-iso-19730/117095524 (accessed on 15 January 2019).
- VDLUFA. Methodenbuch VII Umweltanalytik, 4th ed.; VDLUFA-Verl.: Darmstadt, Germany, 2011. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Hinsinger, P.; Plassard, C.; Tang, C.; Jaillard, B. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant Soil 2003, 248, 43–59. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Szteke, B. Trace Elements in Abiotic and Biotic Environments: Chapter 5; CRC Press LLC: Boca Raton, FL, USA, 2015; ISBN 9781482212792. [Google Scholar]
- LUFA. Vorsorge-, Prüf-und Maßnahmenwerte für Boden Gemäß Bundes-Bodenschutz-und Altlastenverordnung (BBodSchV). 1999. Available online: https://www.landwirtschaftskammer.de/lufa/download/fachinfo/bodengarten/richtwerte-bbodschv.pdf (accessed on 11 February 2019).
- Ilyin, I.; Travnikov, O.; Schütze, G.; Feigenspan, S.; Uhse, K. Country-Scale Assessment of Heavy Metal Pollution: A Case Study for Germany: Technical Report 1/2020. 2020. Available online: http://en.msceast.org/reports/1_2020.pdf (accessed on 11 February 2021).
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef]
- Schaap, M.; Hendriks, C.; Jonkers, S.; Builtjes, P. Impacts of Heavy Metal Emission on Air Quality and Ecosystems across Germany–Sources, Transport, Deposition and potential Hazards: Part 1: Assessment of the Atmospheric Heavy Metal Deposition to Terrestrial Ecosystems in Germany. Available online: http://www.umweltbundesamt.de/publikationen (accessed on 19 March 2021).
- BBodSchV. Bundes-Bodenschutz- und Altlastenverordnung vom 12. Juli 1999 (BGBl. I S. 1554), die Zuletzt Durch Artikel 3 Absatz 4 der Verordnung vom 27. September 2017 (BGBl. I S. 3465) Geändert Worden Ist, Germany. 1999. Available online: https://www.gesetze-im-internet.de/bbodschv/BBodSchV.pdf (accessed on 12 March 2019).
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc absorption in plants: Uptake, transport, translocation and accumulation. Rev. Environ. Sci. Biotechnol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Weissengruber, L.; Möller, K.; Puschenreiter, M.; Friedel, J.K. Long-term soil accumulation of potentially toxic elements and selected organic pollutants through application of recycled phosphorus fertilizers for organic farming conditions. Nutr. Cycl. Agroecosyst. 2018, 110, 427–449. [Google Scholar] [CrossRef] [Green Version]
- Regulation (EC) No. 882/2004 on Official Controls Performed to Ensure the Verification of Compliance with Feed and Food Law, Animal Health and Animal Welfare Rules: EC. 2013. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex:32004R0882 (accessed on 10 February 2019).
- Molina, M.; Escudey, M.; Chang, A.C.; Chen, W.; Arancibia-Miranda, N. Trace element uptake dynamics for maize (Zea mays L.) grown under field conditions. Plant Soil 2013, 370, 471–483. [Google Scholar] [CrossRef]
- Kato, F.H.; Carvalho, M.E.A.; Gaziola, S.A.; Azevedo, R.A. Maize plants have different strategies to protect their developing seeds against cadmium toxicity. Theor. Exp. Plant Physiol. 2020, 32, 203–211. [Google Scholar] [CrossRef]
- Akram, M.A.; Wahid, A.; Abrar, M.; Manan, A.; Naeem, S.; Zahid, M.A.; Gilani, M.M.; Paudyal, R.; Gong, H.Y.; Ran, J.Z.; et al. Comparative study of six maize (Zea mays L.) cultivars concerning cadmium uptake, partitioning and tolerance. Appl. Ecol. Environ. Res. 2021, 19, 2305–2331. [Google Scholar] [CrossRef]
- Song, Y.; Jin, L.; Wang, X. Cadmium absorption and transportation pathways in plants. Int. J. Phytoremediation 2017, 19, 133–141. [Google Scholar] [CrossRef]
- Grant, C.A.; Bailey, L.D.; Harapiak, J.T.; Flore, N.A. Effect of phosphate source, rate and cadmium content and use of Penicillium bilaii on phosphorus, zinc and cadmium concentration in durum wheat grain. J. Sci. Food Agric. 2002, 82, 301–308. [Google Scholar] [CrossRef]
- Gao, X.; Grant, C.A. Cadmium and Zinc Concentration in Grain of Durum Wheat in Relation to Phosphorus Fertilization, Crop Sequence and Tillage Management. Appl. Environ. Soil Sci. 2012, 2012, 817107. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Rehim, A.; Sarwar, N.; Hussain, S. Zinc bioavailability in maize grains in response of phosphorous-zinc interaction. J. Plant Nutr. Soil Sci. 2016, 179, 60–66. [Google Scholar] [CrossRef]
- Jones, M.; Woodward, R.; Stoller, J. Increasing Precision in Agronomic Field Trials Using Latin Square Designs. Agron. J. 2015, 107, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Roberts, T.L. Cadmium and Phosphorous Fertilizers: The Issues and the Science. Procedia Eng. 2014, 83, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.; Zheng, N.; Tang, L.; Ji, X.; Li, Y. Effect of soil pH and organic matter content on heavy metals availability in maize (Zea mays L.) rhizospheric soil of non-ferrous metals smelting area. Environ. Monit. Assess. 2019, 191, 634. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, X.; Li, B.; Wang, Y.; Guo, J. Distribution of Cd and Cu Fractions in Chinese Soils and Their Relationships with Soil pH: A Meta-Analysis. Sustainability 2019, 11, 337. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Z.; Niño-Savala, A.G.; Mi, Z.; Wan, Y.; Su, D.; Li, H.; Fangmeier, A. Cadmium accumulation in wheat and maize grains from China: Interaction of soil properties, novel enrichment models and soil thresholds. Environ. Pollut. 2021, 275, 116623. [Google Scholar] [CrossRef]
- Chen, R.; Gao, T.; Cheng, N.; Ding, G.; Wang, Q.; Shi, R.; Hu, G.; Cai, X. Application of DGT/DIFS to assess bioavailable Cd to maize and its release in agricultural soils. J. Hazard. Mater. 2021, 411, 124837. [Google Scholar] [CrossRef]

| P treatment | Phosphorus | Fertilizer | Nitrogen | Fertilizer | Potassium | Fertilizer |
|---|---|---|---|---|---|---|
| Units | kg (P2O5) ha−1 | kg ha−1 (DAP) | kg N ha−1 | kg ha−1 (urea) | kg K2O ha−1 | kg ha−1 (Patentkali 30% K2O) |
| Low-placed | 114 | 248 | 180 | 294 | 368 | 1127 |
| Low-broad | ||||||
| High-placed | 171 | 372 | 245 | |||
| High-broad | ||||||
| Control | No P applied | No P applied | 391 |
| Variable | Soil pH | Cdsoil | CdNH4NO3 | ZnSoil | ZnNH4NO3 |
|---|---|---|---|---|---|
| Units | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | |
| Block | *** | *** | *** | *** | * |
| I | 5.32 ± 0.03 a | 0.166 ± 0.003 a | 0.005 ± 0.003 a | 66.7 ± 0.57 b | 0.125 ± 0.010 a |
| II | 5.46 ± 0.03 b | 0.149 ± 0.003 b | 0.004 ± 0.003 b | 65.3 ± 0.56 a,b | 0.114 ± 0.010 a,b |
| III | 5.54 ± 0.03 b | 0.145 ± 0.003 b | 0.003 ± 0.003 b | 64.2 ± 0.59 b | 0.094 ± 0.010 a,b |
| IV | 5.54 ± 0.03 b | 0.162 ± 0.003 a | 0.004 ± 0.003 b | 64.2 ± 0.59 b | 0.082 ± 0.010 b |
| Time | *** | *** | ns | *** | ns |
| Sowing | 5.28 ± 0.02 a | 0.145 ± 0.002 a | 0.004 ± 0.002 | 63.8 ± 0.45 a | 0.101 ± 0.007 |
| Harvest | 5.65 ± 0.02 b | 0.166 ± 0.002 b | 0.004 ± 0.002 | 66.3 ± 0.44 b | 0.107 ± 0.007 |
| Rate | ns | ns | ns | ns | ns |
| Place | ns | ns | ns | ns | ns |
| P Treatment/Plant Organ | Leaf | Stem | Grain | Cob | Total | SE |
|---|---|---|---|---|---|---|
| Control | 0.0531 a | 0.0449 a,b | <0.020 | <0.020 | 0.0189 d | 0.0028 |
| High-broad | 0.0525 a | 0.0443 a,b | <0.020 | 0.0230 c | 0.0182 d | 0.0028 |
| High-placed | 0.0483 a,b | 0.0401 a,b | <0.020 | 0.0350 c | 0.0141 d | 0.0028 |
| Low-broad | 0.0449 a,b | 0.0367 b | <0.020 | <0.020 | 0.0182 d | 0.0028 |
| Low-placed | 0.0530 a | 0.0448 a,b | <0.020 | <0.020 | 0.0188 d | 0.0028 |
| Total | 0.0504 a | 0.0422 b | <0.020 | 0.0140 c | 0.0161 d | |
| SE | 0.0017 | 0.0017 | <0.020 | 0.0017 | 0.0017 |
| P Treatment/Plant Organ | Leaf | Stem | Grain | Cob | Total | SE |
|---|---|---|---|---|---|---|
| Control | 24.55 d | 7.65 a | 15.37 c | 11.04 b | 13.70 c | 0.63 |
| High-broad | 23.70 d | 6.80 a | 14.51 c | 10.19 b | 12.84 c | 0.63 |
| High-placed | 24.03 d | 7.13 a | 14.85 c | 10.52 b | 13.18 c | 0.63 |
| Low-broad | 24.10 d | 7.20 a | 14.92 c | 10.59 b | 13.25 c | 0.63 |
| Low-placed | 25.22 d | 8.32 a | 16.03 c | 11.71 b | 14.36 c | 0.63 |
| Total | 24.32 d | 7.42 a | 15.14 c | 10.81 b | 13.47 c | |
| SE | 0.47 | 0.47 | 0.47 | 0.48 | 0.47 |
| Stage | Equation | R2 | |
|---|---|---|---|
| Leaf development | With Zn | CdNH4NO3 = −0.0011 + 0.0178CdSoil + 0.0275ZnNH4NO3 | 0.90 |
| CdMaize = 0.2273 − 0.0922pH + 0.0041ZnSoil + 0.0044ZnMaize | 0.42 | ||
| Without Zn | CdNH4NO3 = 0.0320 − 0.0053pH | 0.34 | |
| Ripening | CdNH4NO3 = 0.0299 − 0.0050pH + 0.0159CdSoil | 0.66 | |
| CdMaize = 0.1099 − 0.0184pH + 0.0605CdSoil | 0.38 | ||
| CdMaize = −0.4988 + 3.7540CdSoil + 0.0888pH − 0.6504pH:CdSoil | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niño-Savala, A.G.; Weishaar, B.; Franzaring, J.; Liu, X.; Fangmeier, A. Cd and Zn Concentrations in Soil and Silage Maize following the Addition of P Fertilizer. Agronomy 2021, 11, 2336. https://doi.org/10.3390/agronomy11112336
Niño-Savala AG, Weishaar B, Franzaring J, Liu X, Fangmeier A. Cd and Zn Concentrations in Soil and Silage Maize following the Addition of P Fertilizer. Agronomy. 2021; 11(11):2336. https://doi.org/10.3390/agronomy11112336
Chicago/Turabian StyleNiño-Savala, Andrea Giovanna, Benedikt Weishaar, Jürgen Franzaring, Xuejun Liu, and Andreas Fangmeier. 2021. "Cd and Zn Concentrations in Soil and Silage Maize following the Addition of P Fertilizer" Agronomy 11, no. 11: 2336. https://doi.org/10.3390/agronomy11112336
APA StyleNiño-Savala, A. G., Weishaar, B., Franzaring, J., Liu, X., & Fangmeier, A. (2021). Cd and Zn Concentrations in Soil and Silage Maize following the Addition of P Fertilizer. Agronomy, 11(11), 2336. https://doi.org/10.3390/agronomy11112336







