1. Introduction
Industrial hemp belongs to the Cannabaceae family, and while three species:
C. sativa,
C. indica, and
C. ruderalis have been previously proposed [
1],
C. sativa has recently been recommended as the only recognized species [
2,
3]. The plants produce a unique group of compounds collectively called cannabinoids. Its cultivars can be classified based on their chemical profile, a genetic presence of alleles for contrasting cannabinoids, the psychoactive compound Delta-9-tetrahydrocannabinol (THC), and cannabidiol (CBD) [
4]. Marijuana contains 3–15% THC by weight, while industrial hemp and other fiber and oil-seed
Cannabis generally contain less than 1% THC and high CBD content. Hemp is naturally dioecious [
5], but monoecious hemp has been introduced and is found spontaneously at a very low frequency (0.1%) in dioecious hemp farms [
6]. All
Cannabis are interfertile, and no known sexual incompatibility has been reported [
7].
A uniform phenological coding system for hemp is important for efficient planning and design of crop management strategies [
8]. A noncomprehensive decimal code introduced by Mediavilla et al. [
9] divides the hemp life cycle into four principal stages: germination and emergence, vegetative growth, flowering, and seed formation and senescence, each with secondary stages. Later a BBCH (Biologische Bundesantalt, Bundessortenamt und Chemische Industrie) coding system was introduced for hemp by Mishchenko et al. [
8] based on the general BBCH scale, a standard coding system used to identify plants’ phenological development stages [
10,
11,
12]. Based on Mishchenko et al. [
8], the hemp life cycle is divided into nine principal growth stages, including germination and sprouting (0), leaf development (1), the formation of lateral shoots (2), stem elongation (3), inflorescence emergence (5), flowering (6), development of fruit (7), ripening of fruit (8), and senescence (9). Each stage is subdivided into secondary stages based on its main distinctive features [
8]. Prediction of plant growth stages is possible by use of growing degree days (GDD) [
13]. The calculation of cumulative growing degree days (CGDD) using a biological temperature minimum (a value which indicates the beginning or end of growth) is essential in the plants’ life and helps to estimate the range time of each growth stage in hemp [
14]. Bócsa and Karus [
1] stated that the GDD requirement is variety dependent. Fiber-hemp plants require 1900–2000 GDD from germination to technical maturity (110–115 days) and seed-hemp plants require 2700–3000 GDD until seed maturity.
Most hemp cultivars are adapted to temperate or equatorial climate [
15], distributed between 25 and 55 degree parallels on either side of the equator [
16], and require around 250–300 mm precipitation that is well distributed during the growing season [
17]. Hemp germinates at soil temperatures of 8–10 °C and within 8–12 days of planting [
17]. Hemp is a very photosensitive plant and flowering is triggered by shortening day length [
18] and delayed by long days [
19]. The critical day length is longer for male plants than for female plants of the same cultivar [
20]. Because flowering depends on cumulative degree days since planting and cultivar’s photoperiodic behavior and sensitivity, differences in cultivar sensitivity to light could impact crop yield [
21]. Planting too early in spring results in low and poor germination and emergence while late sowing reduces the plant growth period and quality and quantity of yields [
17]. After the implantation phase, and when active growth starts, the temperature sum accumulation starts to influence yield [
21] and depending on intended use, crop harvest timing can impact yield and quality of hemp products. For fiber, harvest should be carried out during male flowering or during the flowering for monoecious crops. For essential oil, one to three weeks before seed maturity, and for cannabinoids extraction, inflorescences are harvested at the beginning of seed maturity [
9].
Hemp has been proposed as a plant that mitigates climate change, because of its similarity to fast-growing hardwoods [
22]. It removes carbon dioxide (CO
2) via photosynthesis, thus enhancing its potential as a terrestrial carbon sink, and biomass generated finds multiple uses as fiber for textiles and paper industry and manufacture of building materials. The high cellulose content and its physiology make it an ideal alternative to the starch-based crops as feedstock for ethanol [
23]. Hemp plant is better at extracting heavy metals from the soil than many agricultural crops [
24,
25,
26,
27] and could be an environmentally sound and viable feedstock. When used in rotation cropping, dead hemp roots improve soil structure and are reported to increase yield in the crop following hemp [
28]. Industrial hemp yields of up to 785 kg ha
−1 grain, 468 L ha
−1 oil, 594 kg ha
−1 meal, 5941 kg ha
−1 straw, or 1457 kg ha
−1 fiber have been reported in Canada [
29]. Hemp seed is a good source of oil (50%), 90% of which is unsaturated in some Chinese cultivars [
30]. Different cultivars vary in their seed content of gamma-linolenic acid (GLA) and omega-6 to omega-3 ratio [
31] and anti-nutrition attributes like phytic acid levels [
32]. Hemp polyunsaturated fatty acid contents, and stability against oxidation, make hemp oil good for the cosmetic industry [
33]. Hemp seed contains high-quality storage proteins rich in all essential amino acids and especially arginine, has high, easily digestible fiber, and is gluten-free [
34]. The left-over cake from grain oil extraction can be used to substitute soybean and other high protein cakes as livestock feed supplement [
34,
35]. A laying chicken fed a diet containing hemp seed or oil produced eggs with increased omega-3 fatty acids contents [
36]. Other studies report hemp seed to be rich in iron, manganese, and zinc—critical elements in human and animal health [
27]. Therefore, there is a need to determine performance in Virginia of grain cultivars with high protein and oil content, reduced anti-nutrient qualities, and high micronutrient element contents for use in animal and human food supplements and other industrial products.
In spite of hemp production prohibition in the US, the annual sale in North America of hemp-based products, derived predominantly from imported material, was approximately
$400 million [
37]. It is reported that the combined US income from hemp seed for food and pharmaceutical products alone amounted to 40.5 million dollars in 2012 [
38]. The estimates from Vote Hemp (a nonprofit organization which works on hemp in the U.S.) show that the total retail value of hemp products (food and body products, clothing, auto parts, building materials, and other products) in the U.S. in 2017 was increased to
$820 million [
39]. Because these sales are met with imports, there is a need to promote locally produced hemp to meet local market demands and the production of industrial hemp has the potential to positively impact the US economy. While hemp cultivar development and production continued elsewhere in the world, the long period of legal prohibition in the United States has led to low availability of local genetic materials [
40]. Subsequently, even as legislative action in recent years removed the legal prohibition and made hemp a commodity crop, there exist challenges to its production due to the low availability of adapted genetic materials. However, the removal of prohibition allowed for the establishment of this project to evaluate foreign bred hemp cultivars for their adaptability and productivity potentials in the mid-central part of the Commonwealth of Virginia.
4. Discussion
The relationship between growth stages and GDD is useful for the timing of planting and management practices (fertilizing, pest, disease and weed control, pollination) and assessing cultivar performance under different climates [
8].
In this study all hemp cultivars showed the same GDD requirements from planting to appearance of the first pair of leaves, but there were some differences in CGDD effect on later phenological and morphological growth phases. A similar finding was obtained in a study conducted on 14 cultivars in Latvia, the Czech Republic, France, and Italy [
48]. In the study, a variation in stem and seed yield among genotypes was mainly determined by the difference in flowering time, a growth phase under the control of temperature and photoperiod. Amaducci et al. [
49] also reported that the flowering time in hemp could vary greatly with the latitude of production site.
Cultivars that need more GDD to complete every growth stage of its life cycle may be successfully produced in areas with the longer growing season. In our study, while grain-type cultivar like ‘USO 31’ shows an early transition to the reproductive phase (flower primordia formation at stage 51 BBCH), fiber-type cultivar ‘Carmagnola’ transitioned later. Similar results were reported for ‘USO 31’ in Italy where among eight cultivars, it flowered earlier [
50]. The early flowering in ‘USO 31’ was reportedly due to requirement of short GDD for both pre- and post-flowering periods [
51]. A late flowering cultivar results in lower grain yield while stem biomass at flowering was highest for late flowering cultivar and when planted early [
48]. A late monoecious cultivar, which, if sown early enough, could provide appreciable biomass and seed yield may be a good dual crop [
52].
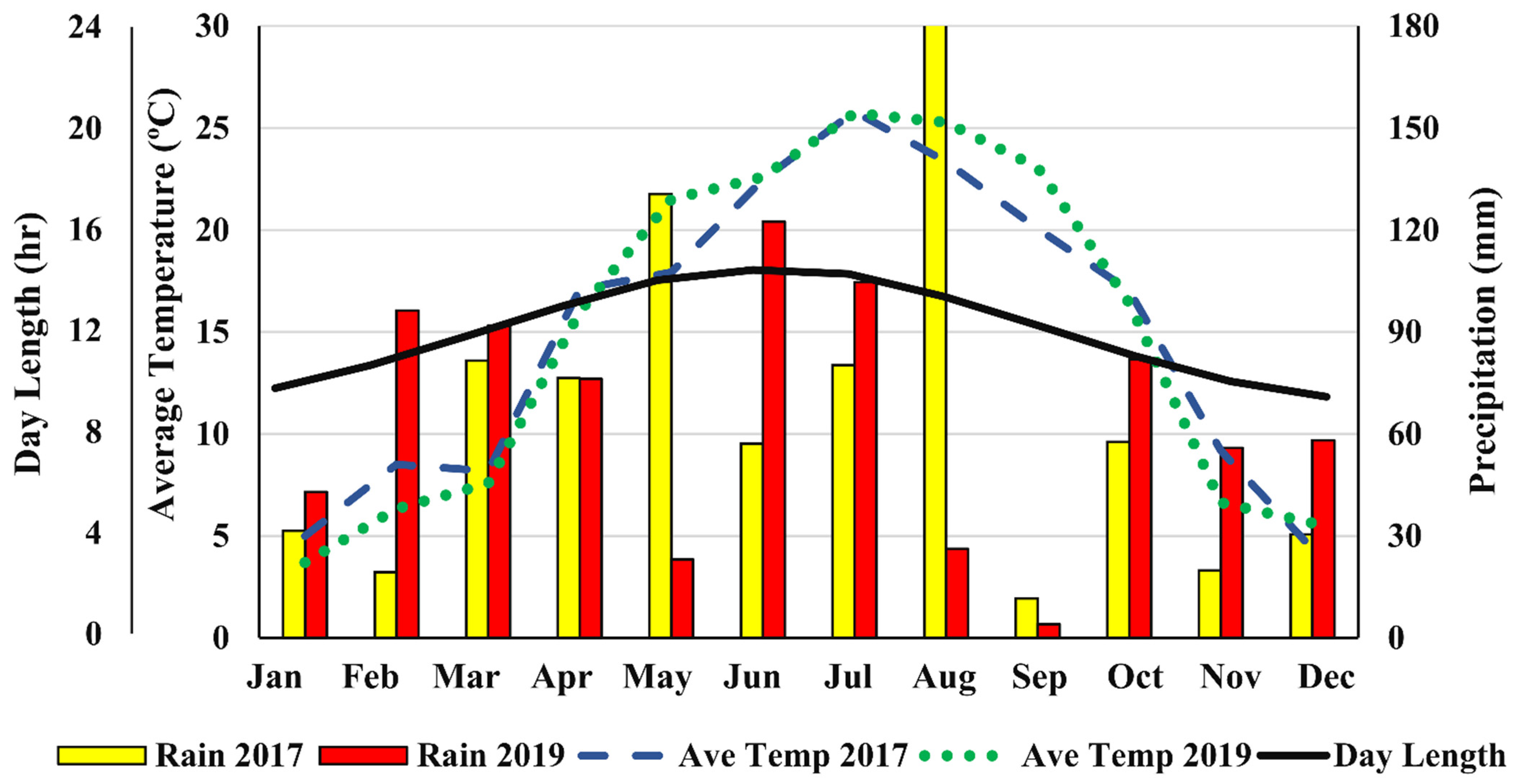
In our study, among grain hemp cultivars, ‘Bialobrzeskie’ showed the highest plant density while ‘Tygra’ had the worst, and in general, plant survival in 2017 was lower than in 2019. High rainfall in May of 2017 could have increased fungal infections and death of germinated seeds and young seedlings and may have contributed to reduced plant density compared to 2019. In addition, the initial poor germination potential of some cultivars may have affected density because some cultivars had no germination percent information, and no germination test was carried out prior to planting the seeds. Similarly, soil conditions at planting may have affected seed germination and emergence and therefore subsequent plant population. Excessive soil moisture results in poor aeration and increased potential soil-borne fungal infestation, while dry condition leads to poor germination and poor seedling emergence through the soil crushed.
Low plant density may have contributed to the observed reduction in grain yield and biomass yield in some cultivars in 2017. Differences in 1000 seed weight among cultivars, which ranges from 17.50 g (‘Canda’) to 12.10 g (‘Bialobrzeskie’), can be attributed to plant genetics. However, low seed weight for late-planted crops may be a result of accelerated seed maturity and poor seed-fill that occurred during the hot summer. The seed weight for crops planted early was comparable to that found for the same cultivars in New York [
53].
‘Fedora 17’ grain yield of 770.9 kg ha
−1 in 2019 was slightly lower, and ‘USO 31’ was far lower than those reported in France [
54]; this indicates that potential exists for improving yield at this location. ‘Felina 32’ gave a relatively good yield during both production years, a trend previously reported in New York when compared to 13 other cultivars [
55]. Baldini et al. [
50] reported ‘Fedora 17’ to produce greater seed yield than eight other cultivars across multiple years. In our study, a lot of seeds were lost in shattering and to feeding birds; this could contribute to the observed low yields. Cultivar ‘USO 31’ is known as a very early variety and its growing season (from sowing date till the first matured seed) was 18–20 weeks while for the other eight hemp cultivars was 20–22 weeks in Lithuania [
56]. ‘USO 31’ showed a short growth period to seed maturity in Virginia (
Figure 2), and this may have increased its potential for early bird infestation, resulting in comparatively greater seed loss and the lower seed yield compared to other cultivars. The seed protein and oil contents, the nutritional components of hemp seeds, and the fatty acid composition are mainly affected by genetic factors [
57]. In our study, seed protein, oil, and mineral elements iron, manganese, and zinc contents were comparable to those reported in other studies [
27].
Differences in heights at harvest may be due to differences in genetic background. However, cultivars were generally shorter than those reported in other studies [
58]. This may be an indication that conditions at this location may be suboptimal for these cultivars’ performance and that there exists room for improved performance through better management. Planting early allowed for a longer vegetative growth prior to switching to the reproductive phase, and a crop grown in April to mid-May was taller than those grown in June. Ferfuia et al. [
52] have reported that delayed sowing contributes to reduced plant height in Italy. Mid-late and late flowering varieties such as Bialobrzeskie and Futura 75 scored intermediate for fiber yield [
59]. In our study for the fiber-type cultivars like ‘Carmagnola’ and ‘Futura 75’, yield was less than 50% of that reported by others [
60,
61]. While these previous studies occurred in Europe, a continent with a long history of industrial hemp production, it is an indication that there exists a huge potential for greater yield here at Virginia. The low biomass observed with greater plant density in this study supports previous findings [
60] on the relationship between plant density and biomass. Reduced biomass at high plant populations has been reported [
62] and dense plants had a tendency to flower short and had smaller diameter, factors that will result in a reduction in biomass yield [
63].