Establishment of an Efficient Micropropagation System for Humulus lupulus L. cv. Cascade and Confirmation of Genetic Uniformity of the Regenerated Plants through DNA Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. In Vitro Propagation
2.2.1. In Vitro Culture Initiation and Proliferation of Shoots
2.2.2. Rooting and Acclimatization
2.3. Genetic Fidelity Assessment of In Vitro Raised Plants Using RAPD and SCoT Markers
2.3.1. DNA Isolation
2.3.2. RAPD and SCoT Analysis
2.4. Data Analysis
3. Results
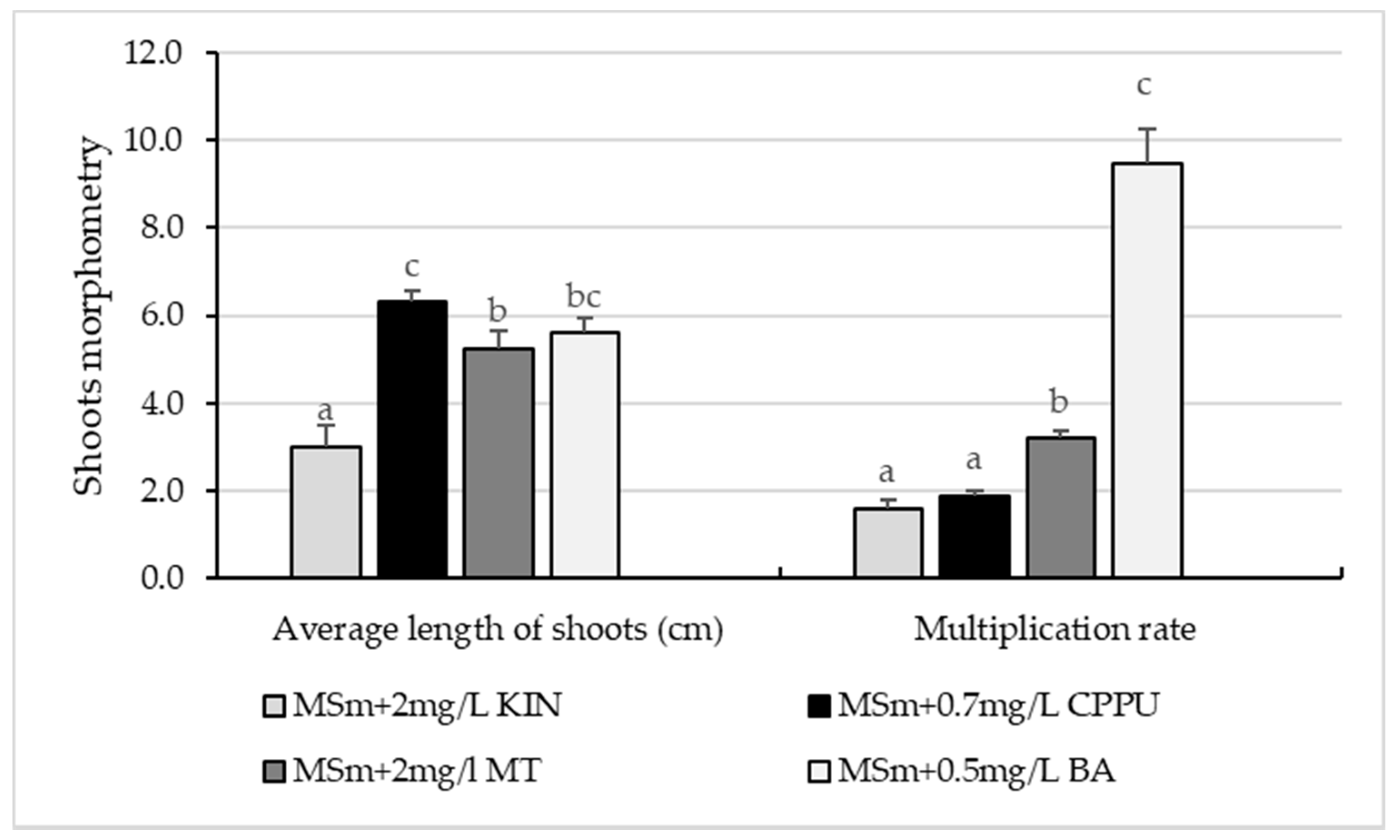
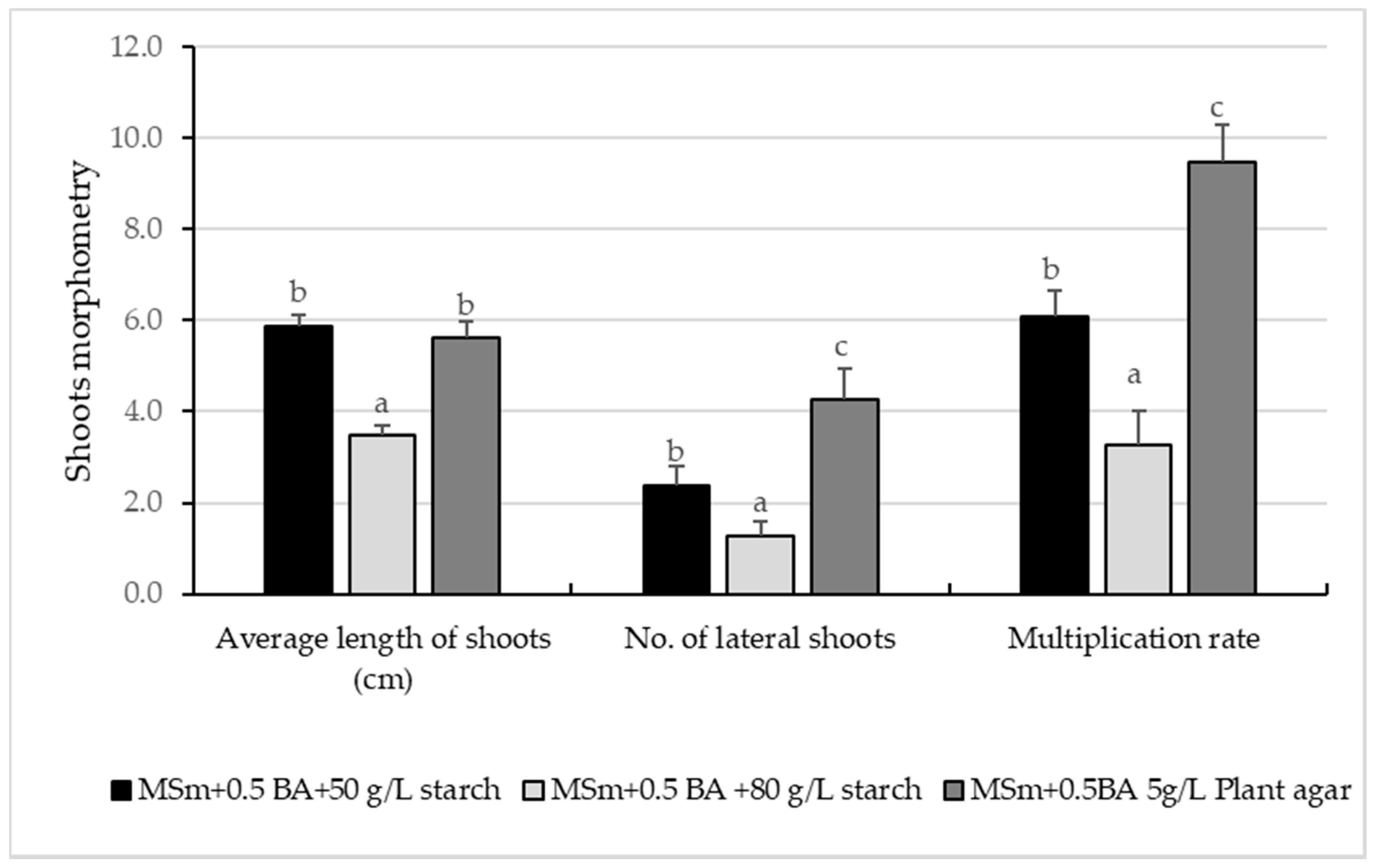
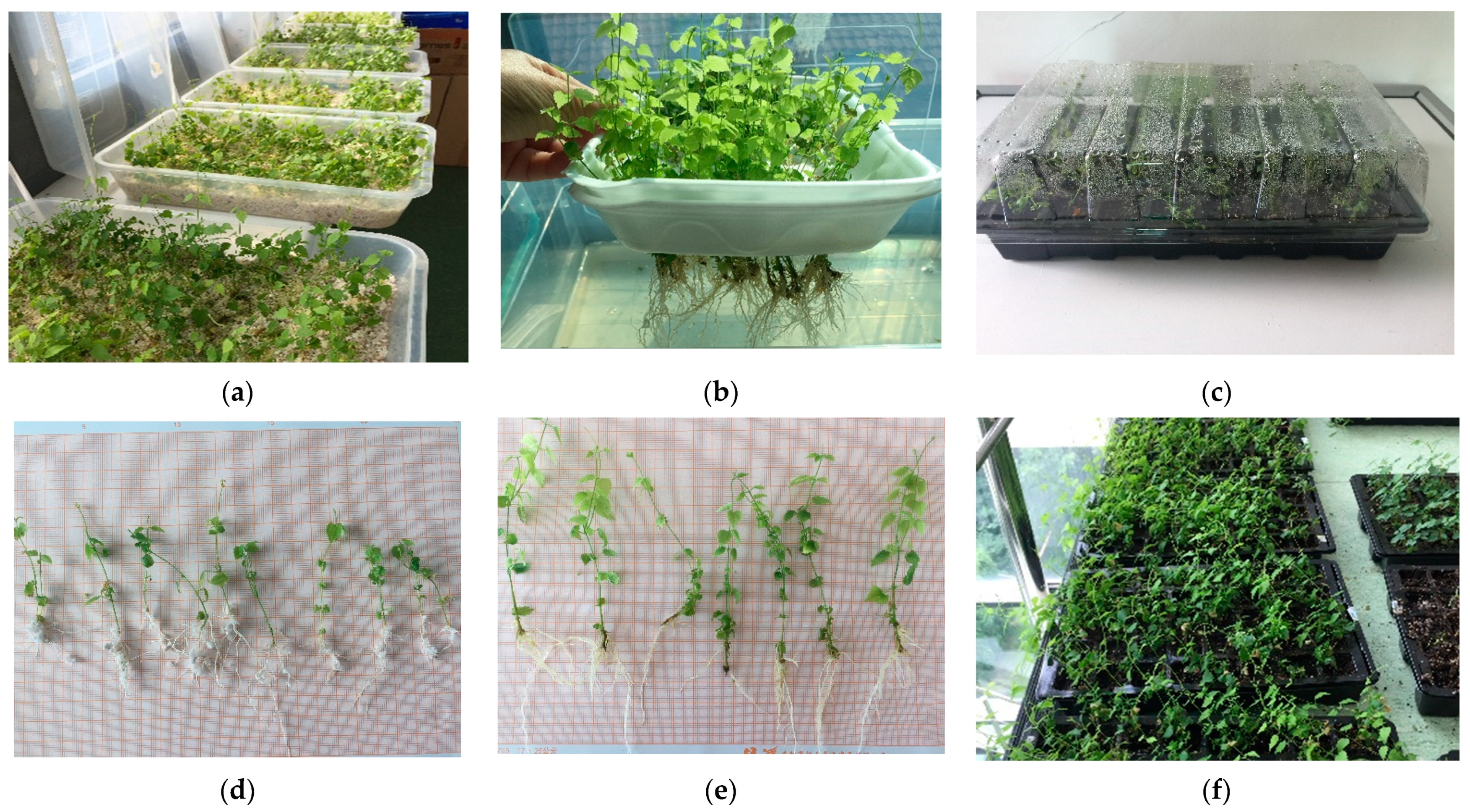
3.1. In Vitro Culture Initiation and Proliferation of Shoots
3.2. Rooting and Acclimatization
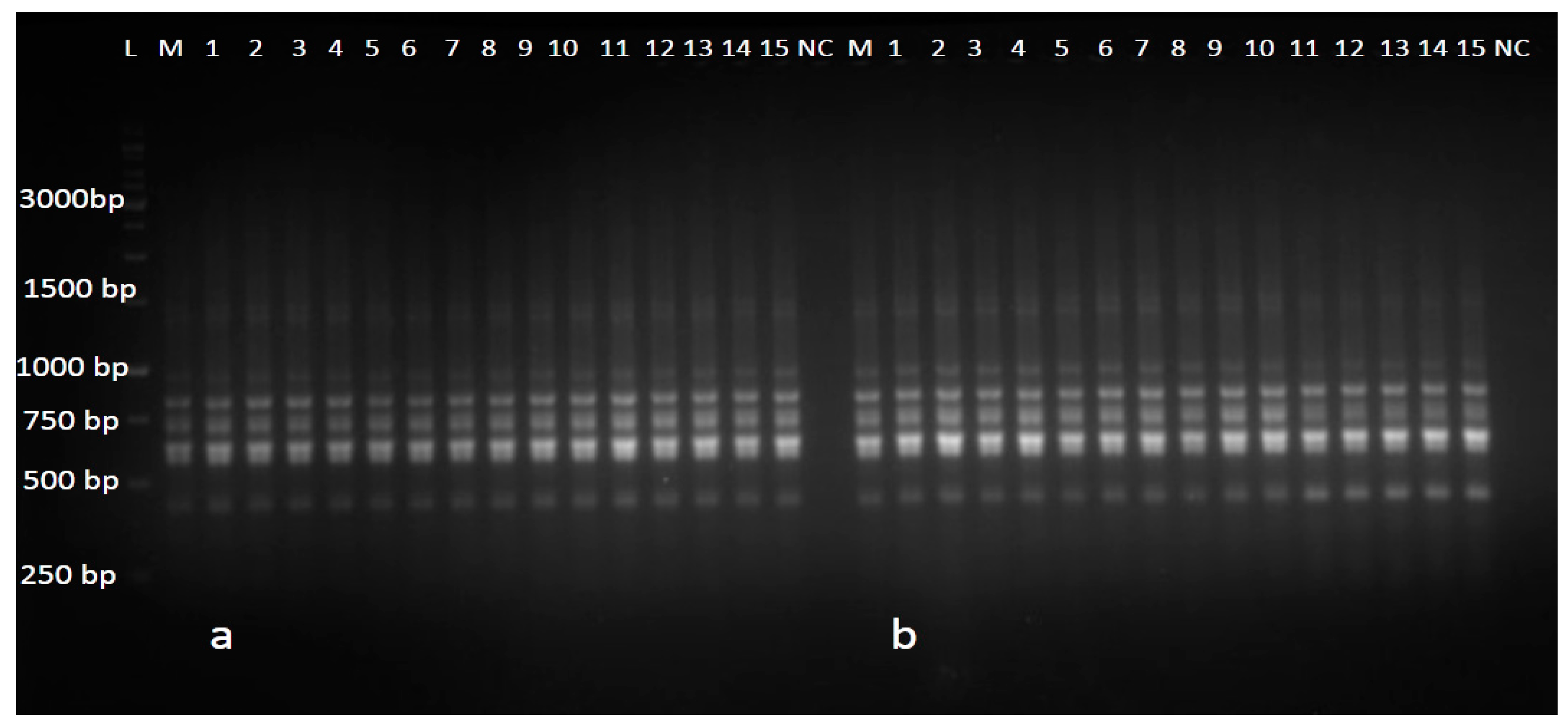
3.3. Genetic Uniformity Assessment Based on RAPD and ScoT Markers
4. Discussion
4.1. In Vitro Culture Initiation and Proliferation of Shoots
4.2. Rooting and Acclimatization
4.3. Genetic Uniformity Assessment Based on RAPD and ScoT Markers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Small, E. A numerical and nomenclatural analysis of morpho-geographic taxa of Humulus. Syst. Bot. 1978, 3, 37–46. Available online: www.jstor.org/stable/2418532 (accessed on 23 August 2021). [CrossRef]
- Fortes, A.M.; Santos, F.; Pais, M.S. Organogenic nodule formation in hop: A tool to study morphogenesis in plants with biotechnological and medicinal applications. J. Biomed. Biotechnol. 2010, 13, 583691. [Google Scholar] [CrossRef]
- Rossini, F.; Virga, G.; Loreti, P.; Iacuzzi, N.; Ruggeri, R.; Provenzano, M.E. Hops (Humulus lupulus L.) as a novel multipurpose crop for the Mediterranean region of Europe: Challenges and opportunities of their cultivation. Agriculture 2021, 11, 484. [Google Scholar] [CrossRef]
- Magalhães, P.J.; Carvalho, D.O.; Cruz, J.M.; Guido, L.F.; Barros, A.A. Fundamentals and health benefits of xanthohumol, a natural product derived from hops and beer. Nat. Prod. Commun. 2009, 4, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Carbone, K.; Macchioni, V.; Petrella, G.; Cicero, D.O. Exploring the potential of microwaves and ultrasounds in the green extraction of bioactive compounds from Humulus lupulus for the food and pharmaceutical industry. Ind. Crops Prod. 2020, 156, 112888. [Google Scholar] [CrossRef]
- Chadwick, L.R.; Pauli, G.F.; Farnsworth, N.R. The pharmacognosy of Humulus lupulus L. (hops) with an emphasis on estrogenic properties. Phytomedicine 2006, 13, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Keiler, A.; Zierau, O.; Kretzschmar, G. Hop extracts and hop substances in treatment of menopausal complaints. Planta Med. 2013, 79, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Milligan, S.; Kalita, J.C.; Pocock, V.; Van de Kauter, V.; Stevens, J.F.; Deinzer, M.L.; Rong, H.; De Keukeleire, D. The endocrine activities of 8-prenylnaringenin and related hop (Humulus lupulus L.) flavonoids. J. Clin. Endocrinol. Metab. 2000, 85, 4912–4915. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, E.; Archer, R.; Tucknott, M.; Colston, K.; Pirianov, G.; Ramanthan, D.; Dhillon, R.; Sinclair, A.; Skinner, G.A. The synthesis and anticancer effects of a range of natural and unnatural hop bacids on breast cancer cells. Phytochem. Lett. 2012, 5, 144–149. [Google Scholar] [CrossRef]
- Yong, W.K.; Ho, Y.F.; Abd Malek, S.N. Xanthohumol induces apoptosis and S phase cell cycle arrest in A549 non-small cell lung cancer cells. Pharmacog. Mag. 2015, 11, S275–S283. [Google Scholar] [CrossRef]
- Negrão, R.; Duarte, D.; Costa, R. Isoxanthohumol modulates angiogenesis and inflammation via vascular endothelial growth factor receptor, tumor necrosis factor alpha and nuclear factor kappa B pathways. BioFactors 2013, 39, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.S.; Minich, D.; Lerman, R.; Darland, G.; Lamb, J.; Tripp, M.; Grayson, N. Isohumulones from hops (Humulus lupulus) and their potential role in medical nutrition therapy. PharmaNutrition 2015, 3, 46–52. [Google Scholar] [CrossRef]
- Bocquet, L.; Sahpaz, S.; Rivière, C. An overview of the antimicrobial properties of hop. In Natural Antimicrobial Agents. Sustainable Development and Biodiversity; Mérillon, J.M., Riviere, C., Eds.; Springer: New York, NY, USA, 2018; Volume 19, pp. 31–54. [Google Scholar] [CrossRef]
- Hege, M.; Jung, F.; Sellmann, C.; Jin, C.; Ziegenhardt, D.; Hellerbrand, C.; Bergheim, I. An iso-α-acid-rich extract from hops (Humulus lupulus) attenuates acute alcohol-induced liver steatosis in mice. Nutrition 2018, 45, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, M.; Russo, D.; Faraone, I.; Sinisgalli, C.; Labanca, F.; Lela, L.; Milella, L. The promising ability of Humulus lupulus L. Iso-α-acids vs. diabetes, inflammation, and metabolic syndrome: A systematic review. Molecules 2021, 26, 954. [Google Scholar] [CrossRef] [PubMed]
- Moir, M. Hops—A Millennium Review. J. Am. Soc. Brew. Chem. 2000, 58, 131–146. [Google Scholar] [CrossRef]
- DeLyser, D.Y.; Kasper, W.J. Hopped beer: The case for cultivation. Econ. Bot. 1994, 48, 166–170. [Google Scholar] [CrossRef]
- Faivre, C.; Ghedira, K.; Goetz, P.; Lejeune, R.; Staub, H. Humulus lupulus L. Phytothérapie 2007, 5, 86–89. [Google Scholar] [CrossRef]
- Almeida, A.D.R.; Maciel, M.V.D.O.B.; Machado, M.H.; Bazzo, G.C.; de Armas, R.D.; Vitorino, V.B.; Vitali, L.; Block, J.M.; Barreto, P.L.M. Bioactive compounds and antioxidant activities of Brazilian hop (Humulus lupulus L.) extracts. Int. J. Food Sci. Tech. 2020, 55, 340–347. [Google Scholar] [CrossRef]
- Mongelli, A.; Rodolfi, M.; Ganino, T.; Marieschi, M.; Caligiani, A.; Dall’Asta, C.; Bruni, R. Are Humulus lupulus L. ecotypes and cultivars suitable for the cultivation of aromatic hop in Italy? A phytochemical approach. Ind. Crops Prod. 2016, 83, 693–700. [Google Scholar] [CrossRef]
- Machado, M.P.; Gomes, E.N.; Francisco, F.; Bernert, A.F.; Bespalhok Filho, J.C.; Deschamps, C. Micropropagation and establishment of Humulus lupulus L. plantlets under field conditions at Southern Brazil. J. Agric. Sci. 2018, 10, 275. [Google Scholar] [CrossRef][Green Version]
- Rajput, B.S.; Jani, M.; Ramesh, K.; Manokari, M.; Jogam, P.; Allini, V.R.; Kher, M.M.; Shekhawat, M.S. Large-scale clonal propagation of Bambusa balcooa Roxb.: An industrially important bamboo species. Ind. Crops Prod. 2020, 157, 112905. [Google Scholar] [CrossRef]
- Gurriarán, M.J.; Revilla, M.A.; Tamés, R.S. Adventitious shoot regeneration in cultures of Humulus lupulus L. (hop) cvs. Brewers gold and Nugget. Plant Cell Rep. 1999, 18, 1007–1011. [Google Scholar] [CrossRef]
- Reed, B.M.; Okut, N.; D’Achino, J.; Narver, L.; DeNoma, J. Cold storage and cryopreservation of hops (Humulus L.) shoot cultures through application of standard protocols. CryoL Lett. 2003, 24, 389–396. [Google Scholar]
- Postman, J.D.; DeNoma, J.S.; Reed, B.M. Detection and elimination of viruses in USDA hop (Humulus lupulus) germoplasm collection. Acta Hortic. 2005, 668, 143–148. [Google Scholar] [CrossRef]
- Fortes, A.M.; Pais, M.S. Organogenesis from internode-derived nodules of Humulus lupulus var. Nugget (Cannabinaceae): Histological and changes in the starch content. Am. J. Bot. 2000, 87, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.T.; Leggett, G.; Koutoulis, A. Development of a shoot multiplication system for hop (Humulus lupulus L.). Vitr. Cell Dev. Biol. Plant 2001, 37, 79–83. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, A. A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Clapa, D.; Fira, A.; Joshee, N. An efficient ex vitro rooting and acclimatization method for horticultural plants using float hydroculture. HortScience 2013, 48, 1159–1167. [Google Scholar] [CrossRef]
- Lodhi, M.A.; Guang-Ning, Z.; Weeden, F.N.F.; Reisch, B.I. A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant Mol. Biol. Rep. 1994, 12, 6–13. [Google Scholar] [CrossRef]
- Pop, R.; Ardelean, M.; Pamfil, D.; Gaboreanu, I.M. The efficiency of different DNA isolation and purification in ten cultivars of Vitis vinifera. Bull. UASVM Anim. Sci. Biotechnol. 2003, 59, 259–261. [Google Scholar]
- Bodea, M.; Pamfil, D.; Pop, R.; Sisea, R.C. DNA isolation from desiccated leaf material from plum tree (Prunus domestica L.) molecular analysis. Bull. UASVM Hortic. 2016, 1, 1–2. [Google Scholar] [CrossRef]
- Williams, J.G.; Kubelik, A.R.; Livak, K.J.; Rafalski, J.A.; Tingey, S.V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990, 18, 6531–6535. [Google Scholar] [CrossRef]
- Pop, R.; Has, I.; Pop, I.F.; Harta, M.; Pamfil, D. Genetic Diversity Analysis of Maize Inbred Lines from SCDA Turda-Romania Revealed by RAPD Molecular Markers. Bull. Univ. Agric. Sci. 2011, 68, 277–283. [Google Scholar]
- Grigoriadou, K.; Leventakis, N. Large scale commercial production of potato minitubers, using in vitro techniques. Potato Res. 1999, 42, 607–610. [Google Scholar] [CrossRef]
- Volk, T.A.; Verwijst, T.; Tharakan, P.J.; Abrahamson, L.P.; White, E.H. Growing Fuel: A Sustainability Assessment of Willow Biomass Crops. Front. Ecol. Environ. 2004, 2, 411. [Google Scholar] [CrossRef]
- Grudzinska, M.; Solarska, E.; Czubacka, A.; Przybys, M.; Fajbus, A. Elimination of Hop Latent Viroid from Hop plants by cold treatment and meristem tip culture. Phytopathol. Pol. 2006, 40, 21–30. [Google Scholar]
- Al-Taleb, M.M.; Hassawi, D.S.; Abu-Romman, S.M. Production of virus free potato plants using meristem culture from cultivars grown under Jordanian environment. J. Agric. Environ. Sci. 2011, 11, 467–472. [Google Scholar]
- Azeez, H.; Ibrahim, K.; Pop, R.; Pamfil, D.; Hârţa, M.; Bobiș, O. Changes induced by gamma ray irradiation on biomass production and secondary metabolites accumulation in Hypericum triquetrifolium Turra callus cultures. Ind. Crops Prod. 2017, 108, 183–189. [Google Scholar] [CrossRef]
- Adhikary, D.; Kulkarni, M.; El-Mezawy, A.; Mobini, S.; Elhiti, M.; Gjuric, R.; Ray, A.; Polowick, P.; Slaski, J.J.; Jones, M.P.; et al. Medical cannabis and industrial hemp tissue culture: Present status and future potential. Front. Plant Sci. 2021, 12, 275. [Google Scholar] [CrossRef]
- Singh, V.; Tyagi, A.; Chauhan, P.K.; Kumari, P.; Kaushal, S. Identification and prevention of bacterial contamination on explant used in plant tissue culture labs. Int. J. Pharm. Pharm. Sci. 2011, 3, 160–163. [Google Scholar]
- Obisesan, I.A.; Sakpere, A.M.; Amujoyegbe, B.J.; Akinropo, M.S. Explants selection for in vitro propagation of Pachyrhizus erosus L. Not. Sci. Biol. 2021, 13, 10844. [Google Scholar] [CrossRef]
- Singh, C.R. Review on problems and its remedy in plant tissue culture. Asian J. Biol. Sci. 2018, 11, 165–172. [Google Scholar] [CrossRef]
- Mafakheri, M.; Hamidoghli, Y. Micropropagation of hop (Humulus lupulus L.) via shoot tip and node culture. Acta Hortic. 2019, 1236, 31–36. [Google Scholar] [CrossRef]
- Batista, D.; Ascensão, L.; Sousa, M.J.; Pais, M.S. Adventitious shoot mass production of hop (Humulus lupulus L.) var. Eroica in liquid medium from organogenic nodule cultures. Plant Sci. 2000, 151, 47–57. [Google Scholar] [CrossRef]
- Horlemann, C.; Schwekendiek, A.; Höhnle, M.; Weber, G. Regeneration and Agrobacterium-mediated transformation of hop (Humulus lupulus L.). Plant Cell Rep. 2003, 22, 210–217. [Google Scholar] [CrossRef]
- Faragó, J.; Pšenáková, I.; Faragová, N. The use of biotechnology in hop (Humulus lupulus L.) improvement. Nova Biotechnol. 2009, 9, 279–293. [Google Scholar]
- Trojak-Goluch, A.; Czarnecka, D. The effects of explant source and hormone content on plant regeneration and induction of tetraploids in Humulus lupulus L. Vitr. Cell. Dev. Biol. Plant 2015, 51, 152–159. [Google Scholar] [CrossRef]
- Gaspar, T.; Kevers, C.; Faivre-Rampant, O.; Cre’vecoeur, M.; Penel, C.; Greppin, H.; Dommes, J. Changing concepts in plant hormone action. Vitr. Cell. Dev. Biol. Plant 2003, 39, 85–106. [Google Scholar] [CrossRef]
- Faisal, M.; Alatar, A.A.; Abdel-Salam, E.M.; Qahtan, A.A. Effects of 4-CPPU on in vitro multiplication and sustainable generation of Hibiscus rosa-sinensis L.‘White Butterfly’. Saudi J. Biol. Sci. 2020, 27, 412–416. [Google Scholar] [CrossRef]
- Li, Q.; Yu, P.; Lai, J.; Gu, M. Micropropagation of the potential blueberry rootstock—Vaccinium arboreum through axillary shoot proliferation. Sci. Hortic. 2021, 280, 109908. [Google Scholar] [CrossRef]
- San José, M.C.; Cernadas, M.J.; Janeiro, L.V. Optimization of Micropropagation Protocols in Some Woody Plants Using Meta-topolin. In Meta-Topolin: A Growth Regulator for Plant Biotechnology and Agriculture; Springer: Singapore, 2021; pp. 221–240. [Google Scholar] [CrossRef]
- Vescan, L.A.; Clapa, D.; Fira, A.; Pamfil, D. Micropropagation of cold resistant blackberry cultivar ‘Gazda’. Bull. USAMV Anim. Sci. Biotechnol. 2012, 69, 282–289. [Google Scholar]
- Fira, A.; Clapa, D.; Cristea, V.; Plopa, C. In vitro propagation of Lonicera kamtschatica. Agric. Sci. Pract. 2014, 1–2, 89–90. [Google Scholar]
- Gentile, A.; Gutiérrez, M.J.; Martinez, J.; Frattarelli, A.; Nota, P.; Caboni, E. Effect of meta-Topolin on micropropagation and adventitious shoot regeneration in Prunus rootstocks. Plant Cell Tiss. Organ. Cult. 2014, 118, 373–381. [Google Scholar] [CrossRef]
- Gentile, A.; Frattarelli, A.; Nota, P.; Condello, E.; Caboni, E. The aromatic cytokinin meta-topolin promotes in vitro propagation, shoot quality and micrografting in Corylus colurna L. Plant Cell Tiss. Organ. Cult. 2017, 128, 693–703. [Google Scholar] [CrossRef]
- Fira, A.; Magyar-Tábori, K.; Hudák, I.; Clapa, D.; Dobránszky, J. Effect of gelling agents on in vitro development of Amelanchier canadensis ‘Rainbow Pillar’. Int. J. Hortic. Sci. 2013, 19, 75–79. [Google Scholar] [CrossRef]
- Fira, A.; Clapa, D.; Simu, M. Studies regarding the micropropagation of some blackberry cultivars. Bull. UASVM Hortic. 2014, 71, 22–37. [Google Scholar] [CrossRef]
- Fira, A.; Joshee, N.; Cristea, V.; Simu, M.; Hârța, M.; Pamfil, D.; Clapa, D. Optimization of Micropropagation Protocol for Goji Berry (Lycium barbarum L). Bull. UASVM Hortic. 2016, 73, 141–150. [Google Scholar] [CrossRef]
- Smýkalová, I.; Ortová, M.; Lipavská, H.; Patzak, J. Efficient in vitro micropropagation and regeneration of Humulus lupulus on low sugar, starch-Gelrite media. Biol. Plant 2001, 44, 7–12. [Google Scholar] [CrossRef]
- Kastrytskaya, M.S.; Kukharchyk, N.V.; Hashenka, O.A. Acclimatization of hop cultivars after in vitro propagation. Soil Sci. Agrochem. 2014, 1, 370–379. [Google Scholar]
- Hussain, A.; Ahmed, I.; Nazir, H.; Ullah, I. Plant Tissue Culture: Current Status and Opportunities. In Recent Advances in Plant In Vitro Culture; Chapter 1; IntechOpen: London, UK, 2012; pp. 1–28. [Google Scholar] [CrossRef]
- Debergh, P.C.; Maene, L.J. A scheme for commercial propagation of ornamental plants by tissue culture. Sci. Hortic. 1981, 14, 335–345. [Google Scholar] [CrossRef]
- Hazarika, B.N. Acclimatization of tissue-cultured plants. Curr. Sci. 2003, 85, 1704–1712. Available online: http://www.jstor.org/stable/24109975 (accessed on 2 September 2021).
- Wozny, A.; Miler, N. LEDs application in ex vitro rooting and acclimatization of chrysanthemum (Chrysanthemum x grandiflorum/Ramat/Kitam). Electron. J. Pol. Agric. Univ. 2016, 19, 1–8. Available online: http://www.ejpau.media.pl/volume19/issue4/art-02.html (accessed on 4 September 2021).
- Singh, A.; Agarwal, P.K. Enhanced micropropagation protocol of ex vitro rooting of a commercially important crop plant Simmondsia chinensis (Link) Schneider. J. For. Sci. 2016, 62, 107–115. [Google Scholar] [CrossRef]
- Dinesh, R.M.; Patel, A.K.; Vibha, J.B.; Shekhawat, S.; Shekhawat, N.S. Cloning of mature pomegranate (Punica granatum) cv. Jalore seedless via in vitro shoot production and ex vitro rooting. Vegetos 2019, 32, 181–189. [Google Scholar] [CrossRef]
- Goto, S.; Thakur, R.C.; Ishii, K. Determination of genetic stability in long-term micropropagated shoots of Pinus thunbergii Parl. using RADP markers. Plant Cell Rep. 1998, 18, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.K.; Chandel, P.; Gupta, S.; Gopal, J.; Singh, B.P.; Bhardwaj, V. Analysis of genetic stability of in vitro propagated potato microtubers using DNA markers. Physiol. Mol. Biol. Plants 2013, 19, 587–595. [Google Scholar] [CrossRef]
- Saha, S.; Adhikari, S.; Dey, T.; Ghosh, P. RAPD and ISSR based evaluation of genetic stability of micropropagated plantlets of Morus alba L. variety S-1. Meta Gene 2016, 7, 7–15. [Google Scholar] [CrossRef]
- Thakur, J.; Dwivedi, M.D.; Sourabh, P.; Uniyal, P.L.; Pandey, A.K. Genetic homogeneity revealed using SCoT, ISSR and RAPD markers in micropropagated Pittosporum eriocarpum royle—An endemic and endangered medicinal plant. PLoS ONE 2016, 11, e0159050. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Rai, M.K.; Shekhawat, N.S.; Kataria, V. Genetic homogeneity revealed in micropropagated Bauhinia racemosa Lam. using gene targeted markers CBDP and SCoT. Physiol. Mol. Biol. Plants 2019, 25, 581–588. [Google Scholar] [CrossRef]
- Tikendra, L.; Koijam, A.S.; Nongdam, P. Molecular markers based genetic fidelity assessment of micropropagated Dendrobium chrysotoxum Lindl. Meta Gene 2019, 20, 100562. [Google Scholar] [CrossRef]
- Nanda, R.M.; Das, P.; Rout, G.R. In vitro clonal propagation of Acacia mangium Willd and its evaluation of genetic stability through RAPD marker. Ann. For. Sci. 2004, 61, 381–386. [Google Scholar] [CrossRef]
- Kumar, S.; Mangal, M.; Dhawan, A.K.; Singh, N. Assessment of genetic fidelity of micropropagated plants of Simmondsia chinensis (Link) Schneider using RAPD and ISSR markers. Acta Physiol. Planta 2011, 33, 2541–2545. [Google Scholar] [CrossRef]
- Saha, S.; Abdul, K.; Parthadeb, G. In vitro propagation of Ocimum gratissimum L. (Lamiaceae) and its evaluation of genetic fidelity using RAPD marker. Am. J. Plant Sci. 2012, 3, 64–74. [Google Scholar] [CrossRef]
- Modi, A.R.; Patil, G.; Kumar, N.; Singh, A.S.; Subhash, N. A Simple and efficient in vitro mass multiplication procedure for Stevia rebaudiana Bertoni and analysis of genetic fidelity of in vitro raised plants through RAPD. Sugar Tech. 2012, 14, 391–397. [Google Scholar] [CrossRef]
- Çetin, B. Evaluation of the genetic fidelity of in vitro raised plants of Origanum majorana L. using Random Amplified Polymorphic DNA. Celal Bayar Univ. J. Sci. 2018, 14, 237–239. [Google Scholar] [CrossRef][Green Version]
- Yadav, A.; Kothari, S.L.; Kachhwaha, S.; Joshi, A. In vitro propagation of chia (Salvia hispanica L.) and assessment of genetic fidelity using random amplified polymorphic DNA and intersimple sequence repeat molecular markers. J. Appl. Biol. Biotechnol. 2019, 7, 42–47. [Google Scholar]
- Penner, G.A.; Bush, A.; Wise, R.; Kim, W.; Domier, L.; Kasha, K.; Laroche, A.; Scoles, G.; Molnar, S.J.; Fedak, G. Reproducibility of random amplified polymorphic DNA (RAPD) analysis among laboratories. Genome Res. 1993, 2, 341–345. [Google Scholar] [CrossRef]
- Micheli, M.R.; Bova, R.; Pascale, E.; D’Ambrosio, E. Reproducible DNA fingerprinting with the random amplified polymorphic DNA (RAPD) method. Nucleic Acids Res. 1994, 22, 1921. [Google Scholar] [CrossRef]
- Jones, C.J.; Edwards, K.J.; Castaglione, S.; Winfield, M.O.; Sala, F.; Van de Wiel, C.; Bredemeijer, G.; Vosman, B.; Matthes, M.; Daly, A.; et al. Reproducibility testing of RAPD, AFLP and SSR markers in plants by a network of European laboratories. Mol. Breed. 1997, 3, 381–390. [Google Scholar] [CrossRef]
- Ramos, J.R.; Telles, M.P.; Diniz-Filho, J.A.; Soares, T.N.; Melo, D.B.; Oliveira, G. Optimizing reproducibility evaluation for random amplified polymorphic DNA markers. Genet. Mol. Res. 2008, 7, 1384–1391. [Google Scholar] [CrossRef]
- Collard, B.C.; Mackill, D.J. Start codon targeted (SCoT) polymorphism: A simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol. Biol Rep. 2009, 27, 86–93. [Google Scholar] [CrossRef]
- Xiong, F.; Zhong, R.; Han, Z.; Jiang, J.; He, L.; Zhuang, W.; Tang, R. Start codon targeted polymorphism for evaluation of functional genetic variation and relationships in cultivated peanut (Arachis hypogaea L.) genotypes. Mol. Bio. Rep. 2011, 38, 3487–3494. [Google Scholar] [CrossRef] [PubMed]
- Chhajer, S.; Jukanti, A.K.; Kalia, R.K. Start codon targeted (SCoT) polymorphism-based genetic relationships and diversity among populations of Tecomella undulata (Sm.) Seem-an endangered timber tree of hot arid regions. Tree Genet. Genomes 2017, 13, 84. [Google Scholar] [CrossRef]

| Rooting Method | Shoots’ and Roots’ Morphometry | Rooting (%) | Survival under Greenhouse Conditions (%) | |||
|---|---|---|---|---|---|---|
| No.of Shoots | Length of Shoots (cm) | No. of Roots | Length of Roots (cm) | |||
| In vitro rooting | 1.92 ± 0.08 c | 5.65 ± 0.21 b | 6.95 ± 0.47 b | 1.13 ± 0.06 b | 86.66 ± 0.39 a | 94 a |
| Ex vitro rooting on perlite | 1.55 ± 0.07 b | 4.89 ± 0.19 a | 6.56 ± 0.62 c | 1.29 ± 0.09 c | 96.00 ± 0.60 b | 100 b |
| Ex vitro rooting on peat + perlite | 1.35 ± 0.06 a | 4.95 ± 0.15 a | 5.56 ± 0.52 a | 1.19 ± 0.19 a | 85.71 ± 0.27 a | 100 b |
| Primer Code | Primer Sequence (5′-3′) | No. of Scorable Bands | Size Range of Bands (bp) |
|---|---|---|---|
| OPA04 | AATCGCGCTG | 3 | 525–1330 |
| OPAB11 | GTGCGCAATG | 5 | 325–1545 |
| OPB08 | GTCCACACGG | 6 | 220–1336 |
| OPC08 | TGGACCGGTG | 6 | 253–1408 |
| OPD16 | AGGGCGTAAG | 5 | 255–1605 |
| OPF13 | GGCTGCAGAA | 6 | 345–1218 |
| OPF20 | GGTCTAGAGG | 6 | 355–1115 |
| OPAB18 | CTGGCGTGTC | 5 | 305–1435 |
| OPE14 | TGCGGCTGAG | 6 | 410–1005 |
| OPF16 | GGAGTACTGG | 5 | 317–1214 |
| OPX03 | TGGCGCAGTG | 7 | 408–1415 |
| OPP18 | GGCTTGGCCT | 6 | 415–1138 |
| Total bands | - | 66 | - |
| SCOT1 | CAACAATGGCTACCACCA | 4 | 554–1028 |
| SCOT2 | CAACAATGGCTACCACCC | 3 | 354–1464 |
| SCOT3 | CAACAATGGCTACCACCG | 5 | 528–2018 |
| SCOT4 | CAACAATGGCTACCACCT | 6 | 536–2520 |
| SCOT5 | CAACAATGGCTACCACGA | 5 | 514–2550 |
| SCOT6 | CAACAATGGCTACCACGC | 5 | 500–1578 |
| SCOT7 | CAACAATGGCTACCACGG | 6 | 564–2514 |
| SCOT8 | CAACAATGGCTACCACGT | 6 | 586–2058 |
| SCOT9 | CAACAATGGCTACCAGCA | 5 | 754–2566 |
| SCoT10 | CAACAATGGCTACCAGCC | 4 | 542–1564 |
| SCOT16 | ACCATGGCTACCACCGAC | 5 | 528–2012 |
| SCOT25 | ACCATGGCTACCACCGGG | 6 | 456–1036 |
| Total bands | - | 60 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clapa, D.; Hârța, M. Establishment of an Efficient Micropropagation System for Humulus lupulus L. cv. Cascade and Confirmation of Genetic Uniformity of the Regenerated Plants through DNA Markers. Agronomy 2021, 11, 2268. https://doi.org/10.3390/agronomy11112268
Clapa D, Hârța M. Establishment of an Efficient Micropropagation System for Humulus lupulus L. cv. Cascade and Confirmation of Genetic Uniformity of the Regenerated Plants through DNA Markers. Agronomy. 2021; 11(11):2268. https://doi.org/10.3390/agronomy11112268
Chicago/Turabian StyleClapa, Doina, and Monica Hârța. 2021. "Establishment of an Efficient Micropropagation System for Humulus lupulus L. cv. Cascade and Confirmation of Genetic Uniformity of the Regenerated Plants through DNA Markers" Agronomy 11, no. 11: 2268. https://doi.org/10.3390/agronomy11112268
APA StyleClapa, D., & Hârța, M. (2021). Establishment of an Efficient Micropropagation System for Humulus lupulus L. cv. Cascade and Confirmation of Genetic Uniformity of the Regenerated Plants through DNA Markers. Agronomy, 11(11), 2268. https://doi.org/10.3390/agronomy11112268







