Resource Recovery from Synthetic Nitrified Urine in the Hydroponic Cultivation of Lettuce (Lactuca sativa Var. capitata L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Urine
2.2. Urine Nitrification
2.3. Hydroponic Set-Up
2.4. Analytical Methods
2.5. Nutrient Recovery from Humans for Cultivation of Lettuce
3. Results
3.1. Yield
3.2. Lettuce Composition
3.3. Nutrient Recovery in the Lettuce from the Urine
4. Discussion
4.1. Yield and Lettuce Composition
4.2. Effects of Excessive Sodium and Chloride
4.3. Nutrient Content Levels
4.4. Nutrient Content of the Treatment Solutions
4.5. Nutrient Recovery and the Pros and Cons of Using Nitrified Urine as Fertilizer
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bongaarts, J. Human population growth and the demographic transition. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2985–2990. [Google Scholar] [CrossRef] [Green Version]
- Cortés-Gutiérrez, E.A.; Herrera-Sancho, O.A. Urban growth tendency of electrical cables in the Costa Rican Metropolitan Area. Phys. A Stat. Mech. Its Appl. 2021, 562, 125371. [Google Scholar] [CrossRef]
- Kovacs, B.; Kotroczó, Z.; Kocsis, L.; Biró, B. Potentials of indoor lettuce production in natural forest soil at limited watering. J. Cent. Eur. Agric. 2020, 21, 531–536. [Google Scholar] [CrossRef]
- Souza, S.V.; Gimenes, R.M.T.; Binotto, E. Economic viability for deploying hydroponic system in emerging countries: A differentiated risk adjustment proposal. Land Use Policy 2019, 83, 357–369. [Google Scholar] [CrossRef]
- Barbosa, G.; Gadelha, F.; Kublik, N.; Proctor, A.; Reichelm, L.; Weissinger, E.; Wohlleb, G.; Halden, R. Comparison of Land, Water, and Energy Requirements of Lettuce Grown Using Hydroponic vs. Conventional Agricultural Methods. Int. J. Environ. Res. Public Health 2015, 12, 6879–6891. [Google Scholar] [CrossRef] [Green Version]
- Domingues, D.S.; Takahashi, H.W.; Camara, C.A.P.; Nixdorf, S.L. Automated system developed to control pH and concentration of nutrient solution evaluated in hydroponic lettuce production. Comput. Electron. Agric. 2012, 84, 53–61. [Google Scholar] [CrossRef]
- Carvalho, R.d.S.C.; Bastos, R.G.; Souza, C.F. Influence of the use of wastewater on nutrient absorption and production of lettuce grown in a hydroponic system. Agric. Water Manag. 2018, 203, 311–321. [Google Scholar] [CrossRef] [Green Version]
- Maestre-Valero, J.F.; Martin-Gorriz, B.; Soto-García, M.; Martinez-Mate, M.A.; Martinez-Alvarez, V. Producing lettuce in soil-based or in soilless outdoor systems. Which is more economically profitable? Agric. Water Manag. 2018, 206, 48–55. [Google Scholar] [CrossRef]
- Karak, T.; Bhattacharyya, P. Human urine as a source of alternative natural fertilizer in agriculture: A flight of fancy or an achievable reality. Resour. Conserv. Recycl. 2011, 55, 400–408. [Google Scholar] [CrossRef]
- Magwaza, S.T.; Magwaza, L.S.; Odindo, A.O.; Mditshwa, A. Hydroponic technology as decentralised system for domestic wastewater treatment and vegetable production in urban agriculture: A review. Sci. Total Environ. 2020, 698, 134154. [Google Scholar] [CrossRef]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamsson, M. Potential use of human urine by greenhouse culturing of microalgae (Scenedesmus acuminatus), zooplankton (Daphnia magna) and tomatoes (Lycopersicon). Ecol. Eng. 2000, 16, 243–254. [Google Scholar] [CrossRef]
- Akpan-Idiok, A.U.; Udo, I.A.; Braide, E.I. The use of human urine as an organic fertilizer in the production of okra (Abelmoschus esculentus) in South Eastern Nigeria. Resour. Conserv. Recycl. 2012, 62, 14–20. [Google Scholar] [CrossRef]
- Heinonen-Tanski, H.; Sjöblom, A.; Fabritius, H.; Karinen, P. Pure human urine is a good fertiliser for cucumbers. Bioresour. Technol. 2007, 98, 214–217. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Nerg, A.M.; Sjöblom, A.; Holopainen, J.K.; Heinonen-Tanski, H. Use of Human Urine Fertilizer in Cultivation of Cabbage (Brassica oleracea)––Impacts on Chemical, Microbial, and Flavor Quality. J. Agric. Food Chem. 2007, 55, 8657–8663. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Holopainen, J.K.; Weisell, J.; Heinonen-Tanski, H. Human Urine and Wood Ash as Plant Nutrients for Red Beet (Beta vulgaris) Cultivation: Impacts on Yield Quality. J. Agric. Food Chem. 2010, 58, 2034–2039. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Holopainen, J.K.; Heinonen-Tanski, H. Stored Human Urine Supplemented with Wood Ash as Fertilizer in Tomato (Solanum lycopersicum) Cultivation and Its Impacts on Fruit Yield and Quality. J. Agric. Food Chem. 2009, 57, 7612–7617. [Google Scholar] [CrossRef]
- Ganrot, Z.; Dave, G.; Nilsson, E.; Li, B. Plant availability of nutrients recovered as solids from human urine tested in climate chamber on Triticum aestivum L. Bioresour. Technol. 2007, 98, 3122–3129. [Google Scholar] [CrossRef]
- Morozov, Y.A.; Trifonov, S.V.; Ushakova, S.A.; Anishchenko, O.V.; Tikhomirov, A.A. Feasibility of incorporating all products of human waste processing into material cycling in the BTLSS. Life Sci. Space Res. 2018, 18, 29–34. [Google Scholar] [CrossRef]
- Sibanda, M.; Mutanga, O.; Magwaza, L.S.; Dube, T.; Magwaza, S.T.; Odindo, A.O.; Mditshwa, A.; Mafongoya, P.L. Discrimination of Tomato Plants (Solanum lycopersicum) Grown under Anaerobic Baffled Reactor Effluent, Nitrified Urine Concentrates and Commercial Hydroponic Fertilizer Regimes Using Simulated Sensor Spectral Settings. Agronomy 2019, 9, 373. [Google Scholar] [CrossRef] [Green Version]
- Zabel, P.; Bornemann, G.; Tajmar, M.; Schubert, D. Yield of dwarf tomatoes grown with a nutrient solution based on recycled synthetic urine. Life Sci. Space Res. 2019, 20, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Giannis, A.; Chang, V.W.C.; Liu, B.; Zhang, J.; Wang, J.Y. Application of hydroponic systems for the treatment of source-separated human urine. Ecol. Eng. 2015, 81, 182–191. [Google Scholar] [CrossRef]
- Tikhomirov, A.; Ushakova, S.; Tikhomirova, N.; Velichko, V.; Trifonov, S.; Anishchenko, O. Establishing cycling processes in an experimental model of a closed ecosystem. Acta Astronaut. 2020, 166, 537–544. [Google Scholar] [CrossRef]
- Volpin, F.; Jiang, J.; El Saliby, I.; Preire, M.; Lim, S.; Johir, M.A.H.; Choc, J.; Hand, D.S.; Phuntshoa, S.; Shon, H.K. Sanitation and dewatering of human urine via membrane bioreactor and membrane distillation and its reuse for fertigation. J. Clean. Prod. 2020, 270, 122390. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Geelen, D.; De Paepe, J.; Clauwaert, P.; De Pascale, S.; Rouphael, Y. An Appraisal of Urine Derivatives Integrated in the Nitrogen and Phosphorus Inputs of a Lettuce Soilless Cultivation System. Sustainability 2021, 13, 4218. [Google Scholar] [CrossRef]
- Feng, D.L.; Wu, Z.C. Culture of Spirulina platensis in human urine for biomass production and O2 evolution. J. Zhejiang Univ. Sci. B 2006, 7, 34–37. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.S.; Ewert, M.K.; Keener, J.F.; Wagner, S.A. Life Support Baseline Values and Assumptions Document NASA/TP-2015-218570/REV1; NASA: Washington, DC, USA, 2018.
- Janiak, K.; Jurga, A.; Wizimirska, A.; Miodoński, S.; Muszyński-Huhajło, M.; Ratkiewicz, K.; Zięba, B. Urine nitrification robustness for application in space: Effect of high salinity and the response to extreme free ammonia concentrations. J. Environ. Manag. 2021, 279, 111610. [Google Scholar] [CrossRef]
- Koller, D.; Ritter, S.; Heller, E. Light-driven movements of the primary leaves of bean (Phaseolus vulgaris L.): A kinetic analysis. Isr. J. Plant Sci. 2001, 49, 1–7. [Google Scholar] [CrossRef]
- Bie, Z.; Ito, T.; Shinohara, Y. Effects of sodium sulfate and sodium bicarbonate on the growth, gas exchange and mineral composition of lettuce. Sci. Hortic. 2004, 99, 215–224. [Google Scholar] [CrossRef]
- Avgoustaki, D.D.; Xydis, G. How energy innovation in indoor vertical farming can improve food security, sustainability, and food safety? Adv. Food Secur. Sustain. 2020, 5, 1–51. [Google Scholar] [CrossRef]
- Adrover, M.; Moyà, G.; Vadell, J. Use of hydroponics culture to assess nutrient supply by treated wastewater. J. Environ. Manag. 2013, 127, 162–165. [Google Scholar] [CrossRef]
- Roosta, H.R. Interaction between water alkalinity and nutrient solution pH on the vegetative growth, chlorophyll fluorescence and leaf magnesium, iron, manganese, and zinc concentrations in lettuce. J. Plant Nutr. 2011, 34, 717–731. [Google Scholar] [CrossRef]
- Roosta, H.R.; Hamidpour, M. Effects of foliar application of some macro-and micro-nutrients on tomato plants in aquaponic and hydroponic systems. Sci. Hortic. 2011, 129, 396–402. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef] [Green Version]
- López-Millán, A.F.; Grusak, M.A.; Abadía, A.; Abadía, J. Iron deficiency in plants: An insight from proteomic approaches. Front. Plant Sci. 2013, 4, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in soils, water and food crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic press: Cambridge, MA, USA, 2011. [Google Scholar]
- Simon, E.W. The symptoms of calcium deficiency in plants. New Phytol. 1978, 80, 1–15. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Tang, B.; Gu, M. Growth Responses and Root Characteristics of Lettuce Grown in Aeroponics, Hydroponics, and Substrate Culture. Horticulturae 2018, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Hospido, A.; i Canals, L.M.; McLaren, S.; Truninger, M.; Edwards-Jones, G.; Clift, R. The role of seasonality in lettuce consumption: A case study of environmental and social aspects. Int. J. Life Cycle Assess. 2009, 14, 381–391. [Google Scholar] [CrossRef]
- Długosz-Grochowska, O.; Wojciechowska, R.; Kruczek, M.; Habela, A. Supplemental lighting with LEDs improves the biochemical composition of two Valerianella locusta (L.) cultivars. Hortic. Environ. Biotechnol. 2017, 58, 441–449. [Google Scholar] [CrossRef]
- Kleiber, T.; Krzesinski, W.; Przygocka-Cyna, K.; Spizewski, T. The response of hydroponically grown lettuce under Mn stress to differentiated application of silica sol. J. Elem. 2015, 20. [Google Scholar] [CrossRef] [Green Version]
- Fallovo, C.; Rouphael, Y.; Rea, E.; Battistelli, A.; Colla, G. Nutrient solution concentration and growing season affect yield and quality of Lactuca sativa L. var. acephala in floating raft culture. J. Sci. Food Agric. 2009, 89, 1682–1689. [Google Scholar] [CrossRef]
- Mills, H.A.; Jones, J.B.; Benton, J. Plant Analysis Handbook II, 2nd ed.; MicroMacro Publishing: Athens, GA, USA, 1996; pp. 1–420. [Google Scholar]
- Wojciechowska, E.; Nawrot, N.; Matej-Łukowicz, K.; Gajewska, M.; Obarska-Pempkowiak, H. Seasonal changes of the concentrations of mineral forms of nitrogen and phosphorus in watercourses in the agricultural catchment area (Bay of Puck, Baltic Sea, Poland). Water Supply 2019, 19, 986–994. [Google Scholar] [CrossRef]
- De Kreij, C.; Sonneveld, C.; Warmenhoven, M.G.; Straver, N.A. Guide values for nutrient element contents of vegetables and flowers under glass. Voedingsoploss. Glas. 1992, 15, 20–25. [Google Scholar]
- Winsor, G.; Adams, P. Glasshouse Crops. Volume 3. Diagnosis of Mineral Disorders in Plants; Her Majesty’s Stationery Office: London, UK, 1987; pp. 1–168. [Google Scholar]
- Singer, S.M.; Hamza, A.E.; Abd El-Samadl, E.H.; Sawan, O.M.; El-Behairy, U.A.; Abou-Hadid, A.F. Growth, Yield and Mineral Contents of Lettuce Cultivars Grown in Nutrient Film Technique (NFT) at Different Transplanting Dates. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 172–183. [Google Scholar]
- Rietra, R.P.J.J.; Heinen, M.; Dimkpa, C.O.; Bindraban, P.S. Effects of nutrient antagonism and synergism on yield and fertilizer use efficiency. Commun. Soil Sci. Plant Anal. 2017, 48, 1895–1920. [Google Scholar] [CrossRef] [Green Version]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Kozik, E.; Tyksiński, W.; Komosa, A. Effect of chelated and mineral forms of micronutrients on their content in leaves and the yield of lettuce. Part I. Manganese. Acta Sci. Pol. Hortorum Cultus 2008, 7, 73–82. [Google Scholar]
- Ylivainio, K.; Jaakkola, A.; Aksela, R. Effects of Fe compounds on nutrient uptake by plants grown in sand media with different pH. J. Plant Nutr. Soil Sci. 2004, 167, 602–608. [Google Scholar] [CrossRef]
- Sahin, S.; Kısa, D.; Göksu, F.; Geboloğlu, N. Effects of boron applications on the physiology and yield of lettuce. Annu. Res. Rev. Biol. 2017, 21, 1–7. [Google Scholar] [CrossRef]
- Mampholo, B.M.; Maboko, M.M.; Soundy, P.; Sivakumar, D. Phytochemicals and overall quality of leafy lettuce (Lactuca sativa L.) varieties grown in closed hydroponic system. J. Food Qual. 2016, 39, 805–815. [Google Scholar] [CrossRef]
- Jurga, A.; Janiak, K.; Ratkiewicz, K.; Podstawczyk, D. An overview of blackwater data collection from space life support systems and its comparison to a terrestrial wastewater dataset. J. Environ. Manag. 2019, 241, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Roobeek, A. White Paper on Vertical Horticulture International Overview of Vertical Horticultural Projects, 1st ed.; MeetingMoreMinds: Amsterdam, The Netherlands, 2018; pp. 1–23. [Google Scholar]
- McConville, J.R.; Kvarnström, E.; Jönsson, H.; Kärrman, E.; Johansson, M. Source separation: Challenges & opportunities for transition in the swedish wastewater sector. Resour. Conserv. Recycl. 2017, 120, 144–156. [Google Scholar] [CrossRef]
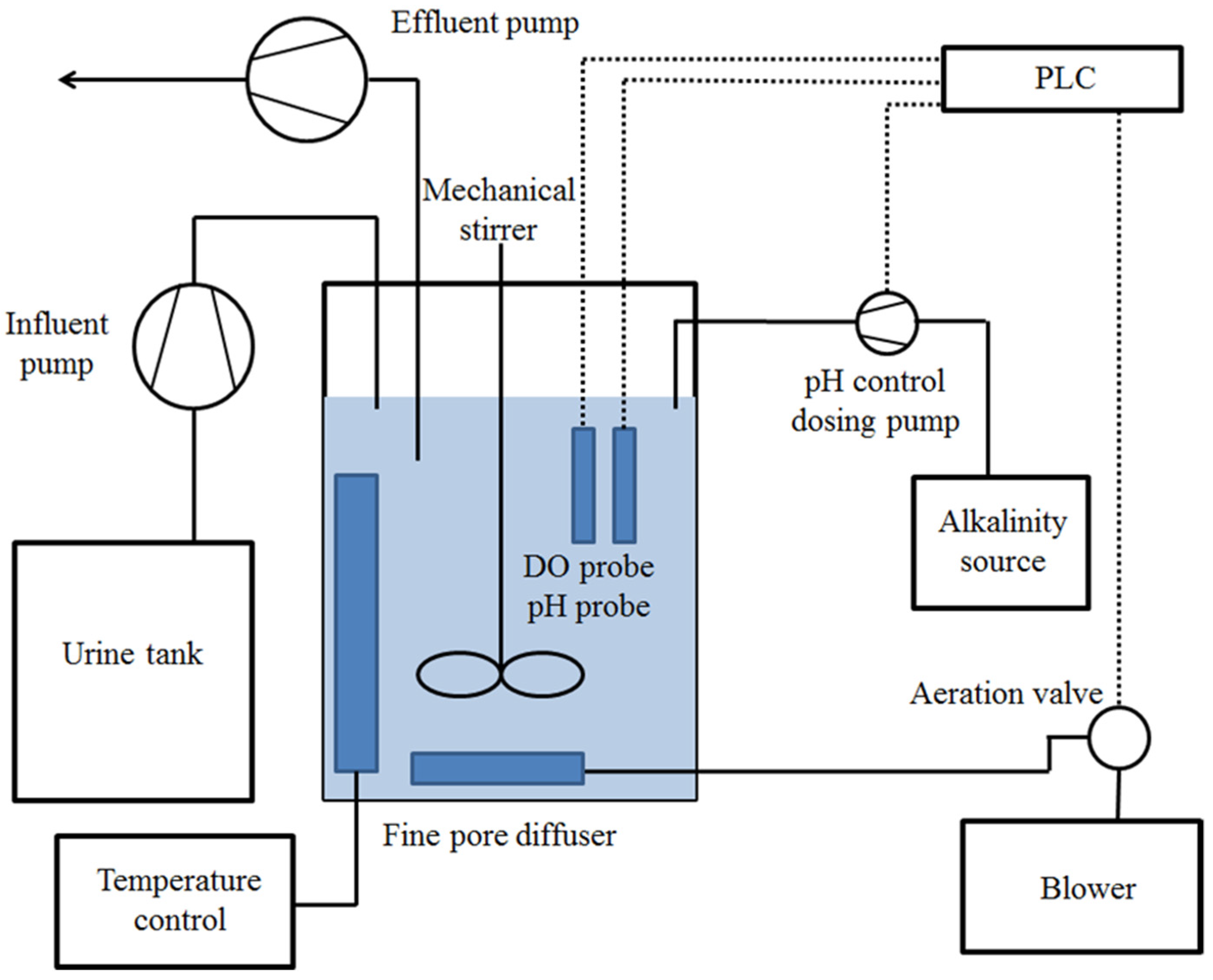
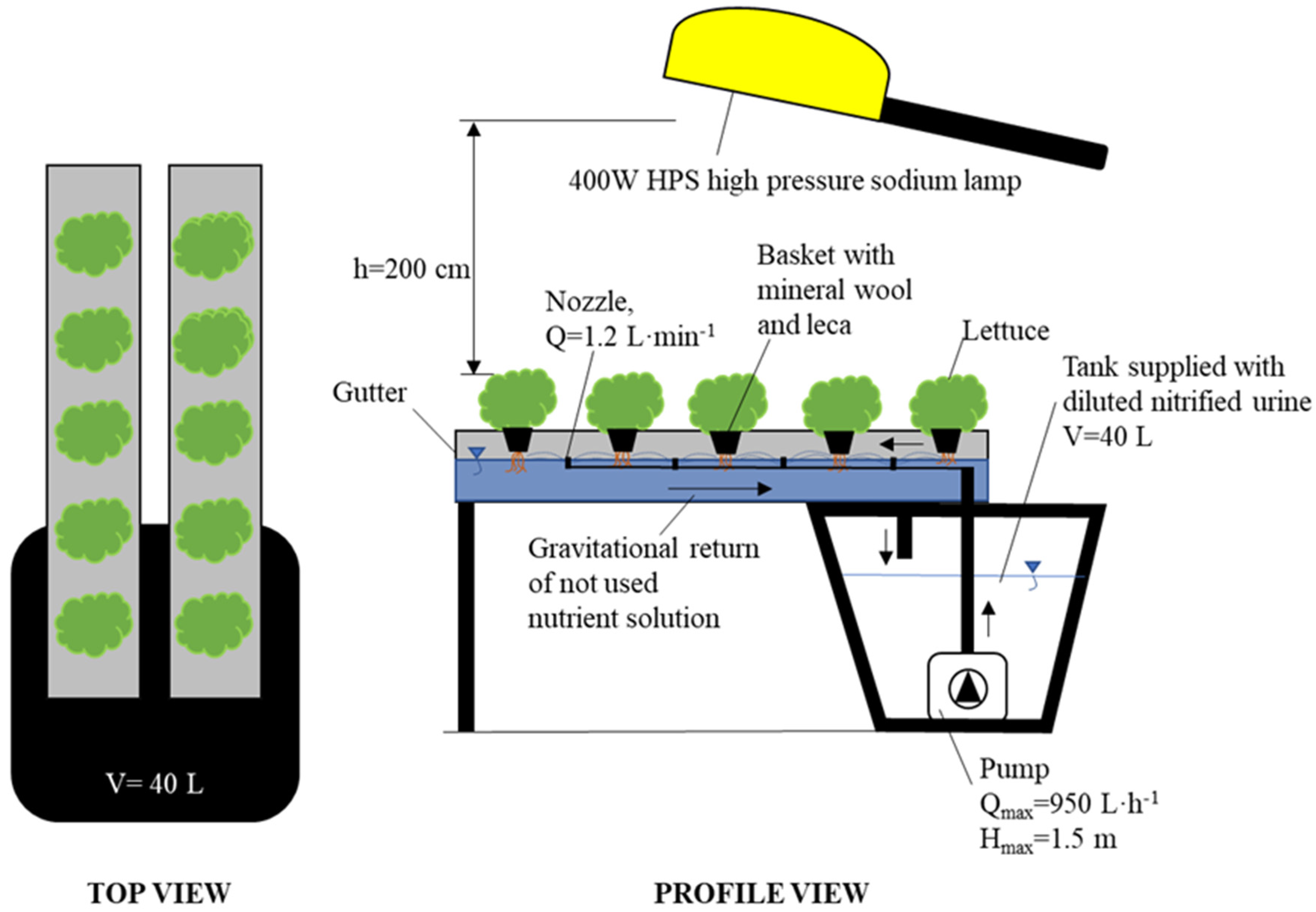
| Module 1 Diluted Nitrified Urine (DNU) | Module 2 Diluted Nitrified Urine Enriched (DNUE) | Module 3 Reference Commercial Fertilizers (Ref) |
|---|---|---|
| 4 L undiluted nitrified urine | 4 L undiluted nitrified urine | 24 g CaNO3 (15.5% N, 18.5% Ca) |
| Topped up with distilled water to 40 L | 12 g CaNO3 (15.5% N, 18.5% Ca) | 40 g Peters Orange fertilizer with ICL Special Fertilizers (16% N, 5% P2O5, 25% K2O, 3.4% MgO, 0.1% Fe, 0.04% Mn, 0.01% B, 0.01% Cu, 0.01% Zn, 0.001% Mo). |
| 10 g K2S (44.8% K, 17% S) | ||
| 1 g Hortisol Micro (7% Fe, 4% Mn, 0.8% Zn, 0.4% Cu, 0.05% Mo, 0.01% Co) | ||
| Topped up with distilled water to 40 L | Topped up with distilled water to 40 L |
| Parameter | Unit | Undiluted Feedstock | Module 1 Diluted Nitrified Urine (DNU) | Module 2 Diluted Nitrified Urine Enriched (DNUE) | Module 3 Reference |
|---|---|---|---|---|---|
| pH | - | 6.50 ± 0.15 | 7.40 ± 0.15 | 7.35 ± 0.15 | 7.15 ± 0.15 |
| EC (electrical conductivity) | mS·cm−1 | 25.60 ± 0.05 | 2.70 ± 0.05 | 3.12 ± 0.05 | 1.85 ± 0.05 |
| N-NO3− | mg·L−1 | 2140 ± 5 | 214 ± 5 | 261 ± 5 | 168 ± 5 |
| N-NH4+ | mg·L−1 | 0 | 0 | 0 | 82 ± 5 |
| P | mg·L−1 | 174.0 ± 0.5 | 17.4 ± 0.5 | 17.4 ± 0.5 | 22.0 ± 0.5 |
| K+ | mg·L−1 | 906 ± 2.5 | 90.6 ± 2.5 | 200.6 ± 2.5 | 199.6 ± 2.5 |
| Ca2+ | mg·L−1 | 160 ± 1.5 | 16 ± 1.5 | 70.6 ± 1.5 | 105.2 ± 1.5 |
| Mg2+ | mg·L−1 | 64.6 ± 0.2 | 6.5 ± 0.2 | 21.5 ± 0.2 | 20.4 ± 0.2 |
| Na+ | mg·L−1 | 5900 ± 0.5 | 590.0 ± 0.5 | 590.0 ± 0.5 | 5.2 ± 0.5 |
| Cl− | mg·L−1 | 1830 ± 1.5 | 183 ± 1.5 | 184.5 ± 1.5 | 5.2 ± 1.5 |
| SO42− | mg·L−1 | 1890 ± 5 | 189 ± 5 | 265 ± 5 | 152 ± 5 |
| Fe | mg·L−1 | 0.20 ± 0.02 | 0.02 ± 0.02 | 1.75 ± 0.02 | 2.05 ± 0.02 |
| Mn | mg·L−1 | 0.20 ± 0.05 | <0.02 | 1.00 ± 0.05 | 0.94 ± 0.05 |
| Cu | mg·L−1 | 0.20 ± 0.05 | 0.02 ± 0.05 | 0.10 ± 0.05 | 0.08 ± 0.05 |
| Zn | mg·L−1 | 0.20 ± 0.05 | 0.02 ± 0.05 | 0.20 ± 0.05 | 0.21 ± 0.05 |
| B | mg·L−1 | 1.90 ± 0.05 | 0.19 ± 0.05 | 0.25 ± 0.05 | 0.26 ± 0.05 |
| Mo | mg·L−1 | trace | trace | 0.05 ± 0.01 | 0.06 ± 0.01 |
| Module Number | Leaf FM | Leaf DM | Stem FM | Stem DM | Root FM | Harvest Index |
|---|---|---|---|---|---|---|
| - | g·plant−1 | % FM | g·plant−1 | % FM | g·plant−1 | % |
| Module 1 Diluted Nitrified Urine (N = 8) | 29.14 ± 11.52 | 4.71 ± 0.03 | 4.96 ± 1.53 | 8.68 ± 0.37 | 3.27 ± 0.45 | 90 ± 3 |
| Module 2 Diluted Nitrified Urine Enriched (N = 10) | 52.73 ± 10.22 | 4.21 ± 0.02 | 8.39 ± 1.90 | 8.25 ± 0.05 | 2.69 ± 0.45 | 96 ± 1 |
| Module 3 Reference (N = 7) | 57.47 ± 9.55 | 4.06 ± 0.04 | 8.84 ± 1.37 | 8.06 ± 0.03 | 2.62 ± 0.59 | 96 ± 1 |
| Parameter | Module 1 Diluted Nitrified Urine (DNU) (N = 8) | Module 2 Diluted Nitrified Urine Enriched (DNUE) (N = 10) | Module 3 Reference (N = 7) |
|---|---|---|---|
| - | (in g·kg DM−1) | ||
| N | 45.7 ± 0.3 | 46.5 ± 0.3 | 49.4 ± 0.3 |
| P | 7.53 ± 0.03 | 7.58 ± 0.03 | 6.30 ± 0.03 |
| K | 66.30 ± 0.25 | 85.00 ± 0.25 | 81.70 ± 0.25 |
| Ca | 5.91 ± 0.05 | 8.82 ± 0.05 | 14.90 ± 0.05 |
| Mg | 3.23 ± 0.02 | 3.14 ± 0.02 | 4.02 ± 0.02 |
| Na | 50.70 ± 0.02 | 42.8 ± 0.02 | 0.70 ± 0.02 |
| Cl | 2.88 ± 0.05 | 3.90 ± 0.05 | 4.30 ± 0.05 |
| S | 15.60 ± 0.02 | 17.70 ± 0.02 | 4.60 ± 0.02 |
| (in mg·kg DM−1) | |||
| Fe | 42.5 ± 2.5 | 72.8 ± 2.5 | 278 ± 2.5 |
| Mn | 11.1 ± 2.0 | 36.8 ± 2.0 | 182 ± 2 |
| Cu | 10.9 ± 0.25 | 11.5 ± 0.25 | 11.3 ± 0.25 |
| Zn | 56.6 ± 2.5 | 70.8 ± 2.5 | 105.0 ± 2.5 |
| B | 33.1 ± 1.5 | 38.3 ± 1.5 | 42.3 ± 1.5 |
| (in mg·100g FM−1) | |||
| Chlorophyll A | 24.87 ± 1.5 | 49.98 ± 1.5 | 55.83 ± 1.5 |
| Chlorophyll B | 8.9 ± 0.5 | 18.7 ± 0.5 | 21.5 ± 0.5 |
| Carotenoids | 53 ± 5 | 156 ± 5 | 190 ± 5 |
| Parameter (g/Lettuce Edible Mass) | Diluted Nitrified Urine (DNU) | Diluted Nitrified Urine Enriched (DNUE) | |
|---|---|---|---|
| Assimilated from the Urine Content (Recovery) | Assimilated from the Added Supplements | ||
| N | 0.082 | 0.111 | 0.024 |
| P | 0.014 | 0.022 | 0.000 |
| K | 0.120 | 0.112 | 0.136 |
| Ca | 0.011 | 0.006 | 0.020 |
| Mg | 0.006 | 0.003 | 0.006 |
| Cl | 0.028 | 0.052 | 0.000 |
| S | 0.005 | 0.008 | 0.003 |
| Relative Elemental Abundance | DNU Treatment (DNU) | Zabel [21] | Yang [22] | Volpin [24] | The Reference Treatment (Module 3) |
|---|---|---|---|---|---|
| N/N | 1(+) | 1(+) | 1(+) | 1(+) | 1 |
| P/N | 0.08(+) | 0.07(+) | 0.01(+) | 0.07(+) | 0.088 |
| K/N | 0.42(−) | 0.22(−) | 1.38(++) | 0.27(−) | 0.8 |
| Ca/N | 0.07(−) | 0.51(+) | 0.01(−) | 0.01(−) | 0.42 |
| Mg/N | 0.03(−) | 0.02(−) | 0.03(−) | 0.001(−) | 0.08 |
| Na/N | 2.78 (++) | 0.33(++) | 3.48(++) | 0.30(++) | 0.021 |
| Cl/N | 0.86(++) | 0.57(++) | 6.57(++) | 0.43(++) | 0.021 |
| SO4/N | 0.88(+) | 0.21(−) | 4.27(++) | 0.24(−) | 0.61 |
| Fe/N | 0.0027(−) | No data | 0.0003(−) | No data | 0.011 |
| Mn/N | 0.0000(−) | No data | 0.0000(−) | No data | 0.004 |
| Cu/N | 0.0001(−) | No data | 0.0005(+) | No data | 0.0003 |
| Zn/N | 0.0001(−) | No data | 0.0006(+) | No data | 0.0008 |
| B/N | 0.0009(+) | No data | 0.0020(+) | No data | 0.001 |
| Farm/Company Name | Local Roots | Jones Food Company and GE | GE and Mirai | Spread Factory | |
|---|---|---|---|---|---|
| Location | US | UK | Japan | Japan | |
| Number of lettuces cultivated per day * | 269 | 3850 | 10,000 | 20,000 | |
| Saved amount of each element, kg/year ** | N | 10.9 | 156.3 | 406.0 | 812.1 |
| P | 2.2 | 31.1 | 80.8 | 161.6 | |
| K | 11.0 | 157.1 | 408.1 | 816.1 | |
| Ca2+ | 0.6 | 8.2 | 21.2 | 42.4 | |
| Mg2+ | 0.3 | 3.8 | 9.9 | 19.8 | |
| Cl− | 5.1 | 72.4 | 188.1 | 376.3 | |
| SO42− | 0.8 | 11.4 | 29.6 | 59.1 | |
| People required to supply the urine *** | N | 3 | 41 | 106 | 212 |
| P | 7 | 96 | 249 | 497 | |
| K | 12 | 173 | 449 | 898 | |
| Ca2+ | 6 | 83 | 215 | 430 | |
| Mg2+ | 3 | 50 | 129 | 258 | |
| Cl− | 2 | 29 | 75 | 149 | |
| SO42− | 1 | 11 | 28 | 55 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurga, A.; Janiak, K.; Wizimirska, A.; Chochura, P.; Miodoński, S.; Muszyński-Huhajło, M.; Ratkiewicz, K.; Zięba, B.; Czaplicka-Pędzich, M.; Pilawka, T.; et al. Resource Recovery from Synthetic Nitrified Urine in the Hydroponic Cultivation of Lettuce (Lactuca sativa Var. capitata L.). Agronomy 2021, 11, 2242. https://doi.org/10.3390/agronomy11112242
Jurga A, Janiak K, Wizimirska A, Chochura P, Miodoński S, Muszyński-Huhajło M, Ratkiewicz K, Zięba B, Czaplicka-Pędzich M, Pilawka T, et al. Resource Recovery from Synthetic Nitrified Urine in the Hydroponic Cultivation of Lettuce (Lactuca sativa Var. capitata L.). Agronomy. 2021; 11(11):2242. https://doi.org/10.3390/agronomy11112242
Chicago/Turabian StyleJurga, Anna, Kamil Janiak, Anna Wizimirska, Piotr Chochura, Stanisław Miodoński, Mateusz Muszyński-Huhajło, Krzysztof Ratkiewicz, Bartosz Zięba, Marta Czaplicka-Pędzich, Tomasz Pilawka, and et al. 2021. "Resource Recovery from Synthetic Nitrified Urine in the Hydroponic Cultivation of Lettuce (Lactuca sativa Var. capitata L.)" Agronomy 11, no. 11: 2242. https://doi.org/10.3390/agronomy11112242
APA StyleJurga, A., Janiak, K., Wizimirska, A., Chochura, P., Miodoński, S., Muszyński-Huhajło, M., Ratkiewicz, K., Zięba, B., Czaplicka-Pędzich, M., Pilawka, T., & Podstawczyk, D. (2021). Resource Recovery from Synthetic Nitrified Urine in the Hydroponic Cultivation of Lettuce (Lactuca sativa Var. capitata L.). Agronomy, 11(11), 2242. https://doi.org/10.3390/agronomy11112242







