Current and Prospective Strategies in the Varietal Improvement of Chilli (Capsicum annuum L.) Specially Heterosis Breeding
Abstract
:1. Introduction
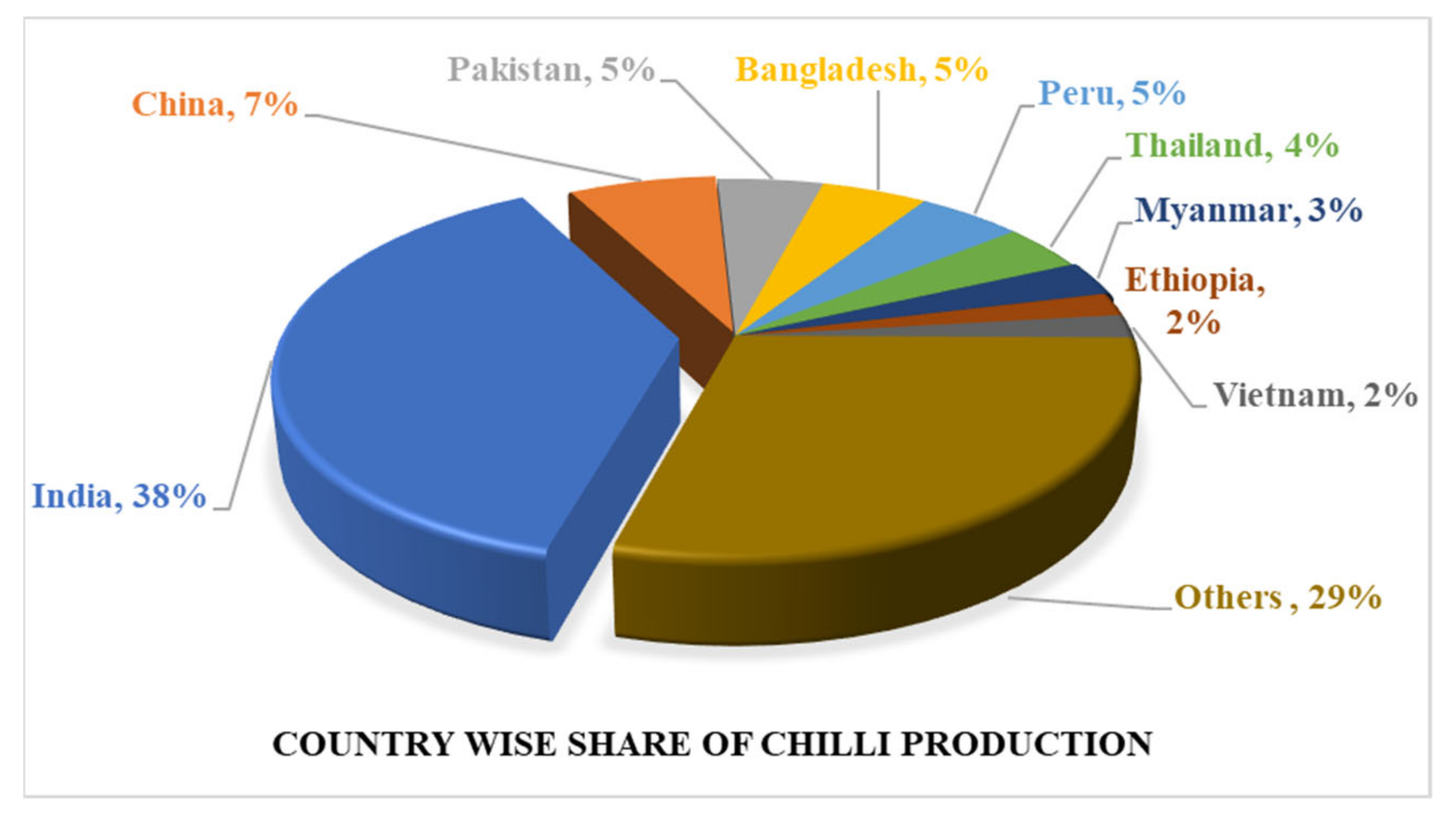
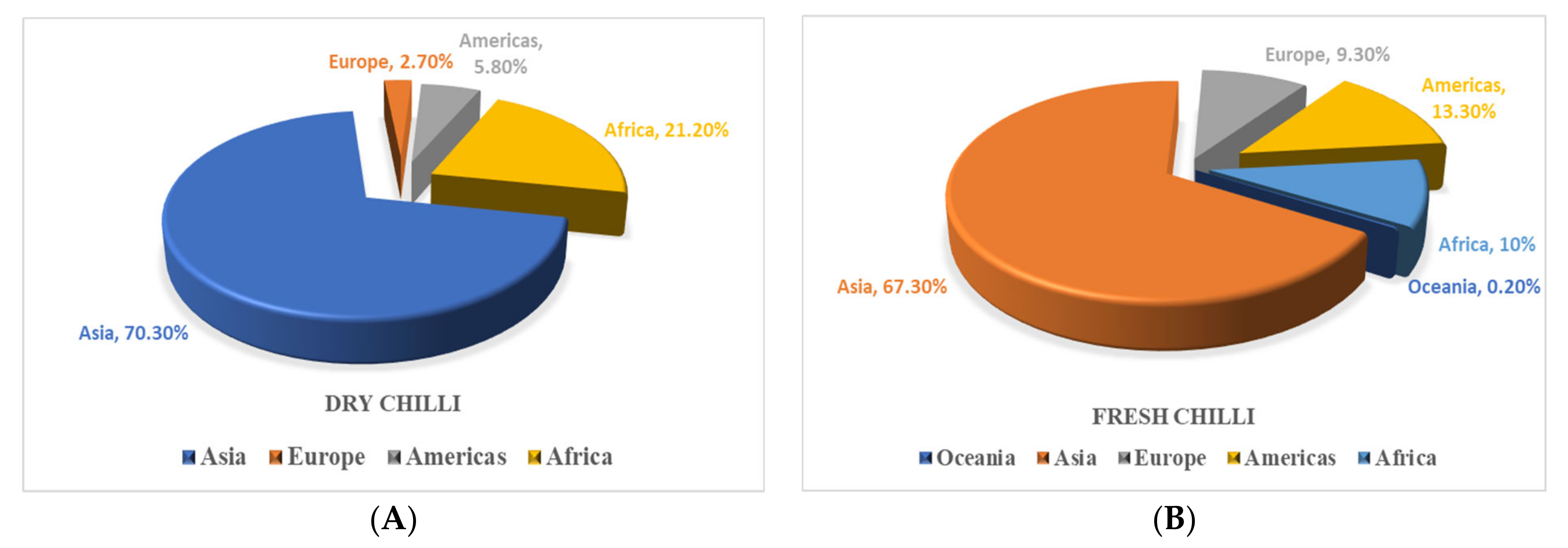
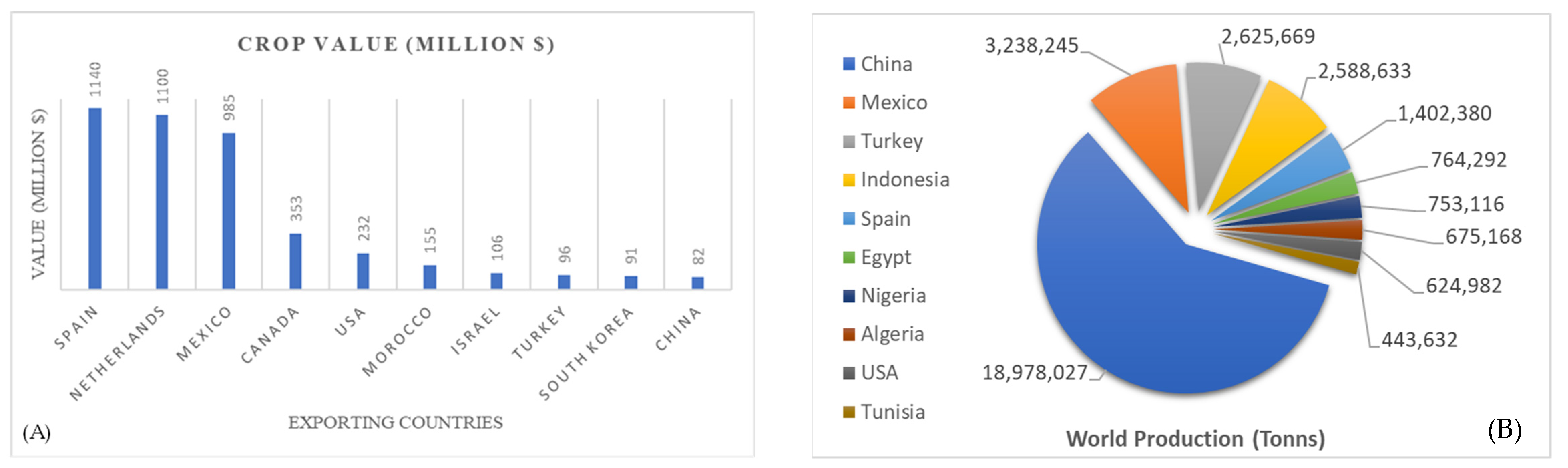
2. Global Production Scenario of Chilli
3. Genetic (Germplasm) Resources and Their Ancestry
4. General Purposes and Classical Breeding Techniques Used for Capsicum Improvement
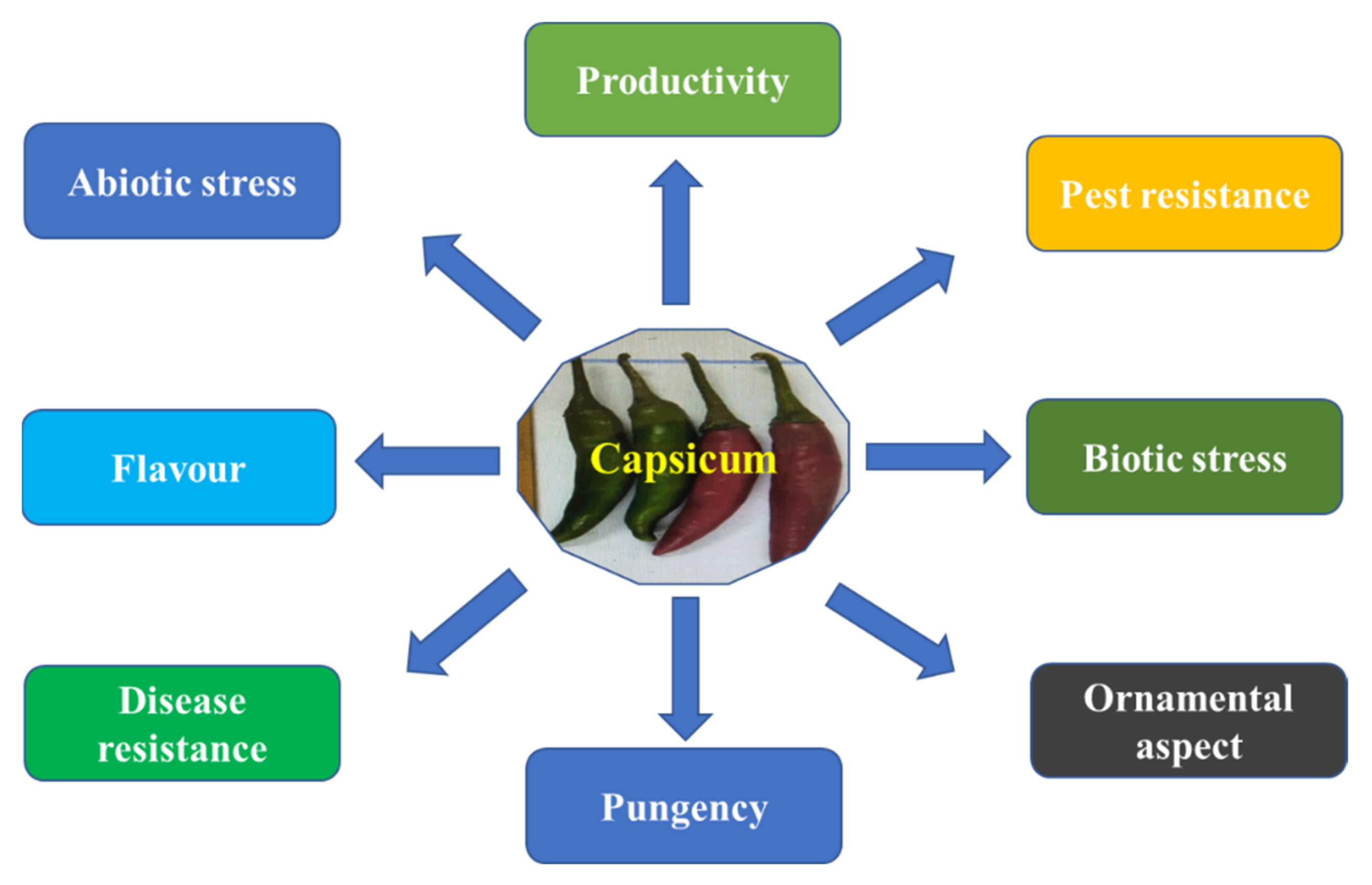
4.1. Common Goal for Capsicum Breeding
4.2. Classical Breeding Techniques Used for Chilli Improvement
4.2.1. Mass Selection
4.2.2. The Pedigree Method
4.2.3. Single Seed Descent Method
4.2.4. Recurrent Selection
4.2.5. Backcross
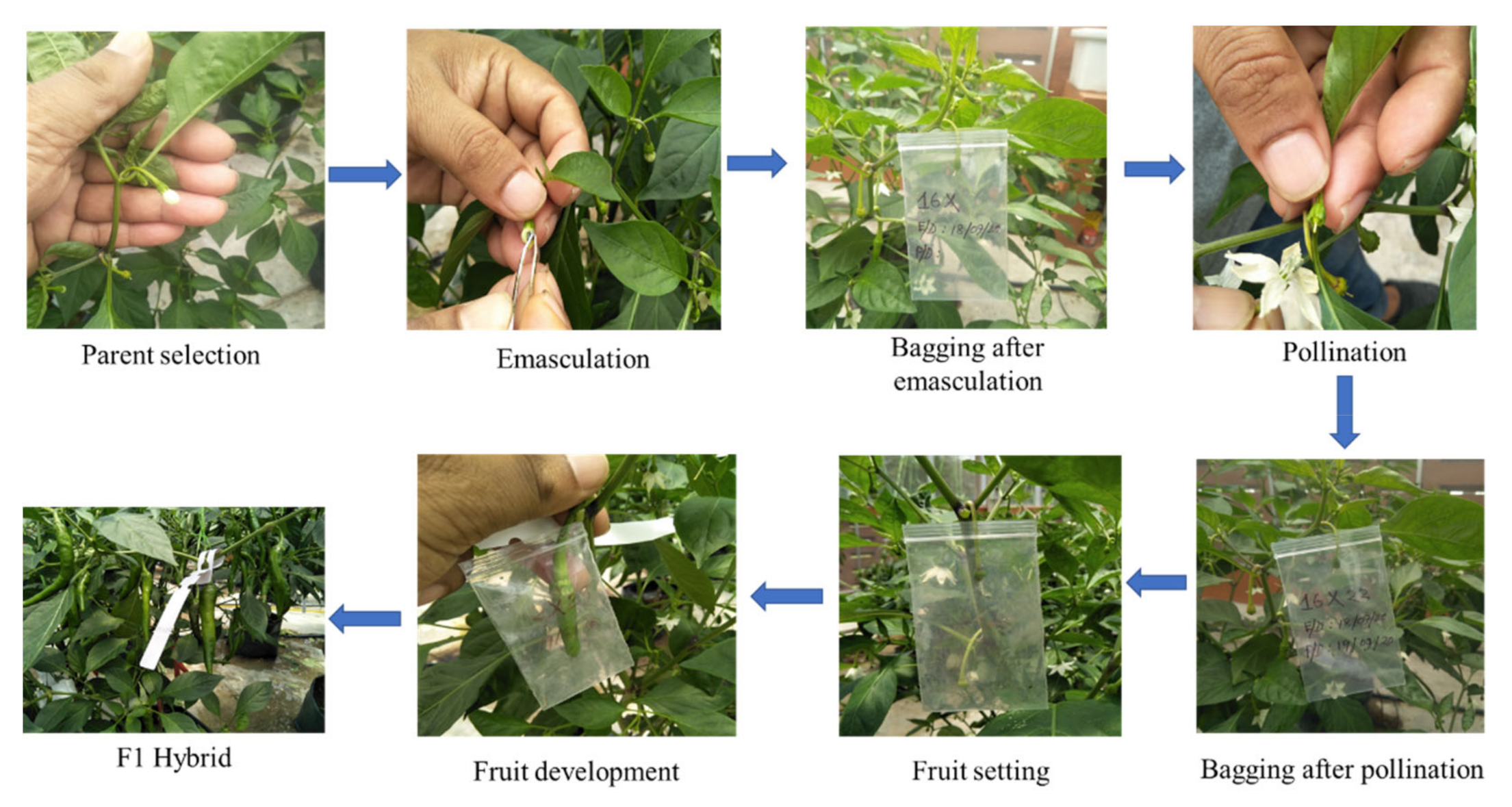
4.2.6. Hybridization
4.2.7. Genetic Basis of Hybridization
4.3. Using the Male Sterile Lines
5. Use of Heterosis for Targeted Genetic Breeding with Specific Traits
5.1. Heterosis for Crop Improvement
5.2. Basis of Heterosis
5.3. Desired Traits of Interest Associated with Chilli
5.4. Utilization of Heterosis in Recent Chilli Breeding Program
5.5. Heterosis through Molecular Approaches
6. Future Projections
- Naturalized Capsicum requires nutrient analysis and medicinal uses. To benefit the local population in the areas of medical, nutrition, and economy, conventional food production techniques must be explored.
- Governments, communities, business-people, and other stakeholders should work together to promote the popularization of native species of the land, by generating high-quality seeds to produce new kinds that are disease resistant and producing high yields.
- An economic cost-benefit analysis of value-added alternatives should be carried out to determine which option would offer farmers the best margin of profit for their capsicum production.
- The genetic variability in local species can be utilized to produce better, environmentally changeable Capsicum cultivars.
- This expertise in the added value of Capsicum should be distributed to local citizens when it comes to surplus Capsicum production to decrease post-harvest waste, to the advantage of the overall socio-economic well-being of the farmers involved.
- The Capsicum production and the many possibilities for Capsicum’s added value, which might contribute to the possible sources of money and the general socio-economic prosperity of both youth and women, must be encouraged and informed.
- A comparative method to genomics can be employed to transmit the information about the genome from tomato to pepper and eggplant. The use of molecular markers in various crops is beginning to be significant. The Marker Assisted Selection (MAS) application can increase the performance, accuracy, and speed of conventional plant breeding significantly. MAS has become available to the Solanaceae breeders as the most potent molecular method for promoting crop advancements.Conventional breeding is still durable and easy in a long-term breeding program to produce a new variety, apart from employing contemporary technology.
- So that this resistance source requires these lines may be successfully used against certain biotic stressors, greater attention should be focused on using wild relatives for the creation of pre-boring lines.
- ▪
- Evolution of less pungent and preferred kinds.
- ▪
- The development of novel hybrids involving male sterility and chemical hybridization.
- ▪
- Introduction of germplasm heat and dryness tolerance as a climate change approach.
- ▪
- Set-specific varieties development.
- ▪
- To create a variety, which can preserve the content of capsaicin after a prolonged time of storage.
- ▪
- Species with greater levels of oleoresin and antioxidants developed.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jarret, R.L.; Barboza, G.E.; da Costa Batista, F.R.; Berke, T.; Chou, Y.-Y.; Hulse-Kemp, A.; Ochoa-Alejo, N.; Tripodi, P.; Veres, A.; Garcia, C.C.; et al. Capsicum-an abbreviated compendium. J. Am. Soc. Hortic. Sci. 2019, 144, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Colney, L.; Tyagi, W.; Rai, M. Morphological and molecular characterization of two distinct chilli cultivars from North Eastern India with special reference to pungency related genes. Sci. Hortic. 2018, 240, 1–10. [Google Scholar] [CrossRef]
- Shetty, P.K.; Ayyappan, S.; Swaminathan, M.S. Climate Change and Sustainable Food Security; NIAS/ICAR: New Delhi, India, 2013. [Google Scholar]
- De Vasconcelos, C.S. Recursos Genéticos de Pimentas (Capsicum, Solanaceae): Qualidade de Frutos Após a Colheita e Ação dos Compostos Antioxidantes na Prevenção da Síndrome Metabólica. Ph.D. Thesis, Universidade Federal de Pelotas, Pelotas, Brazil, 2016; pp. 1–114. [Google Scholar]
- Bosland, P.W.; Votava, E.J.; Votava, E.M. Peppers: Vegetable and Spice Capsicums; CABI: Wallingford, London, UK, 2012. [Google Scholar]
- Sokona, D.; Niamoye, Y.D.; Paul, N.S.; Olagorite, A.; Aminata, D.N.; Kadidiatou, G.T.; Aissata, T.T.R.; Sériba, K.; Daoulé, D.-B. Overview of pepper (Capsicum spp.) breeding in West Africa. African J. Agric. Res. 2013, 8, 1108–1114. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, M.F.N.; Yusop, M.R.; Ismail, S.I.; Ramlee, S.I.; Oladasu, Y.; Hosen, M.; Miah, G. Development of anthracnose disease resistance and heat tolerance chili through conventional breeding and molecular approaches: A review. Biocell 2020, 44, 269–278. [Google Scholar] [CrossRef]
- Moscone, E.A.; Scaldaferro, M.A.; Grabiele, M.; Cecchini, N.M.; Sánchez García, Y.; Jarret, R.; Ehrendorfer, F. The evolution of chili peppers (Capsicum-Solanaceae): A cytogenetic perspective. In Proceedings of the VI International Solanaceae Conference: Genomics Meets Biodiversity, Madison, WI, USA, 23–27 July 2016; pp. 137–170. [Google Scholar]
- Tripodi, P.; Kumar, S. The Capsicum Crop: An Introduction. In The Capsicum Genome; Springer: Cham, Switzerland, 2019; pp. 1–8. [Google Scholar] [CrossRef]
- Herath, H.M.S.N.; Rafii, M.Y.; Ismail, S.I.; Nakasha, J.J.; Ramlee, S.I. Improvement of important economic traits in chilli through heterosis breeding: A review. J. Hortic. Sci. Biotechnol. 2021, 96, 14–23. [Google Scholar] [CrossRef]
- Bhutia, N.D.; Seth, T.; Shende, V.D.; Dutta, S.; Chattopadhyay, A. Estimation of heterosis, dominance effect and genetic control of fresh fruit yield, quality and leaf curl disease severity traits of chilli pepper (Capsicum annuum L.). Sci. Hortic. 2015, 182, 47–55. [Google Scholar] [CrossRef]
- Nagaraju, M.M.; Kumary, I.S.; Celine, V.A.; Devi, C.R.S.; Manju, P. Development of F1 Hybrids in Chilli (Capsicum annuum L.) for Dual Purpose (Green as well as Dry). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Rohini, N.; Lakshmanan, V. Evaluation studies of hot pepper hybrids (Capsicum annuum L.) for yield and quality characters. Electron. J. Plant Breed. 2017, 8, 643–651. [Google Scholar] [CrossRef]
- Sreenivas, M.; Sharangi, A.B.; Banerjee, S.; Kumar Maurya, P.; Bhattacharjee, T. Selecting Parental Lines among Genotypes of Capsicum annuum for Hybridization Aiming at Dry Fruit Yield Improvement. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1881–1899. [Google Scholar] [CrossRef]
- Alok, C.; Rajesh, K.; Solankey, S.S. Estimation of heterosis for yield and quality components in chilli (Capsicum annuum L.). Afr. J. Biotechnol. 2013, 12, 6605–6610. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.K.; Pramod, T.; Jain, S.K. Heterosis studies for growth, flowering, and yield of chilli (Capsicum annuum L.). Pantnagar J. Res. 2012, 10, 61–65. [Google Scholar]
- Ganefianti, D.W.; Fahrurrozi, F. Heterosis and Combining Ability in Complete Diallel Cross of Seven Chili Pepper. AGRIVITA J. Agric. Sci. 2018, 40, 360–370. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Golam, F. Gene actions involved in yield and yield contributing traits of chilli (Capsicum annuum L.). Aust. J. Crop Sci. 2011, 5, 1868–1875. [Google Scholar]
- Do Rêgo, E.R.; do Rêgo, M.M.; Finger, F.L.; Cruz, C.D.; Casali, V.W.D. A diallel study of yield components and fruit quality in chilli pepper (Capsicum baccatum). Euphytica 2009, 168, 275–287. [Google Scholar] [CrossRef]
- Kim, S.; Park, M.; Yeom, S.-I.; Kim, Y.-M.; Lee, J.M.; Lee, H.-A.; Seo, E.; Choi, J.; Cheong, K.; Kim, K.-T.; et al. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat. Genet. 2014, 46, 270–278. [Google Scholar] [CrossRef]
- Chiou, K.L.; Hastorf, C.A.; Bonavia, D.; Dillehay, T.D. Documenting Cultural Selection Pressure Changes on Chile Pepper (Capsicum baccatum L.) Seed Size Through Time in Coastal Peru (7600 B.P.–Present). Econ. Bot. 2014, 68, 190–202. [Google Scholar] [CrossRef]
- Padilha, H.; Barbieri, R. Plant breeding of chili peppers (Capsicum, Solanaceae)—A review. Aust. J. Basic Appl. Sci. 2016, 10, 148–154. [Google Scholar]
- Sarath Babu, B.; Pandravada, S.R.; Prasada Rao, R.D.V.J.; Anitha, K.; Chakrabarty, S.K.; Varaprasad, K.S. Global sources of pepper genetic resources against arthropods, nematodes and pathogens. Crop Prot. 2011, 30, 389–400. [Google Scholar] [CrossRef]
- Chhapekar, S.; Kehie, M.; Ramchiary, N. Advances in Molecular Breeding of Capsicum Species. Biotechnological Tools for Genetic Resources; Daya Publishing House: Darya Ganj, India, 2016; pp. 397–419. [Google Scholar] [CrossRef]
- Hoffmann, A.M.; Noga, G.; Hunsche, M. Acclimations to light quality on plant and leaf level affect the vulnerability of pepper (Capsicum annuum L.) to water deficit. J. Plant Res. 2015, 128, 295–306. [Google Scholar] [CrossRef]
- Baruah, S.; Zaman, M.K.; Rajbongshi, P.; Das, S. A Review on recent researches on Bhutjolokia and pharmacological activity of capsaicin. Int. J. Pharm. Sci. Rev. Res. 2014, 24, 89–94. [Google Scholar]
- Padilha, H.K.M.; Pereira, E.D.S.; Munhoz, P.C.; Vizzotto, M.; Valgas, R.A.; Barbieri, R.L. Genetic variability for synthesis of bioactive compounds in peppers (Capsicum annuum) from Brazil. Food Sci. Technol. 2015, 35, 516–523. [Google Scholar] [CrossRef] [Green Version]
- Ulhoa, A.B.; Pereira, T.N.; Silva, R.N.; Ragassi, C.F.; Rodrigues, R.; Pereira, M.G.; Reifschneider, F.J.B. Caracterização molecular de linhagens de pimenta do tipo Jalapeño amarelo. Hortic. Bras. 2014, 32, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Udaya Prakash, N.K.; Bhuvaneswari, S.; Sripriya, N.; Prameela, L.; Bhagya, R.; Radhika, B.; Balamurugan, A.; Arokiyaraj, S. Antioxidant activity of common plants of Northern Tamil Nadu, India. Int. J. Pharm. Pharm. Sci. 2014, 6, 128–132. [Google Scholar]
- Negi, R.; Thakur, S.; Sharma, P. Advances in the Breeding of Bell Pepper—A Review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2272–2281. [Google Scholar] [CrossRef]
- Qin, C.; Yu, C.; Shen, Y.; Fang, X.; Chen, L.; Min, J.; Cheng, J.; Zhao, S.; Xu, M.; Luo, Y.; et al. Whole-genome sequencing of cultivated and wild peppers provides insights into Capsicum domestication and specialization. Proc. Natl. Acad. Sci. USA 2014, 111, 5135–5140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norman, A.; Taylor, J.; Edwards, J.; Kuchel, H. Optimising genomic selection in wheat: Effect of marker density, population size and population structure on prediction accuracy. G3 Genes Genomes Genet. 2018, 8, 2889–2899. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.M.; Anilkumar, C. Conventional and Contemporary Approaches to Enhance Efficiency in Breeding Chilli/Hot Pepper. In Accelerated Plant Breeding; Springer: Cham, Switzerland, 2020; Volume 2, pp. 223–269. [Google Scholar]
- Sthapit, B.; Shrestha, P.; Subedi, M.; Castillo-Gonzales, F. Mass selection: A low-cost, widely applicable method for local crop improvement in Nepal and Mexico. In Participatory Approaches to the Con-Servation and Use of Plant Genetic Resources; Friis-Hansen, E., Sthapit, B.R., Eds.; International Plant Genetic Resources Institute: Rome, Italy, 2000; p. 111. [Google Scholar]
- Gosal, S.S.; Pathak, D.; Wani, S.H.; Vij, S.; Pathak, M. Accelerated Breeding of Plants: Methods and Applications. In Accelerated Plant Breeding; Springer: Cham, Switzerland, 2020; Volume 1, pp. 1–29. [Google Scholar]
- De Sá Mendes, N.; Santos, M.C.P.; Santos, M.C.B.; Cameron, L.C.; Ferreira, M.S.L.; Gonçalves, É.C.B.A. Characterization of pepper (Capsicum baccatum)—A potential functional ingredient. LWT 2019, 112, 108209. [Google Scholar] [CrossRef]
- Visalakshi, M.; Pandiyan, M. Crop improvement in chillies: An overview. Int. J. Chem. Stud. 2018, 6, 1736–1744. [Google Scholar]
- Bermejo, C.; Gatti, I.; Cointry, E. In vitro embryo culture to shorten the breeding cycle in lentil (Lens culinaris Medik). Plant Cell Tissue Organ Cult. (PCTOC) 2016, 127, 585–590. [Google Scholar] [CrossRef] [Green Version]
- Barroso, P.A.; Rêgo, M.M.D.; Crispim, J.G.; Costa, M.D.P.S.D.; Rêgo, E.R.D. How to shorten a plant breeding program? A case study with ornamental peppers. Crop. Breed. Appl. Biotechnol. 2019, 19, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Ridzuan, R.; Rafii, M.Y.; Ismail, S.I.; Mohammad Yusoff, M.; Miah, G.; Usman, M. Breeding for anthracnose disease resistance in chili: Progress and prospects. Int. J. Mol. Sci. 2018, 19, 3122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do Rego, E.R.; do Rêgo, M.M.; Finger, F.L. Production and Breeding of Chilli Peppers (Capsicum spp.); Springer International Publishing: Cham, Switzerland, 2016; pp. 57–80. [Google Scholar]
- Chen, J.; Luo, M.; Li, S.; Tao, M.; Ye, X.; Duan, W.; Zhang, C.; Qin, Q.; Xiao, J.; Liu, S. A comparative study of distant hybridization in plants and animals. Sci. China Life Sci. 2018, 61, 285–309. [Google Scholar] [CrossRef] [PubMed]
- Goulet, B.E.; Roda, F.; Hopkins, R. Hybridization in plants: Old ideas, new techniques. Plant Physiol. 2017, 173, 65–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, R.; Gonçalves, L.S.; Bento, C.d.S.; Sudré, C.P.; Robaina, R.R.; do Amaral, A.T., Jr. Combining ability and heterosis for agronomic traits in chili pepper. Hortic. Bras. 2012, 30, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Nsabiyera, V.; Ochwo-Ssemakula, M.; Sseruwagi, P.; Ojiewo, C.; Gibson, P. Combining Ability for Field Resistance to Disease, Fruit Yield and Yield Factors among Hot Pepper (Capsicum annuum L.) Genotypes in Uganda. Int. J. Plant. Breed. 2013, 7, 12–21. [Google Scholar]
- Awachar, A.V.; Pandey, M.K. Review on heterosis & combining ability of Chilli. Int. J. All Res. Educ. Sci. Methods (IJARESM) 2021, 9, 2018–2022. [Google Scholar]
- Singh, P.; Cheema, D.S.; Dhaliwal, M.S.; Garg, N. Heterosis and combining ability for earliness, plant growth, yield and fruit attributes in hot pepper (Capsicum annuum L.) involving genetic and cytoplasmic-genetic male sterile lines. Sci. Hortic. 2014, 168, 175–188. [Google Scholar] [CrossRef]
- Ramesh, M.; Lavanya, C.; Sujatha, M.; Sivasankar, A.; Kumari, J.A.; Meena, H.P. Heterosis and Combining Ability for Yield and Yield Component Characters of Newly Developed Castor (Ricinus Communis L.) Hybrid. Bioscan 2013, 8, 1421–1424. [Google Scholar]
- Rohini, N.; Lakshmanan, V. Heterobeltiosis and inbreeding depression for fruit yield and its components in hot pepper (Capsicum annuum var. annuum). Asian J. Hortic. 2016, 11, 86–92. [Google Scholar] [CrossRef]
- Acquaah, G. Principles of Genetics and Plant Breeding. Classic Methods of Plant Breeding; Blackwell Publishing: Hoboken, NJ, USA, 2012; pp. 281–350. [Google Scholar]
- Robsa Shuro, A. Review Paper on Approaches in Developing Inbred Lines in Cross-Pollinated Crops. Biochem. Mol. Biol. 2017, 2, 40. [Google Scholar] [CrossRef] [Green Version]
- Kaur, D.; Jindal, S.K.; Dhaliwal, M.S.; Meena, O.P. Improving fruit traits in chilli pepper through heterosis breeding. Sabrao J. Breed. Genet. 2017, 49, 26–43. [Google Scholar]
- Sharma, J.; Sharma, P.; Sharma, B.; Chaudhary, P. In-Vitro Estimation of Antioxidant Activity in Green Chilli (Capsicum annuum) and Yellow Lantern Chilli (Capsicum chinense). Int. J. Res. Rev. 2017, 4, 54. [Google Scholar]
- Kaya, Y.; Balalic, I.; Milic, V. Eastern Europe Perspectives on Sunflower Production and Processing. In Sunflower; AOCS Press: Urbana, IL, USA, 2015; pp. 575–637. [Google Scholar]
- Thilak, J.C.; Pant, S.C.; Veena, A.M.; Paliwal, A. Studies on general vs. specific combining ability estimates from diallel analysis for yield and its component traits in chilli (Capsicum annuum L. var. acuminatum). Int. J. Chem. Stud. 2019, 7, 1747–1749. [Google Scholar]
- Singh, P. Genetic distance, heterosis and combing ability studies in maize for predicting F1 hybrid performance. Sabrao J. Breed. Genet. 2015, 47, 21–28. [Google Scholar]
- Griffing, B. Concept of General and Specific Combining Ability in Relation to Diallel Crossing Systems. Aust. J. Biol. Sci. 1956, 9, 463. [Google Scholar] [CrossRef]
- Ahmed, N.; Hurra, M.; Wani, S.A.; Khan, S.H. Gene action and combining ability for fruit yield and its component characters in sweet pepper (No. Research). Capsicum Eggplant Newsl. 2003, 22, 55–58. [Google Scholar]
- Janaki, M.; Babu, J.D.; Naidu, L.N.; Ramana, C.V.; Rao, C.K.K.; Krishna, K.U. Combining ability studies for yield and yield components in chilli (Capsicum annuum L.). Electron. J. Plant Breed. 2017, 8, 825. [Google Scholar] [CrossRef]
- Patil, M.Y.; Sawant, G.B.; Jadhav, S.M. Combining ability and gene action studies in chilli (Capsicum annuum L.). Environ. Ecol. 2018, 36, 52–56. [Google Scholar]
- Jindal, S.K.; Kaur, D.; Dhaliwal, S.; Chawla, N. Combining ability and heterosis for qualitative traits in chili pepper (Capsicum annuum L.). Int. J. Hortic. 2015, 5, 1–13. [Google Scholar]
- Ajith, P.M.; Manju, P. Genetic Parameters and Character Association for Yield and Anthracnose Resistance in Chilli (Capsicum annuum L.). Int. J. Agric. Innov. Res. 2015, 4, 1473–2319. [Google Scholar]
- Fujimoto, R.; Uezono, K.; Ishikura, W.; Osabe, K.; Peacock, W.J.; Dennis, E.S. Recent research on the mechanism of heterosis is important for crop and vegetable breeding systems. Breed. Sci. 2018, 68, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, N.; Kumar, R.; Paliwal, R.; Kumar, U.; Kumar, S.; Singh, M.; Singh, R.K. QTL mapping for important horticultural traits in pepper (Capsicum annuum L.). J. Plant Biochem. Biotechnol. 2015, 24, 154–160. [Google Scholar] [CrossRef]
- Hill, T.A.; Chunthawodtiporn, J.; Ashrafi, H.; Stoffel, K.; Weir, A.; Van Deynze, A. Regions Underlying Population Structure and the Genomics of Organ Size Determination in Capsicum annuum. Plant Genome 2017, 10, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, Y.-K.; Tripathi, S.; Cho, Y.-I.; Kim, J.-H.; Lee, H.-E.; Kim, D.-S.; Woo, J.-G. Molecular marker information from de novo assembled transcriptomes of chilli pepper (Capsicum annuum L.) varieties based on next-generation sequencing technology. Plant Genet. Resour. 2014, 12, S83–S86. [Google Scholar] [CrossRef]
| Institute | Country | Description | Web |
|---|---|---|---|
| Banco de Germoplasma de Hortaliças (BGH) | Brazil | By providing BGH text in the name field, you may monitor BGH’s of various collections in the Chileman data base. | http://www.thechileman.org/search.php (accessed on 6 June 2020) |
| The Centre for Genetic Resources, The Netherlands (CGN)) | The Netherlands | In all, 1009 accessions are available with the Institute for enhanced disease resistance and harvest features. The CGN species are listed in the Chileman database as well and may be found in the database by typing CGN text. | http://applicaties.wageningenur.nl/applications/cgngenis/ZoekGewas.aspx?ID=pzlo0t45 and Cropnumber=38 OR http://www.thechileman.org/search.php (accessed on 16 August 2019) |
| The Chile Pepper Institute (New Mexican State University) | Mexico | An elite institute having maximum genetic resources, germplasm collection of different Capsicum species | http://www.chilepepperinstitute.org OR http://www.thechileman.org/search.php (accessed on 6 June 2020) |
| The German Research Centre for Biotechnology (CAP) | Germany | Many of its variations may be discovered in the Chileman database by typing the CAP in the name box | http://www.thechileman.org/search.php (accessed on 6 June 2020) |
| United States Department of Agriculture Research Service (USDA) | USA | They are having most extensive Capsicum genotype collections (4953), resistant to different diseases. | http://www.ars-grin.gov/npgs/acc/acc_queries.html (accessed on 20 January 2020) OR http://www.thechileman.org/search.php (accessed on 6 June 2020) |
| The World Vegetable Center (WorldVeg) (WVC) | Taiwan | A collection (8264) of several Capsicum species resistant to different diseases especially viral with accession number, characterization, and evaluation data. | http://www.avrdc.org (accessed on 26 June 2019) |
| National Bureau of Plant Genetic Resources | India | A collection of germplasm of different Capsicum species with accession number, characterization, and evaluation data | www.nbpgr.ernet.in/pgrportal (accessed on 6 September 2020) |
| ECPGR Pepper Database, Aegean Agricultural Research Institute, AARI | Turkey | A collection of germplasm of different Capsicum | http://www.etae.gov (accessed on 10 November 2018) |
| The Chile variety database | A collection of a large number of variants resistant to most diseases and nematodes | http://www.g6csy.net/chile/database.html (accessed on 10 February 2020) |
| Name of the Approaches | Assumption | Reference |
|---|---|---|
| Mass selection | The next growing year stored seeds of the finest plants; the oldest technique | [42] |
| Pedigree method | Maintaining matings records and progenies. This comprises the selection and self-pollination of single plants. | [43] |
| SSD (Single seed descent) | This approach involves advances without selection of generations and is also used for the production of recombinant inbred lines. | [28] |
| Recurrent selection | Keep choosing individuals from a population and then crossing across to establish a new population. | [44] |
| Backcross | Especially for characteristics regulated by one or few genes involving the selection of individual plants and subsequent crossings for recurring parents | [29] |
| Hybridization | From one species genes or variations migrate via the crossover process | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karim, K.M.R.; Rafii, M.Y.; Misran, A.B.; Ismail, M.F.B.; Harun, A.R.; Khan, M.M.H.; Chowdhury, M.F.N. Current and Prospective Strategies in the Varietal Improvement of Chilli (Capsicum annuum L.) Specially Heterosis Breeding. Agronomy 2021, 11, 2217. https://doi.org/10.3390/agronomy11112217
Karim KMR, Rafii MY, Misran AB, Ismail MFB, Harun AR, Khan MMH, Chowdhury MFN. Current and Prospective Strategies in the Varietal Improvement of Chilli (Capsicum annuum L.) Specially Heterosis Breeding. Agronomy. 2021; 11(11):2217. https://doi.org/10.3390/agronomy11112217
Chicago/Turabian StyleKarim, K. M. Rezaul, Mohd Y. Rafii, Azizah Binti Misran, Mohd Firdaus Bin Ismail, Abdul Rahim Harun, Md Mahmudul Hasan Khan, and Mst. Farhana Nazneen Chowdhury. 2021. "Current and Prospective Strategies in the Varietal Improvement of Chilli (Capsicum annuum L.) Specially Heterosis Breeding" Agronomy 11, no. 11: 2217. https://doi.org/10.3390/agronomy11112217
APA StyleKarim, K. M. R., Rafii, M. Y., Misran, A. B., Ismail, M. F. B., Harun, A. R., Khan, M. M. H., & Chowdhury, M. F. N. (2021). Current and Prospective Strategies in the Varietal Improvement of Chilli (Capsicum annuum L.) Specially Heterosis Breeding. Agronomy, 11(11), 2217. https://doi.org/10.3390/agronomy11112217






