Use of Plant Growth Promoting Rhizobacteria in Combination with Chitosan on Maize Crop: Promising Prospects for Sustainable, Environmentally Friendly Agriculture and against Abiotic Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
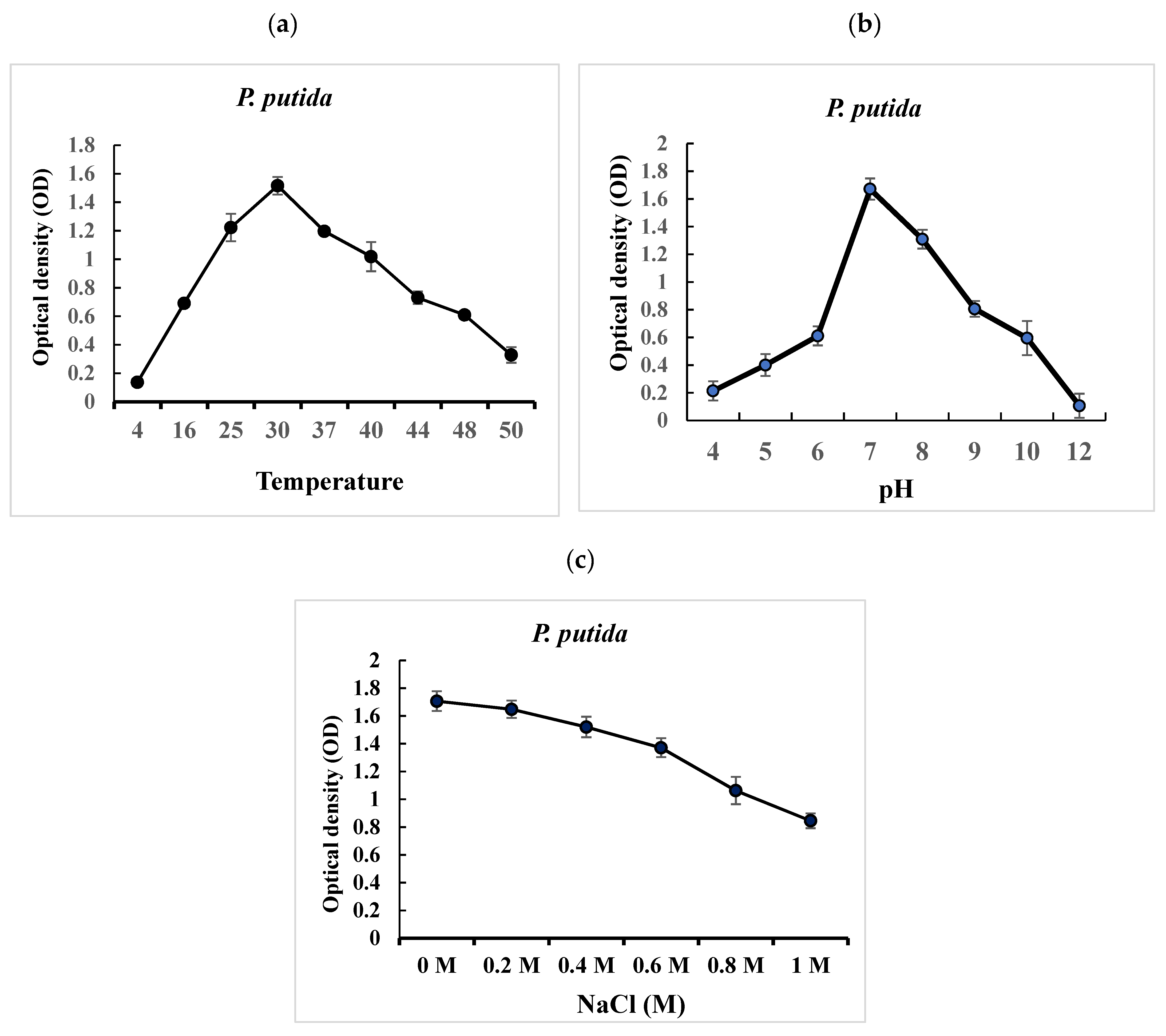
2.2. In Vitro Evaluation of the Ability of P. putida to Grow under Different Environmental Stress Conditions (Temperature, pH, Salt)
2.2.1. Evaluation of the Effects of Temperature Variation on the Growth of P. putida
2.2.2. Evaluation of the Effects of pH Variation on the Growth of P. putida
2.2.3. Evaluation of the Effects of Different Salt Concentrations (NaCl) on the Growth of P. putida
2.3. Evaluation of the Effects of Chitosans Produced in Benin from C. amnicola and C. armatum in Combination with P. putida on the Greenhouse Growth of Maize on Ferrallitic Soil
2.3.1. Extraction of Chitosans from Exoskeletons of C. armatum and C. amnicola
- -
- The samples of land crab (Cardisoma armatum) and freshwater crab (Callinectes amnicola) used were collected from anglers and crab sellers, respectively, in the municipalities of Ajohoun and Abomey-Calavi in Benin. After the crab samples were collected, the shells were separated from the meat. These shells were washed with tap water then with distilled water before being dried. The shells were sprayed with acetone and placed in an oven at 50 °C for 24 h to activate the drying process. Drying was continued in a pasteurized oven at 50 °C for 10 days. After drying, all samples were ground and powdered using a Retsch mill type SM 2000/1430/Upm/Smfet [27].
- -
- The powders of the two crab species obtained were used to obtain chitosan in four steps according to the method of [30] adapted by [27], which consists of a demineralization of the powder in acid medium, followed by a deproteinization of the powder in basic medium, then a bleaching of the powder, and finally a deacetylation of the powder by hydrothermochemical process in basic medium.
2.3.2. Refreshing of the Strain and Preparation of P. putida Suspensions
2.3.3. Experimental Device
- -
- T1: CTL = Uncoated seed (without chitosan and P. putida) (control);
- -
- T2: Cali = Seeds coated with chitosan extracted from C. amnicola;
- -
- T3: Card = Seeds coated with chitosan extracted from C. armatum;
- -
- T4: Puti = Seeds inoculated with Pseudomonas putida;
- -
- T5: Cali + puti = Seeds coated with the combination of C. amnicola + Pseudomonas putida;
- -
- T6: Card + puti = Seeds coated with the combination of C. armatum + Pseudomonas putida.
2.3.4. Filling the Pots
2.3.5. Coating of Seeds with Extracted Chitosans Based on C. amnicola and C. armatum
2.3.6. Sowing of Coated Seeds, Inoculation, and Maintenance of Pots
2.3.7. Chemical Analysis of Soil Used in Greenhouses
2.3.8. Collection of Growth and Yield Parameters
2.3.9. Statistical Analysis of the Data
3. Results
3.1. In Vitro Effect of P. putida Tolerance to Different Environmental Stress Conditions
3.1.1. Effect of Temperature Variation on the Growth of P. putida
3.1.2. Effect of pH Variation on the Growth of P. putida
3.1.3. Effect of Salt (NaCl) at Different Concentrations on the Growth of P. putida
3.2. Chemical Characteristics of the Soil
3.3. Effects of Chitosan-Based Treatments and Its Combination with P. putida on the Growth Parameters of Maize Plants
3.3.1. Height
3.3.2. Diameter
3.3.3. Leaf Area
3.3.4. Effects of Chitosan-Based Treatments and Its Combination with P. putida on Maize Plant Yield Parameters
3.3.5. Correlation of the Various Parameters
4. Discussion
4.1. Effect of Temperature, pH, and Salinity on the Growth of P. putida Strain
4.2. Effects of Combination Chitosan and P. putida on Growth Parameters
4.3. Effects of Chitosan and P. putida on Biomass Yield Parameters
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhat, M.A.; Kumar, V.; Bhat, M.A.; Wani, I.A.; Dar, F.L.; Farooq, I.; Bhatti, F.; Koser, R.; Rahman, S.; Jan, A.T. Mechanistic Insights of the Interaction of Plant Growth-Promoting Rhizobacteria (PGPR) With Plant Roots toward Enhancing Plant Productivity by Alleviating Salinity Stress. Front. Microbiol. 2020, 11, 1952. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. The endosphere microbial communities, a great promise in agriculture. Int. Microbiol. 2020, 24, 1–17. [Google Scholar] [CrossRef]
- Savci, S. An agricultural pollutant: Chemical fertilizer. Int. J. Environ. Sci. Dev. 2012, 3, 11–14. [Google Scholar] [CrossRef] [Green Version]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Sherman, R.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Jochum, M.D.; McWilliams, K.L.; Borrego, E.J.; Kolomiets, M.V.; Niu, G.; Pierson, E.A.; Jo, Y.-K. Bioprospecting plant growth-promoting rhizobacteria that mitigate drought stress in grasses. Front. Microbiol. 2019, 10, 2106. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B.; Kamilova, F. Plant-growthpromoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noumavo, A.P.; Agbodjato, A.N.; Gachomo, E.W.; Salami, H.A.; Baba-Moussa, F.; Adjanohoun, A.; Kotchoni, S.O.; Baba-Moussa, L. Metabolic and biofungicidal properties of maize rhizobacteria for growth promotion and plant disease resistance. Afr. J. Biotechnol. 2015, 14, 811–819. [Google Scholar]
- Agbodjato, N.A.; Amogou, O.; Noumavo, P.A.; Dagbénonbakin, G.; Salami, H.A.; Karimou, R.; Alladé, A.M.; Baba-Moussa, F.; Adebayo, O.; Adjanohoun, A.; et al. Biofertilising, plant-stimulating and biocontrol potentials of maize plant growth promoting Rhizobacteria isolated in central and northern Benin. Afr. J. Microbiol. Res. 2018, 12, 664–672. [Google Scholar]
- Adeoyo, O.R. Plant Growth-Promoting Potentials of Some Indigenous Bacterial Isolates. IOSR-JPBS 2019, 14, 5–10. [Google Scholar]
- Ojuederie, O.B.; Olanrewaju, O.S.; Babalola, O.O. Plant Growth Promoting Rhizobacterial Mitigation of Drought Stress in Crop Plants: Implications for Sustainable Agriculture. Agronomy 2019, 9, 712. [Google Scholar] [CrossRef] [Green Version]
- Enebe, M.C.; Babalola, O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: A survival strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef] [Green Version]
- Karnwal, A. Isolation and identification of plant growth promoting rhizobacteria from maize (Zea mays L.) rhizosphere and their plant growth promoting effect on rice (Oryza sativa L.). J. Plant Prot. Res. 2017, 57, 144–151. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Boonlertnirun, S.; Meechoui, S.; Sarobol, E. Physiological and morphological responses of field corn seedlings to chitosan under hypoxic conditions. Sci. Asia 2010, 36, 89–93. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef] [Green Version]
- Doğdu, S.A.; Turan, C.; Depci, T. Extraction and Characterization of Chitin and Chitosan from Invasive Alien Swimming Crab Charybdis longicollis. NESciences 2021, 6, 96–101. [Google Scholar]
- Shehata, S.A.; Fawzy, Z.F.; El-Ramady, H.R. Response of cucumber plants to foliar application of chitosan and yeast under greenhouse conditions. Aust. J. Basic. Appl. Sci. 2012, 6, 63–71. [Google Scholar]
- Abdel-Mawgoud, A.M.R.; Tantawy, A.S.; El-Nemr, M.A.; Sassine, T.N. Growth and yield response of strawberry plants to chitosan application. Eur. J. Sci. Res. 2010, 39, 170–177. [Google Scholar]
- Sultana, S.; Islam, M.; Khatun, M.A.; Hassain, M.A.; Huque, R. Effect of Foliar Application of Oligo-chitosan on Growth, Yield and Quality of Tomato and Eggplant. Asian J. Agric. Res. 2017, 11, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Farouk, S.; Mosa, A.A.; Taha, A.A.; Heba, M.I.; ELGahmery, A.M. Protective effect of humic acid and chitosan on radish (Raphanus sativus, L. var. sativus) plants subjected to cadmium stress. J. Stress. Physiol. Biochem. 2011, 7, 99–116. [Google Scholar]
- Farouk, S.; Amany, R.A. Improving growth and yield of cowpea by foliar application of chitosan under water stress. Egypt. J. Biol. 2012, 14, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Abu-Muriefah, S.S. Effect of chitosan on common bean (Phaseolus vulgaris L.) plants grown under water stress conditions. Int. Res. J. Agric. Sci. Soil Sci. 2013, 3, 192–199. [Google Scholar]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef]
- Mishra, S.; Jagadeesh, K.S.; Krishnaraj, P.U.; Prem, S. Biocontrol of Tomato leaf curl virus (ToLCV) in tomato with chitosan supplemented formulation of Pseudomonas sp. under field conditions. Aust. J. Crop. Sci. 2014, 8, 47–355. [Google Scholar]
- Yallou, C.G.; Aïhou, K.; Adjanohoun, A.; Toukourou, M.; Sanni, O.A.; Ali, D. Itinéraires techniques de production de maïs au Bénin. Fiche Tech. Bibliothèque Natl. Du Bénin Dépôt Légal N° 2010, 4922, 18. [Google Scholar]
- Mikpon, T.; Agbodjato, N.A.; Dah-Nouvlessounon, D.; Amogou, O.; Lehman, H.; N’tcha, C.; Noumavo, P.A.; Assogba, S.; Allagbe, M.; Ahissou, H.; et al. Extraction of chitosan from the exoskeletons of two species of crabs (Callinectes amnicola and Cardisoma armatum) and evaluation of its effectiveness on in vitro germination of maize (Zea mays l.) in Benin. J. Glob. Biosci. 2020, 9, 8063–8077. [Google Scholar]
- Adjanohoun, A.; Baba-Moussa, L.; Glele-Kakaï, R.; Allagbe, M.; Yehouenou, B.; Gotoechan-hodonou, H.; Sikirou, R.; Sessou, P.; Sohounhloue, D. Caractérisation des rhizobactéries potentiellement promotrices de la croissance végétative du maïs dans différents agrosystèmes du Sud-Bénin. Int. J. Biol. Chem. Sci. 2011, 5, 433–444. [Google Scholar] [CrossRef] [Green Version]
- Silini-Cherif, H.; Silini, A.; Ghoul, M.; Yadav, S. Isolation and Characterization of Plant Growth Promoting Traits of a Rhizobacteria: Pantoea agglomerans lma2. Pak. J. Biol. Sci. 2012, 15, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Oanh, T.; Robert, H.; Fréderic, M.; Patrick, N. Valorisation des résidus industriels de pêches pour la transformation de chitosane par technique hydrothermo-chimique. Revue Sci. l’eau 2007, 20, 253–262. [Google Scholar]
- Govindappa, M.; Ravishankar, R.V.; Lokesh, S. Screening of Pseudomonas fluorescens isolates for biological control of Macrophomina phaseolina root-rot of safflower. Afr. J. Agric. Res. 2011, 6, 6256–6266. [Google Scholar] [CrossRef]
- Gholami, A.; Shahsavani, S.; Nezarat, S. The effect of Plant Growth Promoting Rhizobacteria (PGPR) on germination, seedling growth and yield of maize. World Acad. Sci. Eng. Technol. Int. J. Agric. Biosyst. Eng. 2009, 3, 9–14. [Google Scholar]
- Etèka, A.C. Contribution des ‘Jachères’ Manioc Dans L’amélioration du Rendement des Cultures et du Prélèvement des Nutriments: Cas de la Succession Culturale Manioc-Maïs au Centre du Benin, Thèse de DEA; Faculté des Sciences Agronomiques, Université d’Abomey Calavi: Calavi, Bénin, 2005; p. 107. [Google Scholar]
- Fernández, F.; Gómez, R.; Vanegas, L.F.; Martínez, M.A.; de la Noval, B.M.; Rivera, R. Producto Inoculante Micorrizógeno; Oficina Nacional de Propiedad Industrial: Cuba, Patente, 2000; p. 22641.
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil. Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Détermination du phosphore organique total et des formes disponibles dans les sols. Soil. Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Thomas, G.W. Exchangeable cations. In Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Ed.; Agronomy, American Society of Agronomy, Soil Science Society of America: Madison, DC, USA, 1982; Volume 9, pp. 159–165. [Google Scholar]
- Ruget, F.; Bonhomme, R.; Chartier, M. Estimation simple de la surface foliaire de plantes de maïs en croissance. Agronomie 1996, 16, 553–562. [Google Scholar] [CrossRef]
- Douglas, C.E.; Michael, F.A. On distribution-free multiple comparisons in the one-way analysis of variance, Communications in Statistics. Theory Methods 1991, 20, 127–139. [Google Scholar] [CrossRef]
- Enagbonma, B.J.; Babalola, O.O. Potentials of termite mound soil bacteria in ecosystem engineering for sustainable agriculture. Ann. Microbiol. 2019, 69, 211–219. [Google Scholar] [CrossRef]
- Ahmad, P. Oxidative Damage to Plants: Antioxidant Networks and Signaling; MA Academic Press: San Diego, CA, USA, 2014; pp. 92101–94495. [Google Scholar]
- Rahman, M.S.; Mubassara, S.; Hoque, S.; Khan, Z.U. Effect of some environmental factors on the growth of Azospirillum Species isolated from Ssaline soils of Satkhira district, Bangladesh. Bangladesh J. Microbiol. 2006, 23, 145–148. [Google Scholar] [CrossRef]
- Costa, E.; Usall, J.; Teixido, N.; Delgado, J.; Vinas, I. Water activity, temperature and pH effects on growth of the biocontrol agent Pantoea agglomerans CPA-2. Can. J. Microbiol. 2020, 48, 1082–1088. [Google Scholar] [CrossRef]
- D’Amato, R.; Del Buono, D. Use of a Biostimulant to Mitigate Salt Stress in Maize Plants. Agronomy 2021, 11, 1755. [Google Scholar] [CrossRef]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Abdelaal, K.A.; Tawfik, S.F. Response of Sugar Beet Plant (Beta vulgaris L.) to Mineral Nitrogen Fertilization and Bio-Fertilizers. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 677–688. [Google Scholar]
- Agbodjato, A.N.; Noumavo, P.A.; Adjanohoun, A.; Agbessi, L.; Baba-Moussa, L. Synergistic Effects of plant growth gromoting rhizobacteria and chitosan on in vitro seeds Germination, Greenhouse growth, and nutrient uptake of Maize (Zea mays L.). Biotech. Res. Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Scott, S.; Housh, A.; Powell, G.; Anstaett, A.; Gerheart, A.; Benoit, M.; Wilder, S.; Schueller, M.; Ferrieri, R. Crop Yield Ferritin, and Fe(II) boosted by Azospirillum brasilense (HM053) in Corn. Agronomy 2020, 10, 394. [Google Scholar] [CrossRef] [Green Version]
- Qaisrani, M.M.; Mirza, M.S.; Zaheer, A.; Malik, K.A. Isolation and identification by 16s rRNA sequence analysis of Achromobacter, Azospirillum and Rhodococcus strains from the rhizosphere of maize and screening for the beneficial effect on plant growth. Pak. J. Agric. Sci. 2014, 51, 91–99. [Google Scholar]
- Alori, E.T.; Babalola, O.O. Microbial Inoculants for Improving Crop Quality and Human Health in Africa. Front. Microbiol. 2018, 9, 2213. [Google Scholar] [CrossRef] [Green Version]
- Uge, E.; Sulandari, S.; Hartono, S.; Somowiyarjo, S. The effect of chitosan application against plant growth and intensity of stunting disease on black pepper (Piper nigrum L.) seedlings. J. Perlindungan Tanam. Indones. 2018, 22, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Zeng, D.; Luo, X.; Tu, R. Application of bioactive coatings based on chitosan for soybean seed protection. Int. J. Carbohydr. Chem. 2012, 2012, 1–5. [Google Scholar] [CrossRef]
- Costales-Menéndez, D.; Falcón-Rodríguez, A.B. Combinación de formas de aplicación de quitosano en el desarrollo de soja biofertilizada. Cultiv. Trop. 2018, 39, 71–79. [Google Scholar]
- Zehnder, G.W.; Murphy, J.F.; Sikora, E.J.; Kloepper, J.W. Application of rhizobacteria for induced resistance. Eur. J. Plant. Pathol. 2001, 107, 39–50. [Google Scholar] [CrossRef]
- Uthairatanakij, A.; Teixeira da Silva, J.A.; Obsuwan, K. Chitosan for improving orchid production and quality. Orchid. Sci. Biotech. 2007, 1, 1–5. [Google Scholar]
- Firmansyah, D.; Widodo, S.; Hendrastuti. Chitosan and Plant Growth Promoting Rhizobacteria Application to Control Squash mosaic virus on Cucumber Plants. Asian J. Plant. Pathol. 2017, 11, 148–155. [Google Scholar] [CrossRef]
- Mahdavi, B. Seed germination and growth responses of Isabgol (Plantago ovata Forsk) to chitosan and salinity. Int. J. Agric. Crop. Sci. 2013, 5, 1084–1088. [Google Scholar]
- Guan, Y.J.; Hu, J.; Wang, X.J.; Shao, C.X. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ. Sci. B 2009, 10, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Mondal, M.M.A.; Malek, M.A.; Puteh, A.B.; Ismail, M.R.; Ashrafuzzaman, M.; Naher, L. Effect of foliar application of chitosan on growth and yield in okra. Aust. J. Crop. Sci. 2012, 69, 18–921. [Google Scholar]
- Igiehon, N.O.; Babalola, O.O. Rhizosphere microbiome modulators: Contributions of nitrogen fixing bacteria towards sustainable agriculture. Int. J. Environ. Res. Public Health 2018, 15, 574. [Google Scholar] [CrossRef] [Green Version]
- Khantsi, M.; Adegboye, M.F.; Babalola, O.O. 1-Aminocyclopropane-1-carboxylate deaminase activity as a marker for identifying plant-growth promoting rhizobacteria in cultivated soil. Asia Life. Sci. 2013, 9, 199–211. [Google Scholar]
- Ndeddy Aka, R.J.; Babalola, O.O. Effect of bacterial inoculation of strains of Pseudomonas aeruginosa, Alcaligenes feacalis and Bacillus subtilis on germination, growth and heavy metal (Cd, Cr, and Ni) uptake of Brassica juncea. Int. J. Phytoremediation 2016, 18, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Lizárraga-Paulín, E.G.; Torres-Pacheco, I.; Moreno-Martínez, E.; Miranda-Castro, S.P. Chitosan application in maize (Zea mays) to counteract the effects of abiotic stress at seedling level. Afri. J. Biotechnol. 2011, 10, 6439–6446. [Google Scholar]




| pH | PA (ppm) | OC (%) | OM (%) | EB (meq/100 g) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Water | KCl | Ca | Mg | K | Na | |||
| Soil | 5.3 | 4.7 | 8 | 0.63 | 1.15 | 2.63 | 0.61 | 0.13 | 0.22 |
| Observed Data | Treatments | ||||||
|---|---|---|---|---|---|---|---|
| CTL | Cali | Card | Puti | Cali + puti | Card + puti | p-Value | |
| Height (cm) | 14.2 a ± 0.12 | 15.0 ab ± 0.03 | 16.6 bc ± 0.23 | 16.2 bc ± 0.58 | 17.0 c ± 0.51 | 18 ± 0.71 | 0.0001 *** |
| Diameter (cm) | 0.58 a ± 0.03 | 0.63 ab ± 0.02 | 0.71 bc ± 0.04 | 0.69 bc ± 0.03 | 0.71 c ± 0.01 | 0.76 ± 0.03 | 0.0006 *** |
| Leaf Area (cm2) | 55.7 a ± 2.09 | 66.1 ab ± 2.57 | 66.7 ab ± 3.58 | 73.9 bc ± 2.32 | 81.4 cd ± 3.10 | 86.7 ± 2.44 | 2.8 × 10−6 *** |
| Observed Data | Treatments | ||||||
|---|---|---|---|---|---|---|---|
| CTL | Cali | Card | Puti | Cali + puti | Card + puti | p-Value | |
| FAB (g) | 8.48 a ± 0.6 | 9.99 a ± 0.6 | 13 b ± 0.76 | 14.3 bc ± 0.56 | 13.3 b ± 0.42 | 16.7 c ± 0.47 | 1.1 × 10−7 *** |
| DAB (g) | 1.03 a ± 0.04 | 1.21 a ± 0.07 | 1.83 b ± 0.03 | 1.94 b ± 0.07 | 1.82 b ± 0.07 | 2.33 c ± 0.15 | 7.5 × 10−9 *** |
| FUB (g) | 3.75 a ± 0.38 | 5.28 ab ± 0.56 | 7.72 c ± 0.30 | 7.85 c ± 0.18 | 6.52 bc ± 0.22 | 7.92 c ± 0.27 | 2.0 × 10−7 *** |
| DUB (g) | 0.66 a ± 0.09 | 1.04 ab ± 0.05 | 1.56 bc ± 0.12 | 1.74 c ± 0.10 | 1.54 bc ± 0.14 | 1.84 c ± 0.2 | 1.6 × 10−5 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agbodjato, N.A.; Mikpon, T.; Babalola, O.O.; Dah-Nouvlessounon, D.; Amogou, O.; Lehmane, H.; Adoko, M.Y.; Adjanohoun, A.; Baba-Moussa, L. Use of Plant Growth Promoting Rhizobacteria in Combination with Chitosan on Maize Crop: Promising Prospects for Sustainable, Environmentally Friendly Agriculture and against Abiotic Stress. Agronomy 2021, 11, 2205. https://doi.org/10.3390/agronomy11112205
Agbodjato NA, Mikpon T, Babalola OO, Dah-Nouvlessounon D, Amogou O, Lehmane H, Adoko MY, Adjanohoun A, Baba-Moussa L. Use of Plant Growth Promoting Rhizobacteria in Combination with Chitosan on Maize Crop: Promising Prospects for Sustainable, Environmentally Friendly Agriculture and against Abiotic Stress. Agronomy. 2021; 11(11):2205. https://doi.org/10.3390/agronomy11112205
Chicago/Turabian StyleAgbodjato, Nadège Adoukè, Toussaint Mikpon, Olubukola Oluranti Babalola, Durand Dah-Nouvlessounon, Olaréwadjou Amogou, Halfane Lehmane, Marcel Yévèdo Adoko, Adolphe Adjanohoun, and Lamine Baba-Moussa. 2021. "Use of Plant Growth Promoting Rhizobacteria in Combination with Chitosan on Maize Crop: Promising Prospects for Sustainable, Environmentally Friendly Agriculture and against Abiotic Stress" Agronomy 11, no. 11: 2205. https://doi.org/10.3390/agronomy11112205
APA StyleAgbodjato, N. A., Mikpon, T., Babalola, O. O., Dah-Nouvlessounon, D., Amogou, O., Lehmane, H., Adoko, M. Y., Adjanohoun, A., & Baba-Moussa, L. (2021). Use of Plant Growth Promoting Rhizobacteria in Combination with Chitosan on Maize Crop: Promising Prospects for Sustainable, Environmentally Friendly Agriculture and against Abiotic Stress. Agronomy, 11(11), 2205. https://doi.org/10.3390/agronomy11112205







