Abstract
Introducing insectary plants along with principal crops is an effective way to increase the biological diversity of beneficial insects and improve the stability of ecological equilibrium in agrocenoses and could be an alternative to chemical plant protection, particularly in organic farming. The goal of this study was to determine the effect of white mustard as a companion plant in broad bean cultivation on the occurrence of Aphis fabae Scop., Syrphidae, and Coccinellidae. The study also aimed at finding the optimum row separation of broad bean plants. It also evaluated the effectiveness of the thinning of mustard in a specific time to eliminate excessive competition with the main crop. The results showed that white mustard contributed to visible suppression of A. fabae abundance on broad bean (to the level similar as with the use of chemical protection). S. alba contributed to an increased abundance of hoverflies and lady beetles on broad bean despite the relatively low abundance of their prey, i.e., aphids. Mustard thinning positively affected abundance of larvae and adults of lady beetles as well as improved predator-prey ratio for hoverfly larvae and adult lady beetles. The most appropriate distance between broad bean rows when white mustard was introduced was 65 cm, with the concomitant conduct of mustard thinning when the broad bean plants reached flower bud formation. White mustard can be recommended as an element increasing the role of natural enemies of aphids in mixed crops, however, its strong growth should be taken into account and the plant density should be properly adjusted to avoid excessive competition with main plant.
1. Introduction
Introducing plants, particularly those producing large amounts of pollen (such as white mustard), along with principal crops is an effective way to increase the biological diversity of beneficial insects and improve the stability of ecological equilibrium in agrocenoses [1,2,3,4]. These plants can also provide an alternative to chemical plant protection, particularly in ecological management schemes [5].
White mustard (Sinapis alba L.) is known to reduce feeding by some soil pests (e.g., beet cyst eelworm Heterodera schachtii Schmidt) [6,7]. Because of their allelopathic properties, the root exudates of this plant have been studied for their potential as proecological herbicides [8]. Introducing white mustard to the cultivation area of faba beans Vicia faba L. var. minor can increase the density of faba beans roots in the deeper layers of soils and thus allow better utilization of nutrients. White mustard has shallow root system, so they do not compete. Nevertheless, emphasis has been given to the high competitiveness of mustard for aboveground parts which leads to a reduction in the yield of faba beans seeds [9]. Therefore, it is necessary to select the appropriate row spacing between plants cultivated under companion planting system [10,11].
Thus far, only limited information is available on the effect of mustard as a companion plant on the occurrence of the pests of cultivated plants and their natural enemies, especially predators. It was shown that when cultivated near common peas, mustard reduced the feeding by pea leaf weevils (Sitona spp.) and pea thrips Kakothrips pisivorus Westwood [12,13]. This was due to the direct adverse effects of the compounds contained in the plants of the Brassicae family (glucosinolates). However, mustards can also indirectly affect herbivores by attracting predators or parasitoids that are natural enemies of those pests. Research to date has largely focused on the positive impact of white mustard on the occurrence, fecundity and longevity of parasitoids of herbivorous insects [14,15,16], whereas its impact on predatory insects remains relatively poorly studied. Mustard plants are characterized by a rapid rate of development and a short period of vegetative growth. Its flowers are intensively yellow, and produce large amounts of pollen [17]. Adult hoverflies (Syrphidae), whose larvae are the natural enemies of the pests (principally aphids) of cultivated plants, feed upon the nectar and pollen; therefore, insectary plants provide them with food and habitat, which results in the increase of their numbers [18]. The flower pollen is a source of amino acids, carbohydrates, sugars, proteins, and other organic and inorganic substances which are indispensable for energy generation and egg-laying. Furthermore, these compounds are necessary for the proper growth and development of other important aphid predators such as lady beetles (Coccinellidae) [19]. Several authors have underlined the effect of the composition of the nectar, pollen and the structure of flowers on the occurrence, fertility, and lifespan of the natural enemies of pests [20,21,22] and flowering mustard was observed to be one of the plants most commonly visited by hoverflies [18].
Although not widely cultivated, broad bean Vicia faba L. var. maior is a very useful model plant to study various factors affecting pests because it is attacked by numerous pests that also have a major economic impact on other cultivated plants (pea leaf weevils, black bean aphid Aphis fabae Scop., and bean weevils Bruchus rufimanus Boh.). Feeding by black bean aphid causes malformation of shoots and affects the formation of flowers and seeds. When plants are severely infested, the yield of seeds can be reduced by 30 to 50 percent depending on the variety [23]. Hoverflies and lady beetles are the main predators, which when abundant can effectively inhibit the development of A. fabae colonies and thus avoid the need to use chemical control.
The objectives of this study were to determine the effect of white mustard as a companion plant in broad bean cultivation on the occurrence of A. fabae, hoverflies, and lady beetles and on the growth of broad bean plants; determine optimum row separation of broad bean plants; and evaluate the effectiveness of thinning of companion plants in a specific time, to eliminate excessive competition with the main crop.
2. Materials and Methods
2.1. Experimental
Field experiment was conducted in 2015–2017 in the Experimental Stations of the Agricultural University in Prusy near Kraków (50°07′ N, 20°05′ E), Poland. The soil on the experimental site is degraded chernozem, formed from loess, with a granulometric composition of silt loam with a pH in H2O = 6.56 and a humus content of 2.28%. The trial area comprised three blocks: untreated homogenous broad bean (21 m × 21 m; served as control), companion planting (39 m × 39 m), and chemically protected (21 m × 21 m) (Figure 1). Division into three blocks was performed to avoid interference of investigated factors (mustard vicinity in companion planting, pesticides drifts in chemically protected treatment) with homogenous, unprotected treatment. Soil conditions within all three blocks as well as their surroundings were similar. The experiment was conducted each year in different part of the same bigger field. Broad bean of the Bartek variety was cultivated in companion planting with white mustard of the Bardena variety with different row spacing. White mustard was sown as a row in the midway between two broad bean rows (Figure 1). In order to find the correct spacing, the main experiment was preceded by a 1-year preliminary experiment. As it was observed in the preliminary experiment (three row spacing were tested: 50 cm (standard distance in broad bean cultivation), 65 cm and 80 cm) that white mustard was competitive toward broad bean, in the main experiment, the standard distance (50 cm) was eliminated and only row spacing of 65 and 80 cm was applied. Additionally, when the broad bean plants reached the phase of flower bud formation, half of the plots in which mustard was cultivated as a companion plant were thinned (every other mustard plant was uprooted). In the initial stage of growth of broad beans, mustard does not show any competitive effect on the main crop. To exert its limiting effect on the pests invading the emerging broad beans (adult pea leaf weevils), the population density of mustard must be high. During the development of both broad bean and mustard plants (both grow fairly fast and at similar rates), competitiveness shows up, and mustard plants limit the flowering of broad bean plants and subsequent pod setting; therefore, at that time, it is necessary to thin the mustard plants to reduce their competitiveness, while still retaining the attractiveness of their flowers.
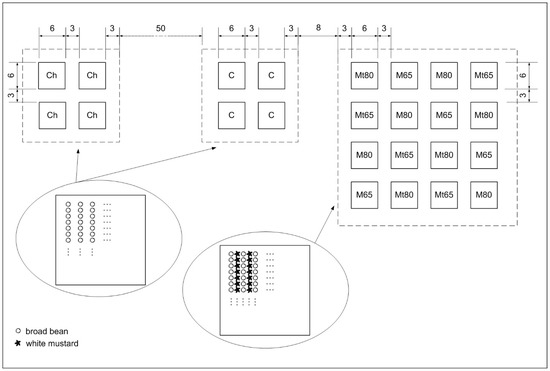
Figure 1.
Experiment scheme. Ch—broad beans in homogeneous cultivation with 50-cm row spacing subjected to standard chemical pest control practice; Control—broad beans in homogeneous cultivation with 50-cm row spacing; M65—broad beans with mustard with 65-cm row spacing, not thinned; M80—broad beans with mustard with 80-cm row spacing, not thinned; Mt65—broad beans with mustard with 65-cm row spacing, thinned; Mt80—broad beans with mustard with 80-cm row spacing, thinned. In Ch and Control were: 13 rows of broad bean per plot, 40 plants of broad bean in each row; in M65 and Mt65: 10 rows of broad bean per plot, 40 plants of broad bean in each row, 9 rows of mustard, 240 plants of mustard in each row (before thinning in Mt65); in M80 and Mt80: 8 rows of broad bean, 40 plants of broad bean in each row, 7 rows of mustard, 240 plants of mustard in each row (before thinning in Mt80).
In this study, the control treatment (unprotected) and chemically protected treatment (Ch) were characterized by homogeneous cultivation of broad beans at a row spacing of 50 cm. During cultivation in the Ch treatment, the following chemical insecticides were applied for protection as per conventional protection practice: Fastac 100 EC (BASF SE, alpha-cypermethrin, 100 g per liter of preparation (10.87%)) against pea leaf weevils (0.09 L/ha when the first damage caused by those agrophages was noticed, repeated after 7 days) and Decis 2.5 EC (Bayer SAS, deltamethrine, 25 g per liter of preparation (2.8%)) to combat broad bean weevils and black bean aphids (0.25 L/ha at the time of appearance of aphids on the broad bean plants, repeated after 7 days).
Each of the three blocks was further divided into experimental plots according to the specific protection method (Figure 1). The surface area of a single plot measured 36 m2 (6 m × 6 m). The experiment was carried out in four repetitions. The position of the plots with companion planting was randomized within the block. The blocks were separated by 8-m-wide oat strips, except for the chemically protected block, which was located 50 m away from the other blocks and separated by oat to avoid the effects of pesticide drift. Due to their neutrality for pests, similar buffer zones with cereals (1.25-m-wide stripes) had been used by other authors in previous studies investigating the effect of crop co-ordinates on natural enemies of pests [3]. The spacing of 3 m between the experimental plots within blocks was maintained mechanically as bare soil. Cereal plants (winter wheat in 2014, spring barley in 2015 and oat in 2016,) were used each year as a forecrop for broad bean plants. Broad bean seeds were sown at the end of March (25 March 2015, 30 March 2016, and 31 March 2017). Spacing of 15 cm within rows was maintained between the broad bean seeds. Seeds were placed in the soil at a depth of 6 cm. White mustard seeds were also sowed simultaneously between the rows of broad beans. The total volume of white mustard seeds was 50% of the volume per hectare normally sown for the seed production. Throughout the experiment, weed control was implemented by mechanical methods.
The following experimental treatments were set up:
- -
- broad beans with mustard with 65-cm row spacing, not thinned (M65);
- -
- broad beans with mustard with 80-cm row spacing, not thinned (M80);
- -
- broad beans with mustard with 65-cm row spacing, thinned (Mt65);
- -
- broad beans with mustard with 80-cm row spacing, thinned (Mt80);
- -
- broad beans in homogeneous cultivation with 50-cm row spacing (control); and
- -
- broad beans in homogeneous cultivation with 50-cm row spacing subjected to standard chemical pest control practice (Ch).
2.2. Black Bean Aphid Abundance
During the monitoring of black bean aphid (only asexual population of A. fabae was investigated [24]), the numbers of particular morphotic forms of aphids (wingless females, winged females, nymphs) were determined. These observations were conducted once every 3–4 days from the time of occurrence of the first winged female migrant up to the end of the period of occurrence of aphids on 30 randomly selected and marked plants on each plot. The aphids were counted on the whole plant (similarly as Almogdad and Semaskiene [25]). Generally, when the number of aphids was less than 100, all specimens were precisely counted, and when the number became higher, the number of aphids was estimated. Initially, the aphids colonise the apexes of plants, and then spread onto shoots and flowers, and subsequently to leaves and pods in the final stage of foraging. In the case of aphids feeding on the apex all individuals were carefully counted (even if their number was higher than 100). Aphids feeding on the shoot (all individuals) were counted on the length of 1 cm of the shoot and multiplied by the length of the colony on the shoot. The same applies to the aphids feeding on the pods. Similarly for aphids feeding on leaves or flowers, all individuals per 1 randomly selected and infested leaf (flower) were counted and multiplied by the number of leaves (flowers) infested by the aphids. The leaves were turned during counting.
2.3. Predator Abundance
The occurrence of predators of aphids (hoverflies Diptera: Syrphidae, lady beetles Coleoptera: Coccinellidae) was assessed simultaneously with aphids, on the same plants selected for assessing the occurrence of A. fabae. The occurrence and numbers of predators were analyzed in particular developmental stages (eggs and larvae for hoverflies; egg clutches, larvae and adult forms for lady beetles). In the case of lady beetles, which oviposit in clutches, the egg clutches rather than individual eggs were counted. Mean clutch size for Harmonia axyridis L. (which was the predominant species in this experiment) is 27.4 (±15.4) eggs [26]. To determine the species composition of hoverflies, all pupae encountered were transferred to the laboratory and placed in Petri dishes at a temperature of 22–24 °C and 70% relative humidity. Adults that emerged were identified to the species level with the use of the identification guides by Bańkowska [27] and van Veen [28]. Hoverfly larvae were not collected to avoid interference with the aphid–predator system.
2.4. Growth and Yield Assessments of Broad Beans
After completion of the assessment of the occurrence of A. fabae and predators (usually mid-July), plant materials (20 randomly selected whole plants from each plot, excluding plants from the outer rows to eliminate the marginal effect on the plant growth) were collected and their growth parameters were measured in a laboratory. This analysis was done once each year. The following characteristics were included in the analyses: mass of leaves, mass of shoots, mass of pods with seeds, and yield of seeds. Masses of leaves, shoots, and pods with seeds were measured for each plant separately, using laboratory balance. Mass of shoots was evaluated after removing leaves and pods with seeds. The seeds were taken from the pods and weighed as well. The average mass of seeds per plant was calculated for each plot separately. To calculate the seed yield, the average weight of seeds per plant in a given plot was multiplied by the number of plants in the plot. Then the seed yield per 1 m2 was calculated.
2.5. Statistical Analysis
The obtained data (number of aphids and individual stages of predators per plant, growth parameters and yield) were checked for normality (Shapiro–Wilk test with Lilliefors correction), and when necessary log-transformed. Then they were analyzed using STATISTICA 12.5 software. The significance of differences between the companion planting treatments was tested firstly by three-factor variance analysis (row spacing, thinning and year of study as factors). Because each of the parameters analyzed was significantly affected by the year of study (Tables S1, S3 and S5) the data from each year was then analyzed separately and two-factor variance analysis (row spacing and thinning as factors) was performed. The means were differentiated by Fisher’s LSD test at a level of p < 0.05. Data from control and Ch treatments were treated as standards and not included in Anova analyses. To determine the quantitative relationships between the components of the assemblage of A. fabae predators, the numerical predator–prey ratios were also calculated (mean data per plot were used, the total number of aphids was divided into three equal parts and then each part (number of aphids) was divided by the number of specific predator—Syrphidae larva, Coccinellidae larva or adult Coccinellidae).
3. Results
3.1. Abundance of Black Bean Aphid, Hoverflies, and Lady Beetles
Aphids were the most abundant in 2015, and the least in 2017 (Table 1). Neither different row spacing nor the thinning had a significant effect on their numbers (Table S2). The respective mean counts of A. fabae in 2015, 2016 and 2017 on broad bean in treatments with accompanying mustard was 2-, 9- and 7-fold lower than in homogenous broad bean crop (control).

Table 1.
Mean numbers of black bean aphid (all morphotic forms) per one broad beans plant in the 2015–2017 seasons.
Thinning and row spacing significantly influenced number of hoverfly larvae only in 2017 (Table S2). Thinning and 65 cm row spacing resulted in increased number of hoverfly larvae in this season (Table 2). Comparing with control treatment number of hoverfly larvae in mustard treatments was similar (2015, 2016) or lower (2017).

Table 2.
Mean numbers of Syrphidae larvae per one broad beans plant in the 2015–2017 seasons.
Thinning resulted in increased number of lady beetle larvae in 2015 (three-fold) and 2016 (by approximately 50%). In 2016 significant interaction between row spacing and thinning in the number of lady beetles larvae was found. This parameter had significantly higher value in Mt65 treatment than in M65 and Mt80 treatments. The number of adult lady beetles was affected by thinning procedure in 2016 and 2017 as well as by row spacing in 2017 (Table S2). Thinning increased the abundance of adults by nearly 50% to 110% (depending of the year of study) while increased row spacing increased this parameter by approximately 70% (Table 3).

Table 3.
Mean numbers of Coccinelidae larvae and adults per one broad beans plant in the 2015–2017 seasons.
To check if the treatments influenced early appearance of eggs of predators the dynamics of their abundance during individual days of observations was presented (Figure 2). Hoverflies eggs were more abundant already at the beginning of aphid development on broad bean plants cultivated in the vicinity of mustard than in control (Figure 2a,c,e). In the case of lady beetles egg clutches there were no such tendencies (Figure 2b,d,f). Counts of hoverfly eggs in the treatments, where broad bean was accompanied by mustard was affected by row spacing in 2015 and 2017 seasons (Table S2). However, in 2015 significantly higher number of hoverfly eggs was found in the treatment with 65 cm spacing, while in 2017 in the treatment with 80 cm. In 2015, significant interaction between row spacing and thinning was found—the number of hoverfly eggs in Mt80 treatment was significantly lower than in all other treatments. Comparing with control treatment number of hoverfly eggs in mustard treatments was similar (2015 and 2017) or higher (2016). No significant impact of thinning and row spacing on the number of lady beetle egg clutches laid could be observed (Table S2).
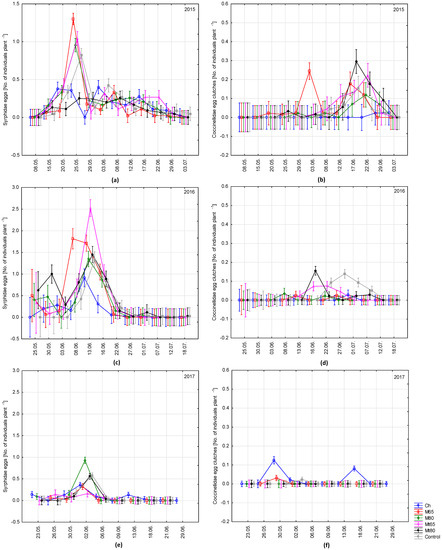
Figure 2.
Mean numbers of Syrphidae eggs (a,c,e) and Coccinellidae egg clutches (b,d,f) per one broad beans plant at individual days of observations in the 2015 (a,b), 2016 (c,d), and 2017 (e,f) seasons. Vertical bars mean ± SE. Symbols as in Figure 1. The scale for Figure 2a different from Figure 2c,e.
3.2. Predator-Prey Ratio and Species Composition of Predators
Predator-prey ratio in the case of hoverfly larvae was significantly affected by thinning in two years of study (2016 and 2017) and by row spacing in one year (2016) (Table S4). Significantly lower (approximately two times) number of aphids per hoverfly larva was observed when mustard was thinned (Table 4). Increased row spacing in 2016 resulted in increased number of aphids per hoverfly larva. Significant interaction between these two factors in 2016 and 2017 was also found—the most favorable predator-prey ratio (i.e., the lowest number of aphids per hoverfly larva) was observed in Mt65 treatment (in 2017). The effect of row spacing and thinning on predator-prey ratio for lady beetle larvae differed between two years of study: in 2016 increased distance between rows (up to 80 cm) and thinning resulted in lowered predator-prey ratio, while in 2017 it was the opposite (Table 4). The interaction of these two factors in 2016 and 2017 was also significant (Table S4). In 2016 the least favorable predator-prey ratio was found in M65 treatment, while in 2017—in M80. The effect of thinning procedure was more distinct for adult lady beetles than for larvae: in each year of study lower number of aphids per adult lady beetle was observed when thinning procedure was performed (effects statistically significant in 2016 and 2017). Similar effect was found under increasing row spacing in 2016.

Table 4.
The number of aphids per predator (larva of Syrphidae or Coccinellidae, adult of Coccinellidae) in the 2015–2017 seasons.
The numbers of aphids per hoverfly larva in control were 2–11 fold higher than in treatments with mustard (depending on the year of study and treatment). In the case of lady beetle larvae the predator-prey ratio was also more favorable in the vicinity of mustard comparing to control (between 1.04 and 11.96-fold lower number of aphids per lady beetle larva dependently on the year of study in different treatments with mustard). In the case of adult lady beetles the predator to prey ratio in mustard treatments was 3.8 to 7.2 fold lower (depending on the year and treatment) than in control, except for M65 treatment in 2016.
Among lady beetles, Harmonia axyridis (Pallas) was the predominant species, while the most abundant representative of hoverflies was Episyrphus balteatus (Deg.) (Table 5).

Table 5.
Species composition of Syrphidae and Coccinellidae.
3.3. Growth and Yield Assessments of Broad Beans
Increased row spacing resulted in increased masses of leaves (all years of study), shoots (2015 and 2017) and mass of pods with seeds (2016 and 2017) (Table 6). Thinning procedure influenced positively mass of leaves (all years of study) and mass of pods with seeds (2016 and 2017). Mass of pods with seeds in all treatments with mustard (except for M65 treatment in 2017) was higher than in the control treatment. Interestingly, this parameter was also higher in some treatments with mustard than in the treatment utilizing chemical protection (see years 2016 and 2017).

Table 6.
Growth parameters of broad beans in the 2015–2017 seasons.
Smaller row spacing (65 cm) contributed to increased seed yield by 10–44% (depending on the year) compared with 80 cm row spacing (Table 7). The effects were significant in 2015 and 2016. Mustard thinning increased the yield significantly in 2017 (by 32%). In other years of study the yield was also higher when thinning was performed, however the differences were not significant (Table 7 and Table S6). In the result, the highest mean seed yield in 2016 and 2017 was in Mt65 treatment. In 2016 it was even higher by 15% than in chemically protected treatment.

Table 7.
Broad bean seed yield [g m−2] in the 2015–2017 seasons.
4. Discussion
4.1. Abundance of Black Bean Aphid, Hoverflies, and Lady Beetles
Results of the conducted study shows that white mustard used as an accompanying plant can be a favorable solution in controlling aphids in broad bean crop. In each treatment tested, clearly fewer aphids were recorded on broad bean plants in the company of S. alba than in homogenous unprotected crop (Table 1). In some years of the study (2016 and 2017), the aphid population size in mixed crop was lower even than in the insecticide-treated broad bean plots. Other studies have shown that diversified cropping systems reduce general aphid abundance as compared with monoculture [29,30]. S. alba used as living mulch in zucchini crop resulted in lowered densities of Aphis gossypii Glover [10]. The cause for the lower density of aphids in mixed crop may involve difficulty in finding the host plant by the incoming migrants. Flying aphids are directed by visual stimuli, resulting from the contrast between soil background and plant foliage [31,32], which is lowered in mixed crop due to greater coverage of soil surface by vegetation. Another significant cause for the lower abundance of aphids in mixed crops may be the increased role of natural enemies, which are provided by the accompanying crop.
In this study, a positive impact of the presence of mustard in broad bean inter-rows on the number of eggs laid by hoverflies was visible (especially in 2016). Synchronous occurrence of predators and prey is crucial to effective biological control. Hoverflies typically appear in aphid colonies with some delay after the aphids [33]. Often-times, their abundance is therefore too low to keep the pest population below the threshold level, especially on broad bean, where the aphid development rate is higher than on other host plants of A. fabae [34]. However, our results point to an early appearance of hoverflies on the broad bean. This was particularly visible in treatments with mustard, where the aphid abundance was clearly lower than in homogenous crop (Figure 2a,c). Hoverflies are well adopted to ovipositing in small colonies of aphids [35], which likely contributes to their effectiveness in suppressing aphid populations. Early appearance is also important because aphid colonies inhabited by lady beetles are less attractive for oviposition by hoverfly females [36]. Our results showed lady beetles oviposited later than hoverflies (Figure 2).
White mustard is characterized by an intense growth and may compete with the main crop, therefore selection of the appropriate row spacing is vital. In the present experiment increasing the spacing between broad bean rows to 80 cm did not influence the abundance of aphids. The effect of this treatment on hoverfly eggs differed depending on the year of study (higher number of eggs in M65 than in M80 in 2015, but lower in 2017), while significant influence of row spacing on hoverfly larvae was exerted only in 2017 (with higher number in M65 than in M80). Lady beetles adults were more abundant as an effect of increased row spacing to 80 cm only in 2017. Very little is known about the effect of different row spacing on the occurrence of aphids and their predators. A study showed that variable soybean row spacing did not affect abundance of predatory insects, including hoverflies and lady beetles [37], while another study on this plant showed greater density of predators in the high density plantings [38]. In potato crop, average daily abundance of lady beetles was similar irrespective of the different spacing used [39]. Spacing did not have a significant impact on the occurrence of Aphis craccivora Koch. on groundnuts, whereas the incidence of hoverfly larvae was strictly correlated with the occurrence of aphids [40]. Also multivariate analysis points to a strict dependency between the counts of aphids and predators and any variation in the occurrence of A. gossypii under the impact of different row spacing in cotton crop affected the presence of predators [41]. However, the present experiment has an additional factor, i.e., the presence of mustard, which could modify the response of predators to the varied row spacing.
A positive impact of the mustard thinning procedure on the number of hoverfly larvae was observed only in one year of study, while on lady beetles larvae and adults in two years of study (Table 2 and Table 3). In 2015 the number of lady beetles larvae increased as a result of thinning to the level similar as in the homogenous broad bean crop, despite considerably poorer diet base, i.e., the aphids. This fact may be explained by an easier access to food sources (flower pollen and aphids) with lower plant density. Although for lady beetles the main attractant is the presence of aphids, the supplementary plant resources (such as pollen) may be of key significance for reproduction and life history even when suitable prey are not limiting [42]. In our experiment there was no significant effect of thinning on aphids abundance.
Our results suggest that the effect of spacing and/or thinning on predator and aphid abundances are interconnected. Lower plant densities favored higher abundance of predators, which suppressed aphids. However, lack of significant decrease of aphids abundance as a result of increased row spacing or thinning, where predators were more abundant, may suggest that lower density of plants is also beneficial for aphids. It was seen in 2015, when along with increased row density the number of aphids and lady beetles larvae increased.
4.2. Predator Species Composition and Predator-Prey Ratio
E. balteatus was the predominant hoverfly species, while H. axyridis and C. septempunctata were the most common lady beetle species. The recorded species composition do not deviate from reports of other authors on hoverflies and lady beetles feeding on A. fabae colonies [35,43]. A positive influence of mustard vicinity on predator-prey ratio was recorded. The number of aphids per 1 predator in treatments with mustard vs. control was lower by 2- to 11-fold for hoverfly larvae; from 1.04- to 11.96-fold for lady beetle larvae and from 3.8 to 7.2-fold for adult lady beetles depending on the year of the study and the treatment. Increased row spacing lowered the predator-prey ratio for lady beetle adults in one of three years of study, while in the same year the number of aphids per hoverfly larva increased as a result of increased row spacing (Table 4). The effect of different row spacing on predator-prey ratio for lady beetles larvae differed between two years of study (in one year it was lower, while in the next it was higher as a result of increased row spacing). Mustard thinning improved predator-prey ratio for hoverfly larvae and adult lady beetles in two out of three years of study. The influence of natural enemies on aphid populations depends on the relation between the pest population growth rate and the rate of prey consumption by the predators. One E. balteatus larva may eat between 660 to 1400 aphid nymphs of the III stage [35] throughout its entire development under laboratory conditions. As calculated by Wnuk [44], in the absence of other natural enemies, one E. balteatus larva can destroy an A. fabae colony, with predator to prey ratio from 1:15 to 1: 50 within 1.5–4 days, while with 1:200 within 7–8 days. At the 1:300 ratio a larva is unable to destroy a colony, but it significantly reduces colony growth rate. In our experiment, the predator-prey ratio in Mt65 in the 2017 season (78) was closest to this ratio (1:50). Predator-prey ratio for lady beetles in present experiment was generally more favorable than for hoverfly larvae (Table 4). According to Hodek et al. [45] lady beetles can significantly reduce or even destroy aphid colony with predator to prey ratio 1:150. Thus, considering the values of predator-prey ratio in the treatments with the vicinity of mustard (particularly those with mustard thinning performed), the hoverflies and lady beetles present in aphid colonies were able to prevent outbreaks of A. fabae.
4.3. Growth and Yield Assessments of Broad Beans
Analysis of the competitive effect of mustard on growth of broad bean in the preliminary experiment confirmed the earlier reports [9]. This effect was observed at all row spacings tested but declined with increasing row spacing. For this reason, the treatment where the distance between broad bean rows was standard 50 cm was abandoned. Thus, in the presented experiment only M65 and M80 treatments were used and in addition mustard thinning was introduced during the period of its most intense growth, for the further reduction of competition with broad bean. This procedure produced the expected results: the mass of leaves in all years of study and the mass of pods with seeds in 2016 and 2017 were significantly higher in treatment with thinning than when thinning was not performed (Table 6). This later parameter was even higher when compared to the chemically protected treatment. However, as row spacing increases the number of plants per unit area declined, such that the highest seed yields in 2016 and 2017 occurred in the mustard treatments with 65 cm row-spacing and thinning (Mt65). In 2015 the yield in this treatment was also higher than in the treatments with 80 cm spacing but it was similar to M65 treatment.
5. Conclusions
- White mustard intercropped with broad bean crop contributed to visible limitation of black bean aphid abundance on broad bean (to the level similar as with the use of chemical protection);
- The presence of white mustard caused increased abundance of hoverflies and lady beetles on broad bean despite the relatively low abundance of prey-aphids and increased hoverfly oviposition early in the development of aphid infestations, which is important to effective biological control;
- Mustard thinning positively affected abundance of larvae and adults of lady beetles as well as improved predator-prey ratio for hoverfly larvae and adult lady beetles;
- Mustard thinning improved mass of leaves and mass of pods with seeds of broad bean, while broad bean seed yield increased only at a row spacing of 65 cm distance and when the mustard was thinned;
- The most appropriate distance between broad bean rows when white mustard was introduced as insectary plant was 65 cm, with the concomitant conduct of mustard thinning when the broad bean plants reached the phase of flower bud formation.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agronomy11112202/s1, Table S1: Anova results for year (Y), row spacing (RS) and thinning (T) on mean numbers of aphids, Syrphidae eggs and larvae, Coccinellidae egg clutches, larvae and adults in broad bean, Table S2: Anova results for spacing (RS) and thinning (T) on mean numbers of aphids, Syrphidae eggs and larvae, Coccinellidae egg clutches, larvae and adults in broad bean in each year of study, Table S3: Anova results for year (Y), row spacing (RS) and thinning (T) on the number of aphids per one predator (larva of Syrphidae or Coccinellidae, adult of Coccinellidae) in broad bean, Table S4: Anova results for spacing (RS) and thinning (T) on the number of aphids per one predator (larva of Syrphidae or Coccinellidae, adult of Coccinellidae) in broad bean in each year of study, Table S5: Anova results for year (Y), row spacing (RS) and thinning (T) on the growth parameters and yield of broad bean, Table S6: Anova results for spacing (RS) and thinning (T) on the growth parameters and yield of broad bean in each year of study.
Funding
This research was funded by a subsidy of the Ministry of Education and Science for the University of Agriculture in Krakow for the years 2014–2017 and 2021.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Acknowledgments
Thank you to Milena Rusin, Barbara Biniaś and Marcin Gospodarek for technical support.
Conflicts of Interest
The author declares no conflict of interest.
References
- Pascual-Villalobos, M.J.; Lacasa, A.; González, A.; Varó, P.; Garcia, M.J. Effect of flowering plant strips on aphid and syrphid populations in lettuce. Eur. J. Agron. 2006, 24, 182–185. [Google Scholar] [CrossRef]
- Sądej, W.; Walerys, G.; Tworkowski, J. Alternative plants as a factor stimulating occurrence of entomophages. Prog. Plant Prot. 2007, 47, 202–211. [Google Scholar]
- Seidenglanz, M.; Huňady, I.; Poslušna, J.; Loes, A.K. Influence of intercropping with spring cereals on the occurrence of pea aphids (Acyrthosiphon pisum Harris, 1776) and their natural enemies in field pea (Pisum sativum L.). Plant Prot. Sci. 2011, 47, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Ouyang, F.; Chen, J.; Yang, Q.; Desneux, N.; Xiao, Y.; Zhang, J.; Ge, F. Biological control of Aphis spiraecola in apples using an insectary plant that attracts and sustains predators. Biol. Control 2021, 155, 104532. [Google Scholar] [CrossRef]
- Sadeghi, H. Abundance of adult hoverflies (Diptera: Syrphidae) on different flowering plants. Casp. J. Env. Sci. 2008, 6, 47–51. [Google Scholar]
- Szymczak-Nowak, J.; Nowakowski, M. Efekt antymątwikowy i plonowanie gorczycy białej, facelii błękitnej i rzodkwi oleistej uprawianych w plonie głównym. Rośl. Oleiste−Oilseed Crop. 2000, XXI, 285–292. [Google Scholar]
- Szymczak-Nowak, J.; Nowakowski, M. Plonowanie gorczycy białej, rzodkwi oleistej i facelii błękitnej uprawianych w plonie głównym oraz ich wpływ na populację mątwika burakowego. Rośl. Oleiste−Oilseed Crop. 2002, XXIII, 223–234. [Google Scholar]
- Sawicka, B.; Kotiuk, E. Gorczyce jako rośliny wielofunkcyjne. Acta Sci. Pol. Agric. 2007, 6, 17–27. [Google Scholar]
- Schröder, D.; Köpke, U. Faba bean (Vicia faba L.) intercropped with oil crops—A strategy to enhance rooting density and to optimize nitrogen use and grain production? Field Crop. Res. 2012, 135, 74–81. [Google Scholar] [CrossRef]
- Hooks, C.R.R.; Valenzuela, H.R.; Defrank, J. Incidence of pests and arthropod natural enemies in zucchini grown with living mulches. Agr. Ecosyst. Environ. 1998, 69, 217–231. [Google Scholar] [CrossRef]
- Singh, K.K.; Rathi, K.S. Dry matter production and productivity as influenced by staggered sowing of mustard intercropped at different row ratios with chickpea. J. Agron. Crop Sci. 2003, 189, 169–175. [Google Scholar] [CrossRef]
- Wnuk, A. Effect of intercropping of pea with tansy phacelia and white mustard on occurrence of pests. Folia Hortic. 1998, 10, 67–74. [Google Scholar]
- Wnuk, A.; Wiech, K. The effect of spacing, date of sowing and intercropping on the occurrence of pea pests. Roczn. Nauk Roln. E Ochr. Roślin 1996, 25, 9–14. [Google Scholar]
- Damien, M.; Le Lann, C.; Desneux, N.; Alford, L.; Al Hassan, D.; Georges, R.; Van Baaren, J. Flowering cover crops in winter increase pest control but not trophic link diversity. Agr. Ecosyst. Environ. 2017, 247, 418–425. [Google Scholar] [CrossRef] [Green Version]
- Jado, R.H.; Araj, S.-E.; Abu-Irmaileh, B.; Shields, M.W.; Wratten, S.D. Floral resources to enhance the potential of the parasitoid Aphidius colemani for biological control of the aphid Myzus persicae. J. Appl. Entomol. 2019, 143, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Arnó, J.; Oveja, M.F.; Gabarra, R. Selection of flowering plants to enhance the biological control of Tuta absoluta using parasitoids. Biol. Control 2018, 122, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Jabłoński, B.; Kołtowski, Z.; Szklanowska, K. Ważniejsze wyniki badań nektarowania, zapylania i plonowania gorczycy białej i rzepiku jarego. In Proceedings of the 38. Beekeeping Scientific Conference, Puławy, Poland; Institute of Horticulture: Puławy, Poland, 1999; pp. 29–30. [Google Scholar]
- Colley, M.R.; Luna, J.M. Relative attractiveness of potential beneficial insectary plants to aphidophagous hoverflies (Diptera: Syrphidae). Environ. Entomol. 2000, 29, 1054–1059. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Ann. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Patt, J.M.; Hamilton, G.C.; Lashomb, J.H. Foraging success of parasitoid wasps on flowers: Interplay of insect morphology, floral architecture and searching behavior. Entomol. Exp. Et Appl. 1997, 83, 21–30. [Google Scholar] [CrossRef]
- Berndt, L.A.; Wratten, S.D.; Hassan, P.G. Effects of buckwheat flowers on leafroller (Lepidoptera: Tortricidae) parasitoids in a New Zealand vineyard. Agric. For. Entomol. 2002, 4, 39–45. [Google Scholar] [CrossRef]
- Vattala, H.D.; Wratten, S.D.; Phillips, C.B.; Wäckers, F.L. The influence of flower morphology and nectar quality on the longevity of a parasitoid biological control agent. Biol. Control. 2006, 39, 179–185. [Google Scholar] [CrossRef]
- Cichocka, E.; Leszczyński, B.; Ciepiela, A.P.; Goszczyński, W. Response of Aphis fabae Scop. to different broad bean cultivars. EJPAU Hortic. 2002, 5. Available online: http://www.ejpau.media.pl (accessed on 7 August 2021).
- Sandrock, C.; Razmjou, J.; Vorburger, C. Climate effects on life cycle variation and population genetic architecture of the black bean aphid, Aphis fabae. Mol. Ecol. 2011, 20, 4165–4181. [Google Scholar] [CrossRef] [PubMed]
- Almogdag, M.; Semaškienė, R. The occurence and control of black bean aphid (Aphis fabae Scop.) in broad bean. Zemdirb.-Agric. 2021, 108, 165–172. [Google Scholar] [CrossRef]
- Stewart, L.A.; Dixon, A.F.G.; Ruzička, Z.; Iperti, G. Clutch and egg size in laybird beetles. Entomophaga 1991, 36, 329–333. [Google Scholar] [CrossRef]
- Bańkowska, R. Klucze do Oznaczania Owadów Polski, Część XXVII. Muchówki–Diptera. Zesz. 24 Syrphidae; PWN: Warszawa, Poland, 1963; p. 236. [Google Scholar]
- Van Veen, M. Hoverflies of Northwest Europe: Identification Keys to the Syrphidae; KNNV Publishing: Utrecht, Germany, 2004; p. 256. [Google Scholar]
- Gontijo, L.M.; Beers, E.H.; Snyder, W.E. Flowers promote aphid suppression in apple orchards. Biol. Control 2013, 66, 8–15. [Google Scholar] [CrossRef]
- Barbir, J.; Badenes-Pérez, F.R.; Fernandez-Quintanilla, C.; Dorado, J. The attractiveness of flowering herbaceous plants to bees (Hymenoptera: Apoidea) and hoverflies (Diptera: Syrphidae) in agro-ecosystems of Central Spain. Agric. For. Entomol. 2015, 17, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, J.S.; Booth, C.O.; Kershaw, W.J.S. Host finding by aphids in the field. II. Aphis fabae Scop. (Gynoparae) and Brevicoryne brassicae L.; with a reappraisal of the role of host finding behaviour in virus spread. Ann. Appl. Biol. 1959, 47, 424–444. [Google Scholar] [CrossRef]
- Kennedy, J.S.; Kershaw, W.J.S.; Booth, C.O. Host finding by aphids in the field. III. Visual attraction. Ann. Appl. Biol. 1961, 49, 1–21. [Google Scholar] [CrossRef]
- Almohamad, R.; Verheggen, F.J.; Haubruge, E. Searching and oviposition behavior of aphidophagous hoverflies (Diptera: Syrphidae): A review. Biotechnol. Agron. Soc. Et Environ. 2009, 13, 467–481. [Google Scholar]
- Schillewaert, S.; Vantaux, A.; Van den Ende, W.; Wenseleers, T. The effect of host plants on genotype variability in fitness and honeydew composition of Aphis fabae. Insect Sci. 2017, 24, 781–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tenhumberg, B.; Poehling, H.M. Syrphids as natural enemies of cereal aphids in Germany: Aspects of their biology and efficacy in different years and regions. Agricult. Ecosys. Environ. 1995, 52, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Alhmedi, A.; Francis, F.; Bodson, B.; Haubruge, E. Intraguild interactions of aphidophagous predators in fields: Effect of Coccinella septempunctata and Episyrphus balteatus occurrence on aphid infested plants. Comm. Appl. Biol. Sci. 2007, 72, 381–390. [Google Scholar]
- Buchanan, A.L.; Zobel, E.; Hinds, J.; Rosario-Lebron, A.; Hooks, C.R.R. Can Row Spacing Influence Arthropod Communities in Soybean? Implications for Early and Late Planting. Environ. Entomol. 2015, 44, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Mayse, M.A. Effects of spacing between rows on soybean arthropod populations. J. Appl. Ecol. 1978, 15, 439–450. [Google Scholar] [CrossRef]
- Boiteau, G. Effect of planting date, plant spacing, and weed cover on populations of insects, arachnids, and entomophthoran fungi in potato fields. Environ. Entomol. 1984, 13, 751–756. [Google Scholar] [CrossRef]
- Booker, R.H. Effect of sowing date and spacing on rosette disease of groundnut in northern Nigeria, with observations on vector, Aphis craccivora. Ann. App. Biol. 1963, 52, 125–131. [Google Scholar] [CrossRef]
- Malaquias, J.B.; Ramalho, F.S.; Dias, C.T.d.S.; Brugger, B.P.; Lira, A.C.S.; Wilcken, C.F.; Pachú, J.K.S.; Zanuncio, J.C. Multivariate approach to quantitative analysis of Aphis gossypii Glover (Hemiptera: Aphididae) and their natural enemy populations at different cotton spacings. Sci. Rep. 2017, 7, 41470. [Google Scholar] [CrossRef] [Green Version]
- Stowe, H.E.; Michaud, J.P.; Kim, T. The Benefits of Omnivory for Reproduction and Life History of a Specialized Aphid Predator, Hippodamia convergens (Coleoptera: Coccinellidae). Environ. Entomol. 2021, 50, 69–75. [Google Scholar] [CrossRef]
- Wnuk, A.; Gospodarek, J. Occurence of aphidophagus Syrphidae (Diptera) in colonies of Aphis fabae Scop., on its various host plants. Ann. Agricult. Sci. Ser. E-Plant Prot. 1999, 28, 7–15. [Google Scholar]
- Wnuk, A. Rola bzygowatych (Syrphidae) w ograniczaniu liczebności mszyc. Ochr. Roślin—Plant Prot. 2000, 9, 6–7. [Google Scholar]
- Hodek, J.; Novak, K.; Skuhravy, V.; Holman, J. The predation of Coccinella septempunctata L. on Aphis fabae Scop. on sugar beet. Acta Entomol. Bohemoslov 1965, 62, 241–253. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).