Interaction of Inherited Microbiota from Cover Crops with Cash Crops
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Crop Management
2.3. Sampling and Analysis
2.4. Statistical Analysis
3. Results
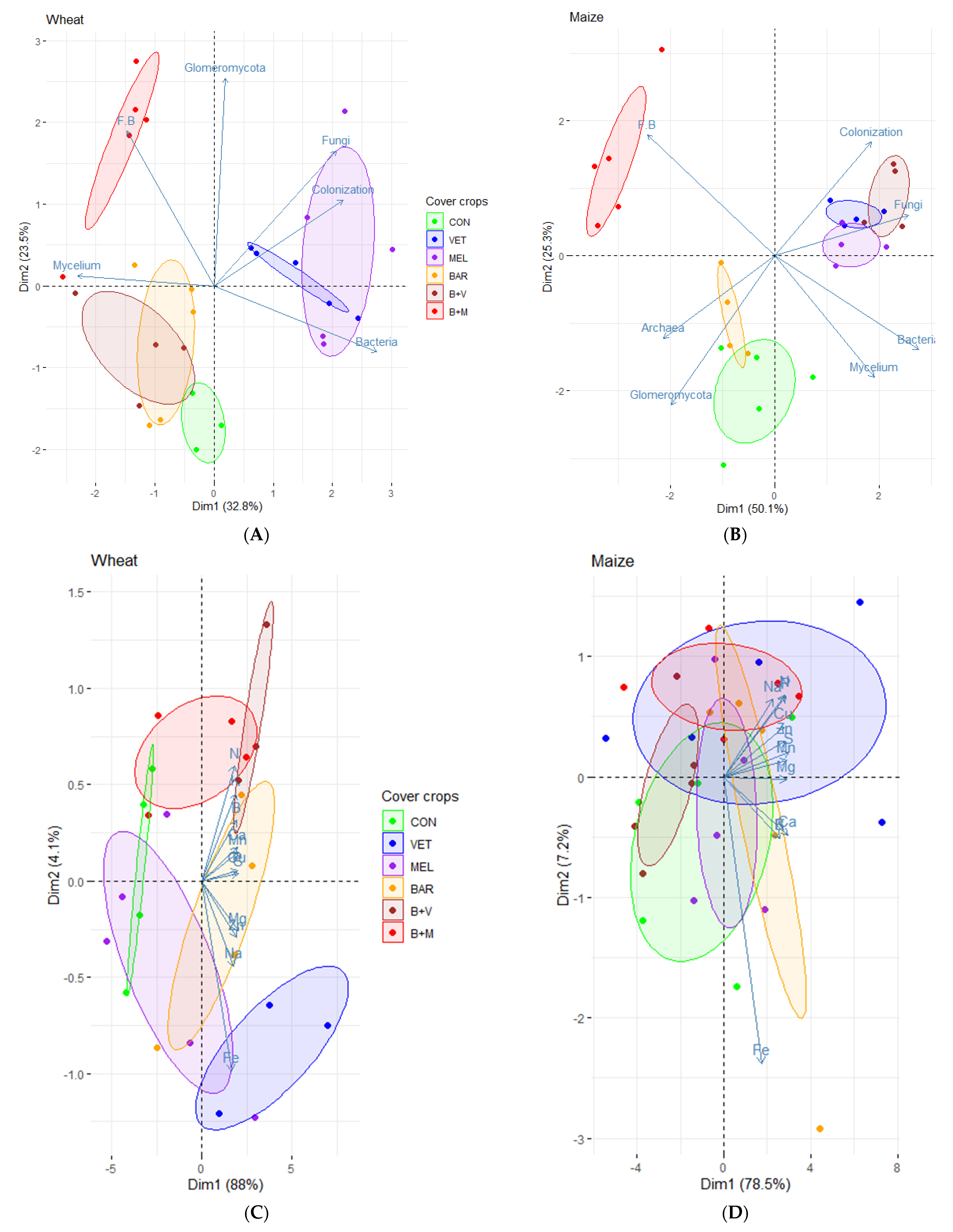
3.1. Effect of Cover Crops on Microbial Variables in Cash Crops
3.2. Effect of Cover Crops on the Nutrition and Growing of Cash Crops
3.3. Wheat and Maize Biomass Modelization
4. Discussion
4.1. Interactions of Crop Identities with the Soil Microbiota
4.2. Relationship of AMF and Nutrients in Main Crops
4.3. Bacteria, Archaea, and Fungi
4.4. Wheat and Maize Biomass
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finney, D.M.; Buyer, J.S.; Kaye, J.P. Living cover crops have immediate impacts on soil microbial community structure and function. J. Soil Water Conserv. 2017, 72, 361–373. [Google Scholar] [CrossRef] [Green Version]
- Thapa, R.; Mirsky, S.B.; Tully, K.L. Cover crops reduce nitrate leaching in agroecosystems: A global meta-analysis. J. Environ. Qual. 2018, 47, 1400–1411. [Google Scholar] [CrossRef]
- Guardia, G.; Aguilera, E.; Vallejo, A.; Sanz-Cobena, A.; Alonso-Ayuso, M.; Quemada, M. Effective climate change mitigation through cover cropping and integrated fertilization: A global warming potential assessment from a 10-year field experiment. J. Clean. Prod. 2019, 241, 118307. [Google Scholar] [CrossRef]
- Brust, J.; Claupein, W.; Gerhards, R. Growth and weed suppression ability of common and new cover crops in Germany. Crop. Prot. 2014, 63, 1–8. [Google Scholar] [CrossRef]
- Cordeau, S.; Guillemin, J.; Reibel, C.; Chauvel, B. Weed species differ in their ability to emerge in no-till systems that include cover crops. Ann. Appl. Biol. 2015, 166, 444–455. [Google Scholar] [CrossRef]
- Kim, N.; Zabaloy, M.C.; Guan, K.; Villamil, M.B. Do cover crops benefit soil microbiome? A meta-analysis of current research. Soil Biol. Biochem. 2020, 142, 107701. [Google Scholar] [CrossRef]
- Schipanski, M.E.; Barbercheck, M.; Douglas, M.R.; Finney, D.M.; Haider, K.; Kaye, J.P.; Kemanian, A.R.; Mortensen, D.A.; Ryan, M.R.; Tooker, J.; et al. A framework for evaluating ecosystem services provided by cover crops in agroecosystems. Agric. Syst. 2014, 125, 12–22. [Google Scholar] [CrossRef]
- García-González, I.; Quemada, M.; Gabriel, J.L.; Alonso-Ayuso, M.; Hontoria, C. Legacy of eight-year cover cropping on mycorrhizae, soil, and plants. J. Plant. Nutr. Soil Sci. 2018, 181, 818–826. [Google Scholar] [CrossRef]
- Tiecher, T.; Calegari, A.; Caner, L.; Rheinheimer, D.d.S. Soil fertility and nutrient budget after 23-years of different soil tillage systems and winter cover crops in a subtropical Oxisol. Geoderma 2017, 308, 78–85. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010; pp. 110–144. [Google Scholar]
- Köpke, U.; Nemecek, T. Ecological services of faba bean. Field Crops Res. 2010, 115, 217–233. [Google Scholar] [CrossRef]
- McLeod, M.L.; Bullington, L.; Cleveland, C.C.; Rousk, J.; Lekberg, Y. Invasive plant-derived dissolved organic matter alters microbial communities and carbon cycling in soils. Soil Biol. Biochem. 2021, 156, 108191. [Google Scholar] [CrossRef]
- Kabir, Z.; Koide, R.T. Effect of autumn and winter mycorrhizal cover crops on soil properties, nutrient uptake and yield of sweet corn in Pennsylvania, USA. Plant Soil 2002, 238, 205–215. [Google Scholar] [CrossRef]
- Requena, N.; Serrano, E.; Ocón, A.; Breuninger, M. Plant signals and fungal perception during arbuscular mycorrhiza establishment. Phytochemistry 2007, 68, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Gianinazzi, S.; Gollotte, A.; Binet, M.; van Tuinen, D.; Redecker, D.; Wipf, D. Agroecology: The key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 2010, 20, 519–530. [Google Scholar] [CrossRef]
- Cameron, D.D.; Neal, A.L.; van Wees, S.C.; Ton, J. Mycorrhiza-induced resistance: More than the sum of its parts? Trends Plant Sci. 2013, 18, 539–545. [Google Scholar] [CrossRef] [Green Version]
- Benitez, M.; Taheri, W.I.; Lehman, R.M. Selection of fungi by candidate cover crops. Appl. Soil Ecol. 2016, 103, 72–82. [Google Scholar] [CrossRef]
- Xiao, D.; Tan, Y.; Liu, X.; Yang, R.; Zhang, W.; He, X.; Wang, K. Effects of different legume species and densities on arbuscular mycorrhizal fungal communities in a karst grassland ecosystem. Sci. Total Environ. 2019, 678, 551–558. [Google Scholar] [CrossRef] [PubMed]
- García-González, I.; Quemada, M.; Gabriel, J.L.; Hontoria, C. Arbuscular mycorrhizal fungal activity responses to winter cover crops in a sunflower and maize cropping system. Appl. Soil Ecol. A Sect. Agric. Ecosyst. Environ. 2016, 102, 10–18. [Google Scholar] [CrossRef]
- Blesh, J. Functional traits in cover crop mixtures: Biological nitrogen fixation and multifunctionality. J. Appl. Ecol. 2018, 55, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Vukicevich, E.; Lowery, T.; Bowen, P.; Úrbez-Torres, J.; Hart, M. Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A review. Agron. Sustain. Dev. 2016, 36, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ball, K.R.; Baldock, J.A.; Penfold, C.; Power, S.A.; Woodin, S.J.; Smith, P.; Pendall, E. Soil organic carbon and nitrogen pools are increased by mixed grass and legume cover crops in vineyard agroecosystems: Detecting short-term management effects using infrared spectroscopy. Geoderma 2020, 379, 114619. [Google Scholar] [CrossRef]
- Abalos, D.; De Deyn, G.B.; Philippot, L.; Oram, N.J.; Oudová, B.; Pantelis, I.; Clark, C.; Fiorini, A.; Bru, D.; Mariscal-Sancho, I.; et al. Manipulating plant community composition to steer efficient N-cycling in intensively managed grasslands. J. Appl. Ecol. 2021, 58, 167–180. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Van Der Putten, W. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Schlatter, D.C.; Bakker, M.G.; Bradeen, J.M.; Kinkel, L.L. Plant community richness and microbial interactions structure bacterial communities in soil. Ecology 2015, 96, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; U.S. Department of Agriculture Natural Resources Conservation Service: Washington, DC, USA, 1999; pp. 163–270. [Google Scholar]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils 1. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Walkley-Black method. Methods Soil Anal. Part 1996, 3, 995–996. [Google Scholar]
- Villalobos, F.J.; Delgado, A.; López-Bernal, Á.; Quemada, M. FertiliCalc: A Decision Support System for Fertilizer Management. Int. J. Plant Prod. 2020, 14, 299–308. [Google Scholar] [CrossRef]
- Enz, M.; Dachler, C.H. Compendio para la Identificación de los Estadios Fenológicos de Especies Mono- y Dicotiledóneas Cultivadas. BBCH; BASF: Limburgerhof, Germany, 1998; pp. 12–25. [Google Scholar]
- Vierheilig, H.; Coughlan, A.P.; Wyss, U.; Piché, Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl. Environ. Microbiol. 1998, 64, 5004–5007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Jakobsen, I.; Abbott, L.K.; Robson, A.D. External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 1. Spread of hyphae and phosphorus inflow into roots. New Phytol. 1992, 120, 371–380. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Young, J.P. Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol. Ecol. 2008, 65, 339–349. [Google Scholar] [CrossRef] [Green Version]
- López-Gutiérrez, J.C.; Henry, S.; Hallet, S.; Martin-Laurent, F.; Catroux, G.; Philippot, L. Quantification of a novel group of nitrate-reducing bacteria in the environment by real-time PCR. J. Microbiol. Methods 2004, 57, 399–407. [Google Scholar] [CrossRef]
- Ochsenreiter, T.; Selezi, D.; Quaiser, A.; Bonch-Osmolovskaya, L.; Schleper, C. Diversity and abundance of Crenarchaeota in terrestrial habitats studied by 16S RNA surveys and real time PCR. Environ. Microbiol. 2003, 5, 787–797. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W. Fungal Barcoding Consortium Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [Green Version]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Bradstreet, R.B. The Kjeldahl Method for Organic Nitrogen; Elsevier Science & Technology: Saint Louis, MO, USA, 1965; pp. 186–188. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; pp. 1–1731. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and visualize the results of Multivariate Data Analyses; R Foundation for Statistical Computing: Vienna, Austria, 2016; pp. 1–74. [Google Scholar]
- Scavo, A.; Abbate, C.; Mauromicale, G. Plant allelochemicals: Agronomic, nutritional and ecological relevance in the soil system. Plant Soil 2019, 442, 23–48. [Google Scholar] [CrossRef]
- Iannucci, A.; Canfora, L.; Nigro, F.; De Vita, P.; Beleggia, R. Relationships between root morphology, root exudate compounds and rhizosphere microbial community in durum wheat. Appl. Soil Ecol. 2021, 158, 103781. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed your friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [Green Version]
- Dietz, S.; Herz, K.; Gorzolka, K.; Jandt, U.; Bruelheide, H.; Scheel, D. Root exudate composition of grass and forb species in natural grasslands. Sci. Rep. 2020, 10, 10691. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Doornbos, R.F.; van Loon, L.C.; Bakker, P.A. Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. A review. Agron. Sustain. Dev. 2012, 32, 227–243. [Google Scholar] [CrossRef]
- Vranova, V.; Rejsek, K.; Skene, K.R.; Janous, D.; Formanek, P. Methods of collection of plant root exudates in relation to plant metabolism and purpose: A review. J. Plant Nutr. Soil Sci. 2013, 176, 175–199. [Google Scholar] [CrossRef]
- Sivaram, A.K.; Subashchandrabose, S.R.; Logeshwaran, P.; Lockington, R.; Naidu, R.; Megharaj, M. Rhizodegradation of PAHs differentially altered by C3 and C4 plants. Sci. Rep. 2020, 10, 16109. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Garcia, B.J.; Furches, A.; Tuskan, G.A.; Jacobson, D. Plant host-associated mechanisms for microbial selection. Front. Plant Sci. 2019, 10, 862. [Google Scholar] [CrossRef] [Green Version]
- Pramanik, M.; Nagai, M.; Asao, T.; Matsui, Y. Effects of temperature and photoperiod on phytotoxic root exudates of cucumber (Cucumis sativus) in hydroponic culture. J. Chem. Ecol. 2000, 26, 1953–1967. [Google Scholar] [CrossRef]
- Drost, S.M.; Rutgers, M.; Wouterse, M.; De Boer, W.; Bodelier, P.L. Decomposition of mixtures of cover crop residues increases microbial functional diversity. Geoderma 2020, 361, 114060. [Google Scholar] [CrossRef]
- Wang, N.; Kong, C.; Wang, P.; Meiners, S.J. Root exudate signals in plant–plant interactions. Plant Cell Environ. 2021, 44, 1044–1058. [Google Scholar] [CrossRef]
- Lehmann, A.; Rillig, M.C. Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops–a meta-analysis. Soil Biol. Biochem. 2015, 81, 147–158. [Google Scholar] [CrossRef]
- Wen, Z.; Li, H.; Shen, Q.; Tang, X.; Xiong, C.; Li, H.; Pang, J.; Ryan, M.H.; Lambers, H.; Shen, J. Tradeoffs among root morphology, exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species. New Phytol. 2019, 223, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; Pascale, S.D.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Khalvati, M.A.; Hu, Y.; Mozafar, A.; Schmidhalter, U. Quantification of water uptake by arbuscular mycorrhizal hyphae and its significance for leaf growth, water relations, and gas exchange of barley subjected to drought stress. Plant. Biol. 2005, 7, 706–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Loiskandl, W.; Kaul, H.; Himmelbauer, M.; Wei, W.; Chen, L.; Bodner, G. Estimation of runoff mitigation by morphologically different cover crop root systems. J. Hydrol. 2016, 538, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Bingham, M.A.; Biondini, M. Mycorrhizal hyphal length as a function of plant community richness and composition in restored northern tallgrass prairies (USA). Rangel. Ecol. Manag. 2009, 62, 60–67. [Google Scholar] [CrossRef]
- Barceló, M.; Van Bodegom, P.M.; Tedersoo, L.; Den Haan, N.; Veen, G.F.; Ostonen, I.; Trimbos, K.; Soudzilovskaia, N.A. The abundance of arbuscular mycorrhiza in soils is linked to the total length of roots colonized at ecosystem level. PLoS ONE 2020, 15, e0237256. [Google Scholar] [CrossRef]
- Verma, P.; Yadav, A.N.; Khannam, K.S.; Saxena, A.K.; Suman, A. Potassium-solubilizing microbes: Diversity, distribution, and role in plant growth promotion. In Microorganisms for Green Revolution; Springer: Berlin, Germany, 2017; pp. 125–149. [Google Scholar]
- Wu, S.C.; Cao, Z.H.; Li, Z.G.; Cheung, K.C.; Wong, M.H. Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: A greenhouse trial. Geoderma 2005, 125, 155–166. [Google Scholar] [CrossRef]
- Priyadharsini, P.; Muthukumar, T. Interactions between arbuscular mycorrhizal fungi and potassium-solubilizing microorganisms on agricultural productivity. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: Berlin, Germany, 2016; pp. 111–125. [Google Scholar]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Raya-Hernández, A.I.; Jaramillo-López, P.F.; López-Carmona, D.A.; Díaz, T.; Carrera-Valtierra, J.A.; Larsen, J. Field evidence for maize-mycorrhiza interactions in agroecosystems with low and high P soils under mineral and organic fertilization. Appl. Soil Ecol. A Sect. Agric. Ecosyst. Environ. 2020, 149, 103511. [Google Scholar] [CrossRef]
- Varma, A.; Hock, B. Mycorrhiza: Structure, Function, Molecular Biology and Biotechnology; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Latz, E.; Eisenhauer, N.; Scheu, S.; Jousset, A. Plant identity drives the expression of biocontrol factors in a rhizosphere bacterium across a plant diversity gradient. Funct. Ecol. 2015, 29, 1225–1234. [Google Scholar] [CrossRef]
- Sivaram, A.K.; Logeshwaran, P.; Lockington, R.; Naidu, R.; Megharaj, M. The impact of low molecular weight organic acids from plants with C3 and C4 photosystems on the rhizoremediation of polycyclic aromatic hydrocarbons contaminated soil. Environ. Technol. Innov. 2020, 19, 100957. [Google Scholar] [CrossRef]
- Naafs, B.; McCormick, D.; Inglis, G.N.; Pancost, R.D. Archaeal and bacterial H-GDGTs are abundant in peat and their relative abundance is positively correlated with temperature. Geochim. Cosmochim. Acta 2018, 227, 156–170. [Google Scholar] [CrossRef]
- Qin, H.; Brookes, P.C.; Xu, J. Arbuscular mycorrhizal fungal hyphae alter soil bacterial community and enhance polychlorinated biphenyls dissipation. Front. Microbiol. 2016, 7, 939. [Google Scholar] [CrossRef]
- Toljander, J.F.; Lindahl, B.D.; Paul, L.R.; Elfstrand, M.; Finlay, R.D. Influence of arbuscular mycorrhizal mycelial exudates on soil bacterial growth and community structure. FEMS Microbiol. Ecol. 2007, 61, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Bailey, V.L.; Smith, J.L.; Bolton, H., Jr. Fungal-to-bacterial ratios in soils investigated for enhanced C sequestration. Soil Biol. Biochem. 2002, 34, 997–1007. [Google Scholar] [CrossRef]
- Malik, A.A.; Chowdhury, S.; Schlager, V.; Oliver, A.; Puissant, J.; Vazquez, P.G.; Jehmlich, N.; von Bergen, M.; Griffiths, R.I.; Gleixner, G. Soil fungal: Bacterial ratios are linked to altered carbon cycling. Front. Microbiol. 2016, 7, 1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Charest, C.; Ton Phan, C. Cold acclimation of wheat (Triticum aestivum): Properties of enzymes involved in proline metabolism. Physiol. Plant 1990, 80, 159–168. [Google Scholar] [CrossRef]
- Leipner, J.; Stamp, P. Chilling stress in maize seedlings. In Handbook of Maize: Its Biology; Springer: Berlin, Germany, 2009; pp. 291–310. [Google Scholar]
- Steduto, P.; Hsiao, T.C.; Fereres, E.; Raes, D. Respuesta del Rendimiento de Los Cultivos al Agua; FAO: Rome, Italy, 2021; Volume 66, pp. 1–530. [Google Scholar]
- Veresoglou, S.D.; Rillig, M.C. Suppression of fungal and nematode plant pathogens through arbuscular mycorrhizal fungi. Biol. Lett. 2012, 8, 214–217. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, A.E.; Johansson, T.; Bengtson, P. Archaeal abundance in relation to root and fungal exudation rates. FEMS Microbiol. Ecol. 2012, 80, 305–311. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Nahar, K.; Hossain, M.; Mahmud, J.A.; Hossen, M.; Masud, A.A.; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Guoyuan, Z.; Zhifu, Y.; Xiaolin, L. Effect of potassium on chilling resistance of maize seedlings. Plant Nutr. Fertil. Sci. 1998, 4, 165–169. [Google Scholar]
- Espinoza, L.; Nathan, S.; Morteza, M. Understanding the Numbers on Your Soil Test Report; University of Arkansas: Fayetteville, AR, USA, 2006. [Google Scholar]
| Cover Crop | Mycorrhizal Colonization (%) | Hyphal Length (cm g−1) | Total Bacteria (Log Copies g−1) | Total Archaea (Log Copies g−1) | Total Fungi (Log Copies g−1) | Glomeromycota (Copies g−1) | F:B |
|---|---|---|---|---|---|---|---|
| CON | 28.0 (±4.47) c | 8.02 (±1.12) cd | 8.20 (±0.07) a | 9.23 (±0.01) d | 4.70 (±0.04) ab | 600 (±47) d | −3.49 (±0.05) b |
| VET | 54.8 (±4.82) a | 7.34 (±1.84) cd | 8.21 (±0.17) a | 9.06 (±0.31) d | 4.87 (±0.03) a | 2513 (±257) cd | −3.34 (±0.15) ab |
| MEL | 51.2 (±5.40) a | 6.22 (±1.52) d | 8.33 (±0.09) a | 11.73 (±0.19) c | 4.90 (±0.29) a | 8271 (±936) b | −3.61 (±0.22) b |
| BAR | 42.8 (±3.35) b | 14.91 (±2.11) a | 8.14 (±0.02) a | 12.56 (±0.09) b | 4.53 (±0.05) b | 8060 (±899) b | −3.61 (±0.06) b |
| B+V | 40.8 (±6.72) b | 9.94 (±1.69) bc | 7.84 (±0.08) b | 7.2 (±0.13) e | 4.52 (±0.13) b | 3936 (±1699) c | −3.32 (±0.16) ab |
| B+M | 39.2 (±3.90) b | 12.63 (±2.54) ab | 7.91 (±0.07) b | 13.78 (±0.02) a | 4.72 (±0.07) ab | 19,807 (±2105) a | −3.19 (±0.10) a |
| Cover Crop | Mycorrhizal Colonization (%) | Hyphal Length (cm g−1) | Total Bacteria (Log Copies g−1) | Total Archaea (Log Copies g−1) | Total Fungi (Log Copies g−1) | Glomeromycota (Copies g−1) | F:B |
|---|---|---|---|---|---|---|---|
| CON | 34.4 (±4.77) c | 12.65 (±2.79) a | 7.78 (±0.09) b | 12.95 (±0.09) b | 4.58 (±0.36) ab | 5219 (±997) a | −3.15 (±0.29) b |
| VET | 58.0 (±7.62) a | 12.00 (±1.15) a | 7.83 (±0.11) b | 12.05 (±0.10) c | 4.85 (±0.05) a | 308 (±43) d | −2.84 (±0.29) b |
| MEL | 54.4 (±6.07) a | 12.28 (±1.32) a | 7.81 (±0.11) b | 9.56 (±0.24) d | 4.77 (±0.10) a | 1343 (±255) c | −2.90 (±0.29) b |
| BAR | 40.4 (±3.85) bc | 9.80 (±2.34) ab | 7.47 (±0.16) c | 14.21 (±0.03) a | 4.62 (±0.04) ab | 3473 (±376) b | −2.93 (±0.20) b |
| B+V | 49.2 (±5.40) ab | 11.68 (±1.61) ab | 8.08 (±0.07) a | 7.29 (±0.06) e | 4.86 (±0.15) a | 28 (±3) e | −3.26 (±0.21) b |
| B+M | 41.6 (±6.39) bc | 7.96 (±1.11) b | 5.44 (±0.13) d | 12.69 (±0.06) b | 4.35 (±0.21) b | 3746 (±1357) b | −1.09 (±0.32) a |
| Cover | N | P | K | S | Ca | Mg |
| crop | (mg microcosm−1) | |||||
| CON | 112.6 (±26.5) c | 5.4 (±2.8) c | 113.7 (±18.4) bc | 5.4 (±0.65) c | 10.8 (±1.2) b | 7.6 (±4.9) a |
| VET | 239.5 (±64.9) a | 11.9 (±4.4) a | 163.9 (±41.5) ab | 14.1 (±4.3) a | 22.3 (±4.5) ab | 16.2 (±4.5) a |
| MEL | 136.3 (±56.2) bc | 5.8 (±3.1) bc | 106.4 (±47.3) c | 6.7 (±03.6) bc | 12.4 (±4.3) b | 7.7 (±4.93) a |
| BAR | 197.7 (±58.3) ab | 7.9 (±2.8) abc | 142.3 (±38.4) abc | 9.3 (±2.4) ab | 18.3 (±2.3) ab | 10.7 (±2.7) a |
| B+V | 242.6 (±14.2) a | 10.6 (±2.2) ab | 189.1 (±24.6) a | 12.7 (±2.3) a | 23.8 (±3.9) a | 15.0 (±4.1) a |
| B+M | 185.3 (±61.6) abc | 8.8 (±4.2) abc | 151.5 (±50.2) abc | 10.2 (±4.1) ab | 17.3 (±5.0) ab | 11.6 (±6.1) a |
| B | Cu | Fe | Mn | Zn | Na | |
| (µg microcosm−1) | ||||||
| CON | 24.87 (±3.44) c | 18.13 (±2.14) c | 364.6 (±84.44) b | 151.0 (±21.44) c | 46.08 (±4.77) c | 0.11 (±0.08) c |
| VET | 75.33 (±27.02) a | 44.71 (±13.04) a | 590.7 (±72.66) a | 445.0 (±139.27) a | 153.35 (±59.68) a | 0.94 (±1.18) a |
| MEL | 32.01 (±12.20) bc | 22.06 (±10.03) bc | 382.7 (±152.28) b | 200.7 (±89.67) bc | 76.45 (±45.73) bc | 0.17 (±0.09) bc |
| BAR | 46.52 (±11.12) abc | 31.08 (±10.04) ab | 460.5 (±52.67) ab | 321.0 (±77.01) ab | 81.44 (±24.39) abc | 0.78 (±1.00) ab |
| B+V | 68.05 (±19.84) a | 40.72 (±7.10) a | 496.0 (±109.63) ab | 428.3 (±81.24) a | 132.39 (±36.44) ab | 0.36(±0.13) abc |
| B+M | 53.29 (±19.37) ab | 31.27 (±12.84) abc | 407.8 (±170.59) ab | 328.9 (±121.54) ab | 73.88 (±26.41) abc | 0.26 (±0.23) bc |
| Cover Crop | Wheat (kg ha−1) | Maize (kg ha−1) |
|---|---|---|
| CON | 1240 (±274.2) b | 480 (±228.2) a |
| VET | 2240 (±564.3) a | 578 (±269.1) a |
| MEL | 1351 (±616.3) b | 505 (±131.2) a |
| BAR | 1904 (±545.3) ab | 607 (±62.2) a |
| B+V | 2297 (±167.1) a | 357 (±97.1) a |
| B+M | 1708 (±554.3) ab | 496 (±178.4) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulcuango, K.; Navas, M.; Centurión, N.; Ibañez, M.Á.; Hontoria, C.; Mariscal-Sancho, I. Interaction of Inherited Microbiota from Cover Crops with Cash Crops. Agronomy 2021, 11, 2199. https://doi.org/10.3390/agronomy11112199
Ulcuango K, Navas M, Centurión N, Ibañez MÁ, Hontoria C, Mariscal-Sancho I. Interaction of Inherited Microbiota from Cover Crops with Cash Crops. Agronomy. 2021; 11(11):2199. https://doi.org/10.3390/agronomy11112199
Chicago/Turabian StyleUlcuango, Kelly, Mariela Navas, Nelly Centurión, Miguel Á. Ibañez, Chiquinquirá Hontoria, and Ignacio Mariscal-Sancho. 2021. "Interaction of Inherited Microbiota from Cover Crops with Cash Crops" Agronomy 11, no. 11: 2199. https://doi.org/10.3390/agronomy11112199
APA StyleUlcuango, K., Navas, M., Centurión, N., Ibañez, M. Á., Hontoria, C., & Mariscal-Sancho, I. (2021). Interaction of Inherited Microbiota from Cover Crops with Cash Crops. Agronomy, 11(11), 2199. https://doi.org/10.3390/agronomy11112199






