A Review on the Role of Silicon Treatment in Biotic Stress Mitigation and Citrus Production
Abstract
:1. Introduction
2. Phytophthora Diseases of Citrus
3. Alternaria Disease
4. Green and Blue Mold
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abraham, A.O. Integrated Use of Yeast, Hot Water and Potassium Silicate Treatments for the Control of Postharvest Green Mould of Citrus and Litchi. Ph.D. Thesis, UKZN, Pietermaritzburg, South Africa, 2010. [Google Scholar]
- Jaouad, M.; Moinina, A.; Ezrari, S.; Lahlali, R. Key pests and diseases of citrus trees with emphasis on root rot diseases. Moroc. J. Agric. Sci. 2020, 1, 149–160. [Google Scholar]
- Liu, Y.Q.; Heying, E.; Tanumihardjo, S.A. History, global distribution, and nutritional importance of citrus fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organisation of the United Nations (FAO)—Statistical Pocketbook World Food and Agriculture. 2015. Available online: http://www.fao.org/3/ai4691e.pdf (accessed on 20 June 2021).
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT Database. 2016. Available online: http://faostat.fao.org/site/291/default.aspx (accessed on 20 June 2021).
- Janisiewicz, W.J.; Korsten, L. Biological control of postharvest diseases of fruits. Ann. Rev. Phytopathol. 2002, 40, 411–441. [Google Scholar] [CrossRef] [Green Version]
- Brown, G.E.; Miller, W.R. Maintaining fruit health after harvest. In Citrus Health Management; Timmer, L.W., Duncan, L.W., Eds.; APS Press: St. Paul, MN, USA, 1999; pp. 175–187. [Google Scholar]
- Macarisin, D.; Cohen, L.; Eick, A.; Rafael, G.; Belausov, E.; Wisniewski, M.; Droby, S. Penicillium digitatum suppresses production of hydrogen peroxide in host tissue during infection of citrus fruit. Phytopathology 2007, 97, 1491–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcet-Houben, M.; Ballester, A.R.; de la Fuente, B.; Harries, E.; Marcos, J.F.; Gonzalez-Candelas, L.; Gabaldon, T. Genome sequence of the necrotrophic fungus Penicillium digitatum, the main postharvest pathogen of citrus. BMC Genom. 2012, 13, 646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fourie, A. Biochemical Mechanisms for Tolerance of Citrus Rootstocks against Phytophthora nicotianae. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2004. [Google Scholar]
- Fourie, P.H.; du Preez, M.; Brink, G.C.; Schutte, G.C. The effect of runoff on spray deposition and control of Alternaria brown spot of mandarins. Australas. Plant Pathol. 2009, 38, 173–182. [Google Scholar] [CrossRef]
- Timmer, L.W.; Menge, J.A. Phytophthora-induced diseases. In Compendium of Citrus Diseases; Whiteside, J.O., Garnsey, S.M., Timmer, L.W., Eds.; APS Press: St. Paul, MN, USA, 1988; pp. 22–24. [Google Scholar]
- Timmer, L.W.; Solel, Z.; Gottwald, T.R.; Ibanez, A.M.; Zitiko, S.E. Environ-mental factors affecting production, release, and field populations of conidia of Alternaria alternata, the Cause of Brown Spot of Citrus. Phytopathology 1998, 88, 1218–1223. [Google Scholar] [CrossRef] [Green Version]
- Epstein, E. SILICON. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 641–664. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Birchall, J. The essentiality of silicon in biology. Chem. Soc. Rev. 1995, 24, 351–357. [Google Scholar] [CrossRef]
- Ma, J.F.; Takahashi, E. Soil, Fertilizer and Plant Silicon Research in Japan; Elsevier Science: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Matichenkov, V.; Bocharnikova, E. The relationship between silicon and soil physical and chemical properties. Stud. Plant Sci. 2001, 8, 209–219. [Google Scholar]
- Richmond, K.E.; Sussman, M. Got silicon? The non-essential beneficial plant nutrient. Curr. Opin. Plant Biol. 2003, 6, 268–272. [Google Scholar] [CrossRef]
- Jones, L.; Handreck, K. Silica in soils, plants, and animals. In Advance in Agronomy; Norman, A.G., Ed.; Academic Press: New York, NY, USA, 1967; pp. 107–149. [Google Scholar]
- Debona, D.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s role in abiotic and biotic plant stresses. Ann. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef] [Green Version]
- Haynes, R.J. A contemporary overview of silicon availability in agricultural soils. J. Plant Nutr. Soil Sci. 2014, 177, 831–844. [Google Scholar] [CrossRef]
- Jones, L.; Handreck, K. Studies of silica in the oat plant: III. Uptake of silica from soils by the plant. Plant Soil 1965, 1, 79–96. [Google Scholar] [CrossRef]
- Tubana, B.S.; Babu, T.; Datnoff, L.E. A review of silicon in soils and plants and its role in US agriculture: History and future perspectives. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef] [Green Version]
- Arnon, D.I.; Stout, P. The essentiality of certain elements in minute quantity for plants with special reference to copper. Plant Physiol. 1939, 14, 371. [Google Scholar] [CrossRef] [Green Version]
- Epstein, E.; Bloom, A. Mineral Nutrition of Plants: Principles and Perspectives, 2nd ed.; Sinauer Associates, Oxford University: Cary, NC, USA, 2005. [Google Scholar]
- Wang, M.; Gao, L.; Dong, S.; Sun, Y.; Shen, Q.; Guo, S. Role of Silicon on Plant–Pathogen Interactions. Front. Plant Sci. 2017, 8, 701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zargar, S.M.; Mahajan, R.; Bhat, J.A.; Muslima Nazir, M.; Deshmukh, R. Role of silicon in plant stress tolerance: Opportunities to achieve a sustainable cropping system. 3 Biotech 2019, 9, 73. [Google Scholar] [CrossRef]
- Dorairaj, D.; Ismail, M.R.; Sinniah, U.R.; Kar Ban, T. Influence of silicon on growth, yield, and lodging resistance of MR219, a lowland rice of Malaysia. J. Plant Nutr. 2017, 40, 1111–1124. [Google Scholar] [CrossRef]
- Marodin, J.C.; Resende, J.T.; Morales, R.G.; Silva, M.L.; Galvão, A.G.; Zanin, D.S. Yield of tomato fruits in relation to silicon sources and rates. Hort. Brasil. 2014, 32, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Sun, W.; Zhu, Y.-G.; Christie, P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ. Pollut. 2007, 147, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Fauteux, F.; Remus-Borel, W.; Menzies, J.G.; Belanger, R.R. Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol. Lett. 2005, 249, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fawe, A.; Abow-Zaid, M.; Menzies, J.G.; Bel-Anger, R.R. Silicon-mediated accumulation of flavonoid phytoalexins in cucumber. Phytopathology 1998, 88, 396–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heine, G.; Tikum, G.; Horst, W.J. The effect of silicon on the infection by and spread of Pythium aphanidermatum in single roots of tomato and bitter gourd. J. Exp. Bot. 2007, 58, 569–577. [Google Scholar] [CrossRef]
- Sakr, N. The role of silicon (Si) in increasing plant resistance against fungal diseases. Hell. Plant Prot. J. 2016, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012. [Google Scholar]
- Datnoff, L.E.; Rodrigrues, F.A.; Seebold, K.W. Silicon and plant disease. In Mineral Nutrition and Plant Disease; Datnoff, L.E., Elmer, W.H., Huber, D.M., Eds.; APS Press: St. Paul, MN, USA, 2007; pp. 233–246. [Google Scholar]
- Bekker, T.; Labuschagne, N.; Aveling, T.; Regnier, T.; Kaiser, C. Effects of soil drenching of water-soluble potassium silicate on commercial avocado (Persea americana Mill.) orchard trees infected with Phytophthora cinnamomi Rands on root density, canopy health, induction and concentration of phenolic compounds. S. Afr. J. Plant Soil 2014, 31, 101–107. [Google Scholar] [CrossRef]
- Menzies, J.G.; Belanger, R.R. Recent advances in cultural management of diseases of greenhouse crops. Can. J. Plant Pathol. 1996, 18, 186–193. [Google Scholar] [CrossRef]
- Chérif, M.; Benhamou, N.; Bélanger, R.R. Defense responses induced by soluble silicon in cucumber roots infected by Pythium spp. Phytopathology 1994, 84, 236–242. [Google Scholar] [CrossRef]
- Seebold, K.W. The Influence of Silicon Fertilization on the Development and Control of Blast Caused by Magnaporthe grisea (Hebert) Barr, in Upland Rice. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 1998. [Google Scholar]
- Seebold, K.W.; Kucharek, T.A.; Datnoff, L.E.; Correa-Victoria, F.J.; Mar-Chetti, M.A. The influence of silicon on components of resistance to blast in susceptible, partially resistant, and resistant cultivars of rice. Phytopathology 2001, 91, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Cui, Y.; Ding, X.; Dai, Q. Stimulation of phenolic metabolism by silicon contributes to rice resistance to sheath blight. J. Plant Nutr. Soil Sci. 2013, 176, 118–124. [Google Scholar] [CrossRef]
- Liang, Y.C.; Sun, W.; Si, J.; Römheld, V. Effects of foliar-and root applied silicon on the enhancement of induced resistance to powdery mildew in Cucumis sativus. Plant Pathol. 2005, 54, 678–685. [Google Scholar] [CrossRef]
- KIM, S.G.; Kim, K.W.; Park, E.W.; Choi, D. Silicon-induced cell wall fortification of rice leaves: A possible cellular mechanism of enhanced host resistance to blast. Phytopathology 2002, 92, 1095–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidane, E.G. Management of Fusarium Wilt Diseases Using Non-Pathogenic Fusarium oxysporum and Silicon. Ph.D. Thesis, University of KwaZulu-Natal, Pietermaritzburg Durban, South Africa, 2008. [Google Scholar]
- Li, Y.C.; Bi, Y.; Ge, Y.H.; Sun, X.J.; Wang, Y. Antifungal activity of sodium silicate on Fusarium sulphureum and its effect on dry rot of potato tubers. J. Food Sci. 2009, 74, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Whan, J.A.; Dann, E.K.; Aitken, E.A. Effects of silicon treatment and inoculation with Fusarium oxysporum f. sp. vasinfectum on cellular defences in root tissues of two cotton cultivars. Ann. Bot. 2016, 118, 219–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chain, F.; Côté-Beaulieu, C.; Belzile, F.; Menzies, J.; Bélanger, R. A comprehensive transcriptomic analysis of the effect of silicon on wheat plants under control and pathogen stress conditions. Mol. Plant Microbe Interact. 2009, 22, 1323–1330. [Google Scholar] [CrossRef] [Green Version]
- Naidoo, P.V.; Mcfarlane, S.; Keeping, M.G.; Caldwell, P.M. Deposition of silicon in leaves of sugarcane (Saccharum spp. hybrids) and its effect on the severity of brown rust caused by Puccinia melanocephala. Proc. South Afr. Sugar Technol. 2009, 82, 542–546. [Google Scholar]
- Ghareeb, H.; Bozso, Z.; Ott, P.G.; Repenning, C.; Stahl, F.; Wydra, K. Transcriptome of silicon induced resistance against Ralstonia solanacearum in the silicon non- accumulator tomato implicates priming effect. Physiol. Mol. Plant Pathol. 2011, 75, 83–89. [Google Scholar] [CrossRef]
- Kurabachew, H.; Stahl, F.; Wydra, K. Global gene expression of rhizobacteriasilicon mediated induced systemic resistance in tomato (Solanum lycopersicum) against Ralstonia solanacearum. Physiol. Mol. Plant Pathol. 2013, 84, 44–52. [Google Scholar] [CrossRef]
- Alves, A.O.; Santos, M.M.B.; Souza, L.J.N.; Souza, E.B.; Mariano, R.L.R. Use of silicon for reducing the severity of bacterial wilt of sweet pepper. J. Plant Pathol. 2015, 97, 419–429. [Google Scholar] [CrossRef]
- Dallagnol, L.J.; Rodrigues, F.A.; Pascholati, S.F.; Fortunato, A.A.; Camargo, L.E.A. Comparison of root and foliar applications of potassium silicate in potentiating post-infection defences of melon against powdery mildew. Plant Pathol. 2015, 64, 1085–1093. [Google Scholar] [CrossRef]
- Zellner, W.; Frantz, J.; Leisner, S. Silicon delays Tobacco ringspot virus systemic symptoms in Nicotiana tabacum. J. Plant Physiol. 2011, 168, 1866–1869. [Google Scholar] [CrossRef]
- Andrade, C.C.L.; Resende, R.S.; Rodrigues, F.A.; Ferraz, H.G.M.; Moreira, W.R.; Oliveira, J.R.; Mariano, R.L.R. Silicon reduces bacterial speck development on tomato leaves. Trop. Plant Pathol. 2013, 38, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Resende, R.S.; Rodrigues, F.; Costa, R.V.; Silva, D.D. Silicon and fungicide effects on anthracnose in moderately resistant and susceptible sorghum lines. J. Phytopathol. 2013, 161, 11–17. [Google Scholar] [CrossRef]
- Cruz, M.F.A.; Debona, D.; Rios, J.A.; Barros, E.G.; Rodrigues, F.A. Potentiation of defense-related gene expression by silicon increases wheat resistance to leaf blast. Trop. Plant Pathol. 2015, 40, 394–400. [Google Scholar] [CrossRef]
- Ferreira, H.A.; do Nascimento, C.W.A.; Datnoff, L.E.; de Sousa Nunes, G.H.; Preston, W.; de Souza, E.B.; de Lima, M.R. Effects of silicon on resistance to bacterial fruit blotch and growth of melon. Crop. Prot. 2015, 78, 277–283. [Google Scholar] [CrossRef]
- van Bockhaven, J.; Steppe, K.; Bauweraert, I.; Kikuchi, S.; Asano, T.; Höfte, M.; de Vleesschauwer, D. Primary metabolism plays a central role in moulding silicon-inducible brown spot resistance in rice. Mol. Plant Pathol. 2015, 16, 811–824. [Google Scholar] [CrossRef]
- Fawe, A.; Menzies, J.G.; Chérif, M.; Bélanger, R.R. Silicon and disease resistance in dicotyledons. Stud. Plant Sci. 2001, 8, 159–169. [Google Scholar] [CrossRef]
- Currie, H.A.; Perry, C.C. Silica in plants: Biological, biochemical and chemical studies. Ann. Bot. 2007, 100, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.A.; Resende, R.S.; Dallagnol, L.J.; Datnoff, L.E. Silicon Potentiates Host Defense Mechanisms against Infection by Plant Pathogens; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Mkhize, N. Effectiveness of Pre- and Postharvest Silicon and Phosphorous Acid Applications in Inhibiting Penicillium Digitatum on Citrus Fruit. Master’s Thesis, UKZN, Pietermaritzburg, South Africa, 2014. [Google Scholar]
- Sakr, N. Silicon-enhanced resistance of plants to biotic stresses—Review article. Acta Phytopathol. Entomol. Hung. 2018, 53, 125–142. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2018, 221, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.A.; Jurick, W.M.; Datnoff, L.E.; Jones, J.B.; Rollins, J.A. Silicon influences cytological and molecular events incompatible and incompatible rice-Magnaporthe grisea interactions. Physiol. Mol. Plant Pathol. 2005, 66, 144–159. [Google Scholar] [CrossRef]
- Samuels, A.L.; Glass, A.D.M.; Menzies, J.G.; Ehret, D.L. Silicon in cell walls and papillae of Cucumis sativus during infection by Sphaerotheca fuliginea. Physiol. Mol. Plant Pathol. 1994, 44, 237–242. [Google Scholar] [CrossRef]
- Chang, S.J.; Tzeng, D.D.; Li, C.C. Effect of silicon nutrient on bacterial blight resistance of rice (Oryza sativa L.). In Proceedings of the Second Silicon in Agriculture Conference, Yamagata, Japan, 22–26 August 2002; pp. 31–33. [Google Scholar]
- Meena, V.D.; Dotaniya, M.L.; Coumar, V.; Rajendiran, S.; Kundu, A.S.; Rao, A.S. A case for silicon fertilization to improve crop yields in tropical soils. Proc. Natl. Acad. Sci. USA India Sect. B Biol. Sci. 2014, 84, 505–518. [Google Scholar] [CrossRef] [Green Version]
- Jayawardana, H.A.R.K.; Weerahewa, H.L.D.; Saparamadu, M.D.J.S. Effect of root or foliar application of soluble silicon on plant growth, fruit quality and anthracnose development of capsicum. Trop. Agric. Res. 2014, 26, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, P.B.; Dayanandan, P.; Franklin, C.I.; Takeoka, Y. Structure and function of silica bodies in the epidermal system of grass shoots. Ann. Bot. 1985, 55, 487–507. [Google Scholar] [CrossRef]
- Vivancos, J.; Labbé, C.; Menzies, J.G.; Bélanger, R.R. Silicon-mediated resistance of Arabidopsis against powdery mildew involves mechanisms other than the salicylic acid (SA)-dependent defence pathway. Mol. Plant Pathol. 2015, 16, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.H.; Menge, J.A. Root health: Fungal diseases. In Citrus Health Management; Timmer, L.W., Duncan, L.W., Eds.; APS Press: St. Paul, MN, USA, 1999; pp. 126–135. [Google Scholar]
- Graham, J.H.; Timmer, L.W. Phytophthora Disease of Citrus. 2003. Available online: http://edis.ifas.ufl.edu (accessed on 20 June 2021).
- Cacciola, S.O.; Magnano di San Lio, G. Management of citrus diseases caused by Phytophthora spp. In Integrated Management of Diseases Caused by Fungi, Phytoplasma and Bacteria; Ciancio, A., Mukerji, K., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 61–84. [Google Scholar]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; APS Press: St. Paul, MN, USA, 1996. [Google Scholar]
- Meitz-Hopkins, J.C.; Pretorius, M.C.; Spies, C.F.J.; Huisman, L.; Botha, W.J.; Langenhoven, S.D.; McLeod, A. Phytophthora species distribution in South African citrus production regions. Eur. J. Plant Pathol. 2014, 138, 733–749. [Google Scholar] [CrossRef]
- Graham, J.; Feichtenberger, E. Citrus phytophthora disease: Management challenges and successes. J. Citrus Pathol. 2015, 2, 1–11. [Google Scholar] [CrossRef]
- Panabières, F.; Ali, G.S.; Allagui, M.B.; Dalio, J.D.; Gudmestad, N.; Kuhn, M.L.; Roy, S.G.; Schena, L.; Zambounis, A. Phytophthora nicotianae diseases worldwide: New knowledge of a long-recognised pathogen. Phytopathol. Mediterr. 2016, 48, 159–188. [Google Scholar] [CrossRef]
- Alvarez, L.A.; Vicent, A.; de la Rosa, E.; Bascón, J.; Abad-Campos, P.; Armengol, J.; García-Jiménez, J. Branch cankers on citrus trees in Spain caused by Phytophthora citrophthora. Plant Pathol. 2008, 57, 84–91. [Google Scholar] [CrossRef]
- Schutte, G.C.; Botha, W.J. Phytophthora citrophthora trunk and branch canker on Clementine mandarins in the Western Cape province of South Africa. Plant Soil 2010, 27, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Graham, J.H.; Timmer, L.W.; Drouillard, D.L.; Peever, T.L. Characterization of Phytophthora spp. causing outbreaks of citrus brown rot in Florida. Phytopathology 1998, 88, 724–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, J.H.; Timmer, L.W.; Dewdney, M.M. Florida Citrus Pest Management Guide: Phytophthora Foot Rot and Root Rot; University of Florida, IFAS Extension: Gainesville, FL, USA, 2012; Volume 156, pp. 1–6. [Google Scholar]
- Graham, J.H. Root regeneration and tolerance of citrus rootstocks caused by Phytophthora nicotianae. Phytopathology 1995, 85, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Bekker, T. Efficacy of Water-Soluble Silicon for Control of Phytophthora Cinnamomi Root Rot of Avocado. Master’s Thesis, University of Pretoria, Pretoria, South Africa, 2007. [Google Scholar]
- Burger, M.C. Tolerance of Citrus Rootstocks to Root Pathogens. Master’s Thesis, University of Pretoria, Pretoria, South Africa, 2001. [Google Scholar]
- Khoshgoftarmanesh, A.H.; Mohaghegh, P.; Sharifnabi, B.; Shirvani, M.; Khalili, B. Silicon nutrition and Phytophthora drechsleri infection effects on growth and mineral nutrients concentration, uptake, and relative translocation in hydroponic-grown cucumber. J. Plant Nutr. 2012, 35, 1168–1179. [Google Scholar] [CrossRef]
- Mohaghegh, P.; Khoshgoftarmanesh, A.H.; Shirvani, M.; Sharifnabi, B.; Nili, N. Effect of silicon nutrition on oxidative stress induced by Phytophthora melonis infection in cucumber. Plant Dis. 2011, 95, 455–460. [Google Scholar] [CrossRef]
- Simmons, E.G. Alternaria: An Identification Manual; CBS Biodiversity: Utrecht, The Netherlands, 2007; Volume 6. [Google Scholar]
- Timmer, L.W.; Darhower, H.M.; Zitko, S.E.; Peever, T.L.; Ibanez, A.M.; Bushong, P.M. Environmental factors affecting the severity of Alternaria brown spot of citrus and their potential use in timing fungicide applications. Plant Dis. 2000, 84, 638–643. [Google Scholar] [CrossRef] [Green Version]
- Asanzi, N.M.; Taylor, N.J.; Vahrmeijer, J.T. Can silicon be used to prevent Alternaria alternata in citrus trees? Technologie 2015, 48, 1–3. [Google Scholar]
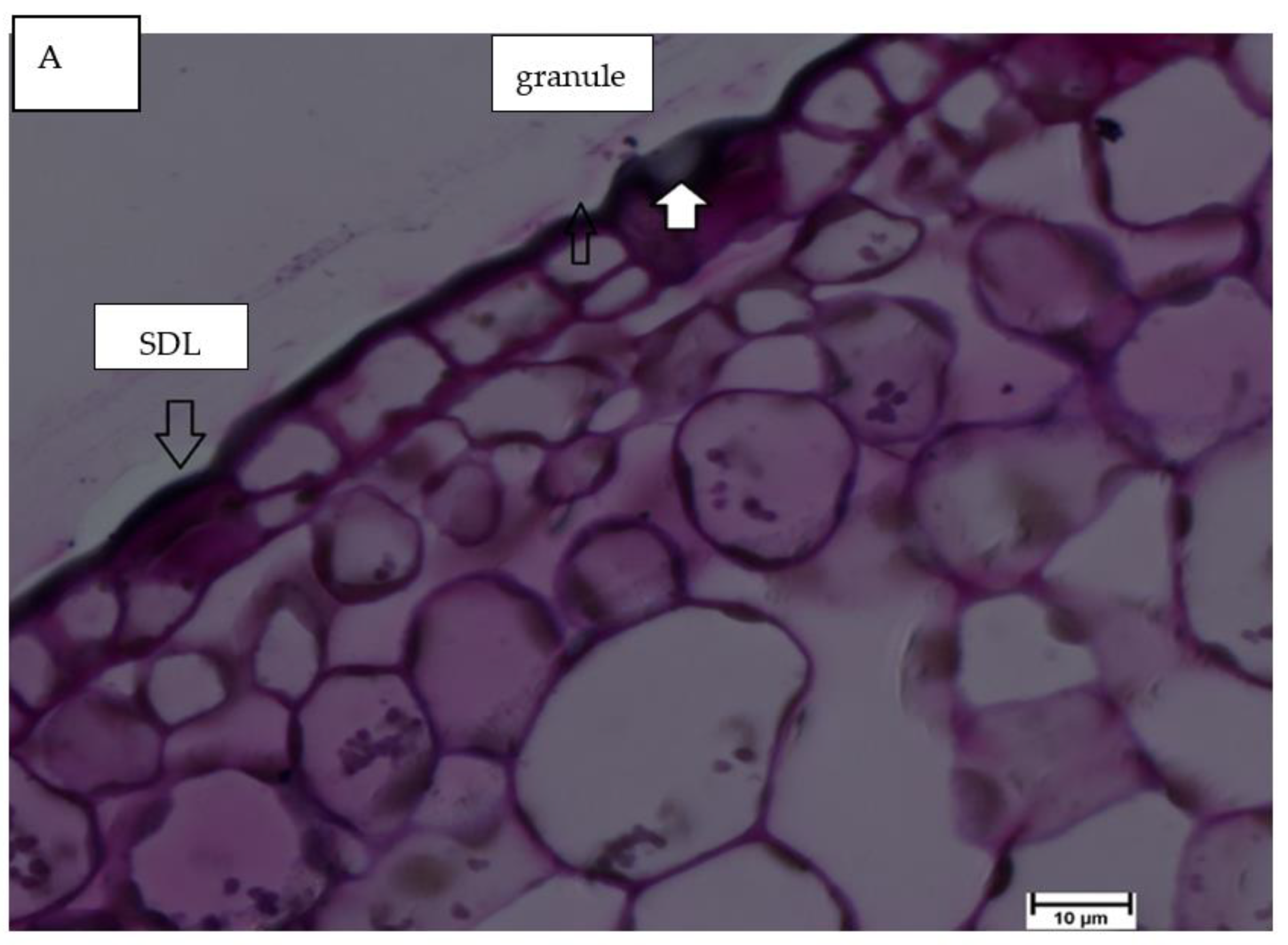
- Mvondo-She, M.A.; Marais, D. The investigation of silicon localization and accumulation in citrus. Plants 2019, 8, 200. [Google Scholar] [CrossRef] [Green Version]
- Matichenkov, V.; Calvert, D.; Snyder, G. Silicon fertilizers for citrus in Florida. Proc. Fla. State Hort. Soc. 1999, 112, 5–8. [Google Scholar]
- Matichenkov, V.; Bocharnikova, E.; Calvert, D. Response of citrus to silicon soil amendments. Proc Fla. State Hort. Soc. 2001, 114, 94–97. [Google Scholar]
- Tian, S.; Qin, G.; Li, B.; Wang, Q. Synergistic effects of combining microbial biocontrol agents with silicon against postharvest diseases of fruits. In Proceedings of the International Congress on Novel Approaches for the Control of Postharvest Diseases and Disorders, Bologna, Italy, 3–5 May 2007; pp. 38–46. [Google Scholar]
- Tian, S.; Torres, R.; Ballester, A.R.; Li, B.; Vilanova, L.; Gonzalez-Candelas, L. Molecular aspects in pathogen-fruit interactions: Virulence and resistance. Postharvest. Biol. Technol. 2016, 122, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Wuryatmo, E.; Klieber, A.; Scott, E.S. Inhibition of citrus postharvest pathogens by vapor of citral and related compounds in culture. J. Agric. Food Chem. 2003, 51, 2637–2640. [Google Scholar] [CrossRef]
- Chen, P.-S.; Peng, Y.-H.; Chung, W.-C.; Chung, K.-R.; Huang, H.-C.; Huang, J.-W. Inhibition of Penicillium digitatum and citrus green mold by volatile compounds produced by Enterobacter cloacae. J. Plant Pathol. Microbiol. 2016, 7, 339. [Google Scholar] [CrossRef] [Green Version]
- Eckert, J.W. Dynamics of benzimidazole resistant Penicillium in the development of postharvest decays of citrus and pome fruit. In Fungicide Resistance in North America; APS Press, American Phytopathological Society: St. Paul, MN, USA, 1988; pp. 31–35. [Google Scholar]
- Rodrigues, F.A.; Datnoff, L.E.; Korndörfer, G.H.; Seebold, K.W.; Rush, M.C. Effect of silicon and host resistance on sheath blight development in rice. Plant Dis. 2001, 85, 827–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mkhize, N.J.; Bower, J.P.; Bertling, I.; Mathaba, N. Response of citrus physiology to phosphorus acid and silicon as elicitors of induced disease resistance. Acta Hort. 2013, 1007, 135–142. [Google Scholar] [CrossRef]
| Product Name | Available Si (%) | Regulations | Manufacturer Website | Cost/ha in Citrus Orchards Monthly Application |
|---|---|---|---|---|
| AgSil K 50 | 21% Si | Non-toxic agricultural input with no MRL requirements (Reg. No. B3756, Act 36 of 1947 | Madumbi.co.za (accessed on 20 June 2021) | U$2 |
| Si granules | 98–99% Si | Products standardized according to FDA guidelines. SAR Agrochemicals and fertilizers Pvt. Ltd, Pune, India. | www.saragro.co.in/silicon-product.html (accessed on 20 June 2021) | U$1.64 |
| AgSil 21 | 26.5% silica liquid | No regulations issues SO certified (9001; 14001; 18001; 45001; 50001; 22716) low hazard to water | www.pqcorp.com | |
| AgSil 25 | 21% silica liquid | |||
| AgSil 16 | 53% silica hydrous powder |
| Biotic Stress | Crop | Treatments | Si Effects under Stress | Reference |
|---|---|---|---|---|
| Crown and root rot Pythium ultimum Trow | Cucumber | Potassium silicate (KaSil, 23.6% SiO2) | Increase in β-glucosidase activity and fungi-toxic aglycones | Chérif et al. [40] |
| Powdery mildew Podosphaera fulginea Braun and Takam. | Cucumber | Potassium silicate (K2SiO3) | Peroxidase, polyphenoloxidase and chitinase levels enhanced | Fawe et al. [33]; Liang et al. [44] |
| Rice blast (P. grisea) | Rice | (0, 50, 100 and 200 mg L−1 Si) | Fortification of cell wall | Kim et al. [45] |
| Fusarium wilt Fusarium oxysporum f.sp. cubense Snyder and Hansen | Banana | K2SiO3 (0, 490 and 7840 mg L−1 Si) and inoculation ofnon-pathogenic F. oxysporum strains | Combination of silicon and non-pathogenic F. oxysporum strains reduced the rate of infection, inhibiting hyphal growth | Kidane [46] |
| Dry rot of potato tubers (Fusarium sulphureum Schltdl.) | Potato | Sodium silicate | Fungitoxic effect by the thickening of the hyphal cell walls, cell distortion, and deposition of electron-dense material in hyphal cells. | Li et al. [47] |
| Fusarium wilt Fusarium oxysporum f. sp. vasinfectum Snyder and Hansen) | Cotton | Potassium silicate | Increase in phenolic content and lignin formation | Whan et al. [48] |
| Blumeria graminis f. sp. tritici Speer | Wheat | K2SiO3 | Disease in resistance correlated with a reduced expression of gene induced by the pathogen. | Chain et al. [49] |
| Brown rust Puccinia melanocephala Syd. and Syd. | Sugarcane (Saccharum spp. hybrids) | K2SiO3 | Si deposited in the lower epidermis, the upper epidermis and the mesophyll reduced rust infection. | Naidoo et al. [50] |
| Bacterial wilt (Ralstonia solanacearum (Smith) Yabuuchi) | Tomato | Silicon | Induced changes in gene expression which prime host resistance. | Ghareeb et al. [51] |
| Bacterial wilt (R. solanacearum) | Tomato | Silicon + rhizobacteria | The up-regulated genes were involved in signal transduction, defense, protein synthesis and metabolism, while a large proportion of down regulated genes were involved in photosynthesis, lipid metabolism. | Kurabachew et al. [52] |
| Bacterial wilt (R. solanacearum) | Sweet pepper | Calcium silicate | Increased production of chitinase, superoxide dismutase, ascorbate, peroxidase, β-1,3-glucanase, lignin and total protein. | Alves et al. [53] Dallagnol et al. [54] |
| Tobacco ringspot virus | Tobacco | Potassium silicate Si (0, 0.1 and 1 mmol L−1) | Enhanced Si levels delayed development of systemic ringspot symptoms, | Zellner et al. [55] |
| Bacterial speck (Pseudomonas syringae van Hall) | Tomato | Supa Silica (SS) (Agrichem; 23.7% K2O + 10% Si, pH 9.42) | Increased activity of peroxidase, polyphenoloxidase and glucanase | Andrade et al. [56] |
| Anthracnose (Glomerella graminicola Politis) | Sorghum | Calcium silicate | Si plus fungicide reduced anthracnose severity by 90% | Resende et al. [57] |
| Blast (P. oryzae) | Wheat | Silicon | Partly decrease the negative effects of salinity by increasing SOD and CAT activities, chlorophyll content and photochemical efficiency of PSII, but reduced H2O2 and MDA | Cruz et al. [58] |
| Bacterial fruit blotch (Acidovorax citrulli Williams et al.) | Melon | Silicon | The significant increase of Ca and Mg in Si treated melon inhibited bacterial botch. | Ferreira et al. [59] |
| Brown spot (Cochliobolus miyabeanus (Ito and Kurib.) Drechsler ex Dastur) | Rice | Silicon | Si application increased photorespiration rates, and enhanced disease resistance, maintaining photosynthetic activity. | Van Bockhaven et al. [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mvondo-She, M.A.; Gatabazi, A.; Laing, M.D.; Ndhlala, A.R. A Review on the Role of Silicon Treatment in Biotic Stress Mitigation and Citrus Production. Agronomy 2021, 11, 2198. https://doi.org/10.3390/agronomy11112198
Mvondo-She MA, Gatabazi A, Laing MD, Ndhlala AR. A Review on the Role of Silicon Treatment in Biotic Stress Mitigation and Citrus Production. Agronomy. 2021; 11(11):2198. https://doi.org/10.3390/agronomy11112198
Chicago/Turabian StyleMvondo-She, Mireille Asanzi, Auges Gatabazi, Mark Delmege Laing, and Ashwell Rungano Ndhlala. 2021. "A Review on the Role of Silicon Treatment in Biotic Stress Mitigation and Citrus Production" Agronomy 11, no. 11: 2198. https://doi.org/10.3390/agronomy11112198
APA StyleMvondo-She, M. A., Gatabazi, A., Laing, M. D., & Ndhlala, A. R. (2021). A Review on the Role of Silicon Treatment in Biotic Stress Mitigation and Citrus Production. Agronomy, 11(11), 2198. https://doi.org/10.3390/agronomy11112198







