Evaluation of Complete Fertilizer in the Aspect of the Antioxidant Enzyme System of Maize Hybrids
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Field Experiment
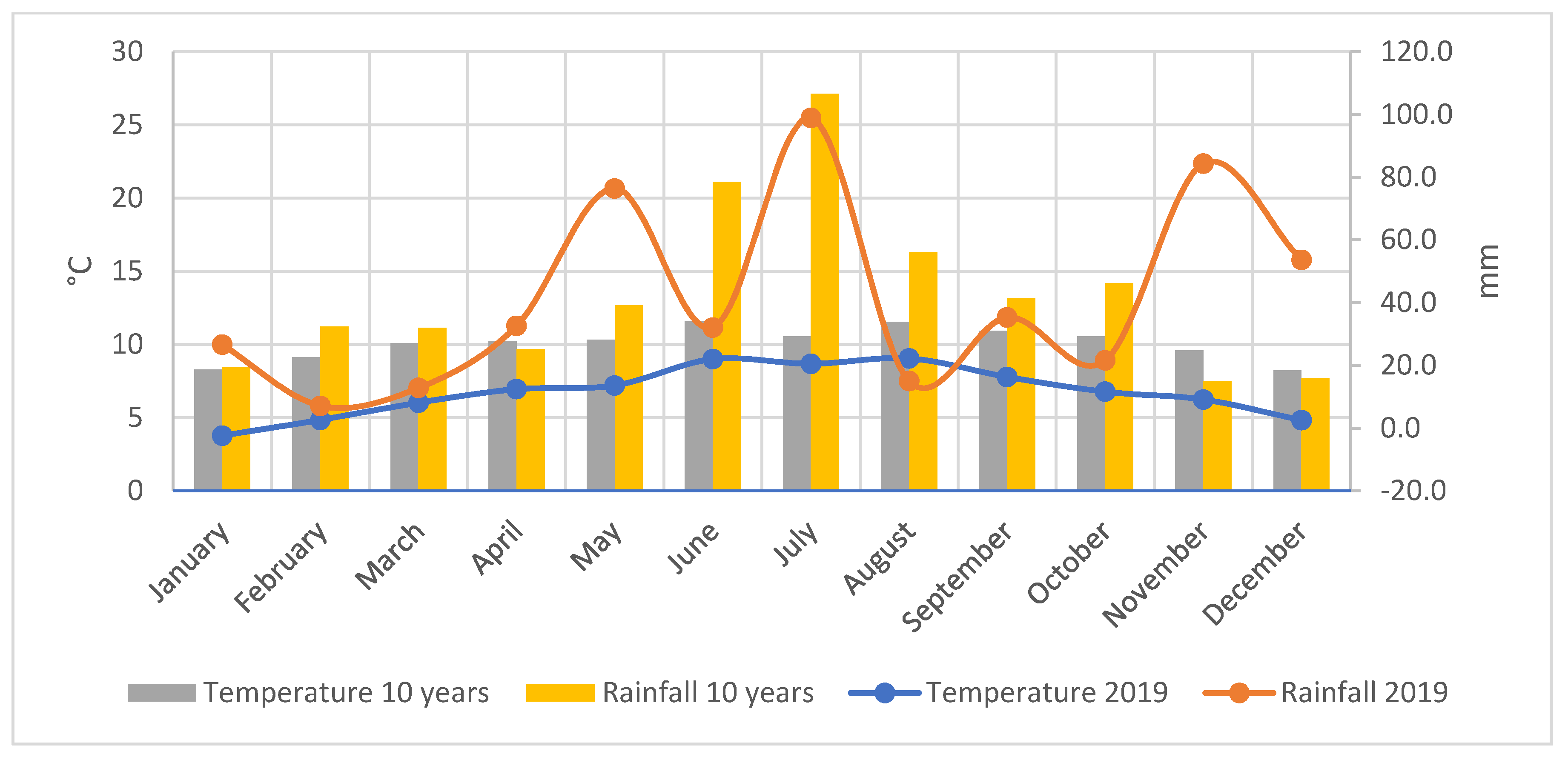
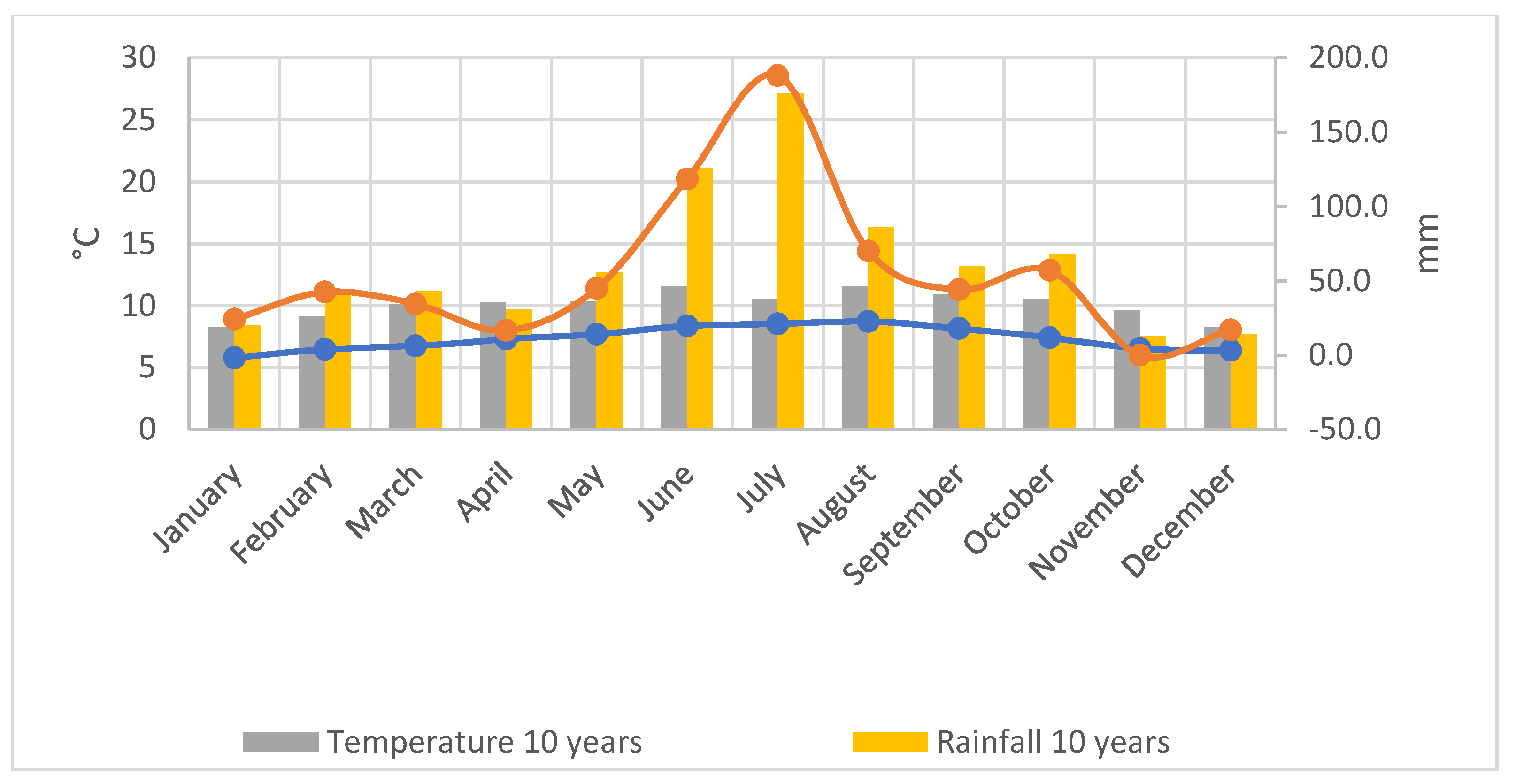
2.2. Climatic Conditions of the Experimental Site (2019–2020)
2.3. Rate of Lipid Peroxidation
2.4. Enzyme Assays
2.5. Statistical Analysis
3. Results
3.1. The Pure Database of the Values in the Control and the Two Highest N Fertilizer Levels
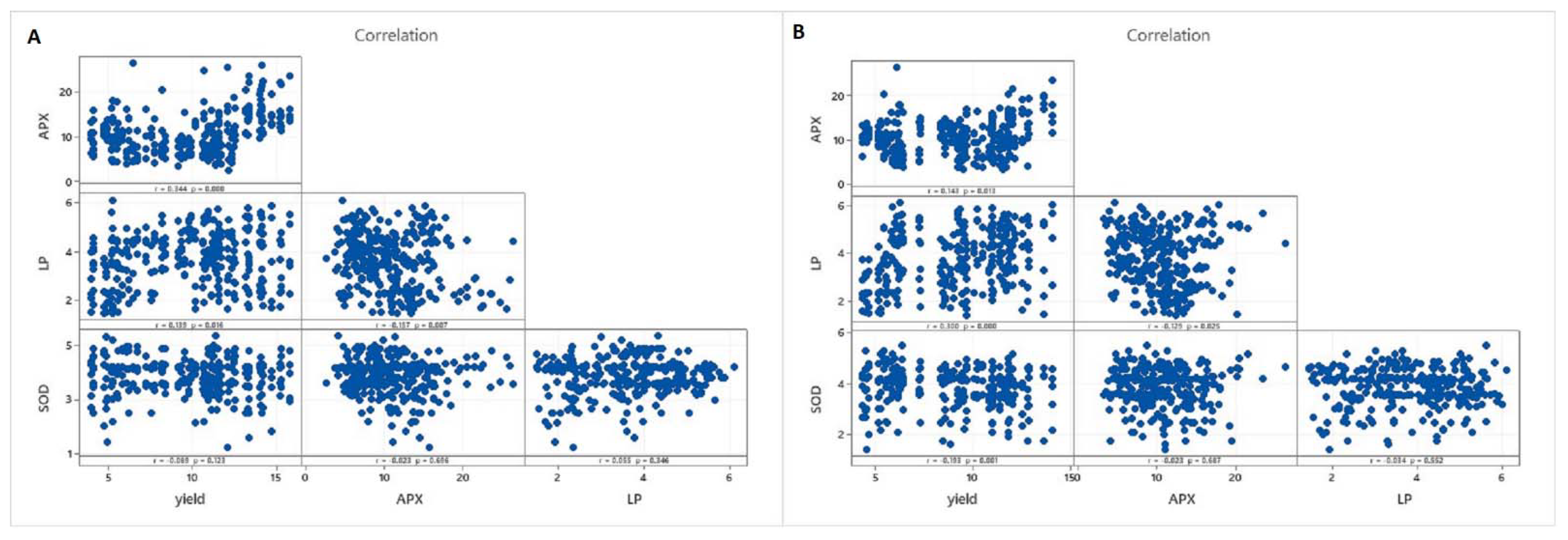
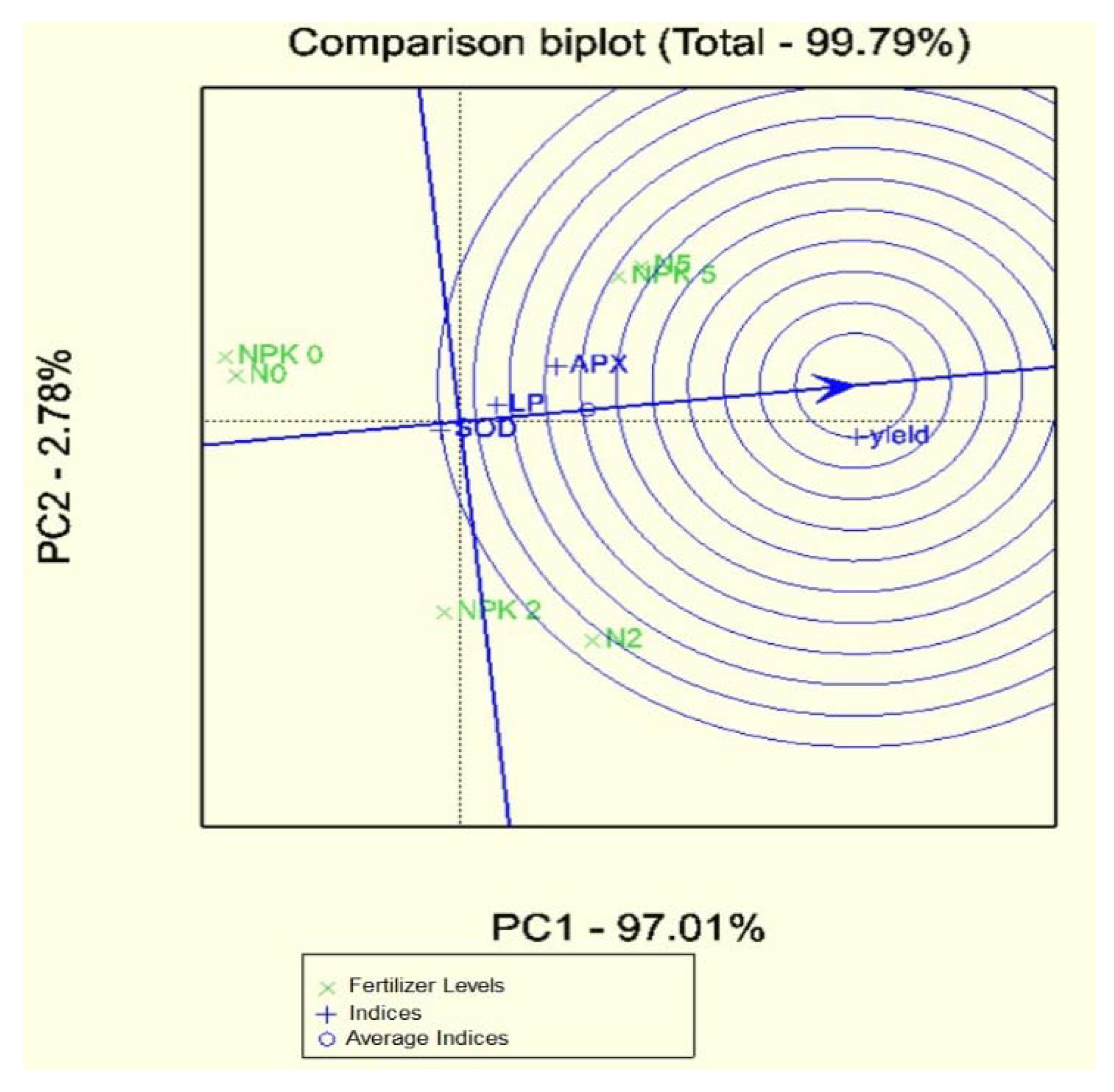
3.2. Statistical Analyses of the Database
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nagy, J. Maize Production; Akadémiai Kiadó: Budapest, Hunguary, 2006. [Google Scholar]
- Mousavi, S.M.N.; Illés, Á.; Bojtor, C.; Nagy, J. The impact of different nutritional treatments on maize hybrids morphological traits based on stability statistical methods. Emir. J. Food Agric. 2020, 32, 666–672. [Google Scholar] [CrossRef]
- Bojtor, C.; Illés, Á.; Nasir Mousavi, S.M.; Széles, A.; Tóth, B.; Nagy, J.; Marton, C.L. Evaluation of the Nutrient Composition of Maize in Different NPK Fertilizer Levels Based on Multivariate Method Analysis. Int. J. Agron. 2021, 2021. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar]
- Gratão, P.L.; Polle, A.; Lea, P.J.; Azevedo, R.A. Making the life of heavy metal-stressed plants a little easier. Funct. Plant Biol. 2005, 32, 481–494. [Google Scholar]
- Zhang, X.; Ervin, E.; Evanylo, G.; Sherony, C.; Peot, C. Biosolids impact on tall fescue drought resistance. J. Residuals Sci. Technol. 2005, 2, 174–180. [Google Scholar]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [PubMed]
- Jubany-Marí, T.; Munné-Bosch, S.; López-Carbonell, M.; Alegre, L. Hydrogen peroxide is involved in the acclimation of the Mediterranean shrub, Cistus albidus L., to summer drought. J. Exp. Bot. 2009, 60, 107–120. [Google Scholar]
- Banu, M.N.A.; Hoque, M.A.; Watanabe-Sugimoto, M.; Matsuoka, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J. Plant Physiol. 2009, 166, 146–156. [Google Scholar] [PubMed]
- Hozayn, M.; Qados, A.A. Magnetic water application for improving wheat (Triticum aestivum L.) crop production. Agric. Biol. J. N. Am. 2010, 1, 677–682. [Google Scholar]
- Cakmak, T.; Cakmak, Z.E.; Dumlupinar, R.; Tekinay, T. Analysis of apoplastic and symplastic antioxidant system in shallot leaves: Impacts of weak static electric and magnetic field. J. Plant Physiol. 2012, 169, 1066–1073. [Google Scholar]
- Vasconcelos, A.C.F.D.; Zhang, X.; Ervin, E.H.; Kiehl, J.D.C. Enzymatic antioxidant responses to biostimulants in maize and soybean subjected to drought. Sci. Agric. 2009, 66, 395–402. [Google Scholar]
- Tittal, M.; Mir, R.A.; Jatav, K.S.; Agarwal, R.M. Supplementation of potassium alleviates water stress-induced changes in Sorghum bicolor L. Physiol. Plant. 2021, 172, 1149–1161. [Google Scholar]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotech. 2010, 30, 161–175. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 26. [Google Scholar] [CrossRef] [Green Version]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar]
- Jiang, Z.W.; Lin, W.X.; Li, Y.Z.; Zhuo, C.Y.; Yang, H.J.; Xie, H.A. Effects of nitrogen fertilizer rates on some physiological characteristics of ratoon rice. Fujian J. Agric. Sci. 2005, 20, 168–171. [Google Scholar]
- Zhang, L.X.; Li, S.X.; Zhang, H.; Liang, Z.S. Nitrogen rates and water stress effects on production, lipid peroxidation and antioxidative enzyme activities in two maize (Zea mays L.) genotypes. J. Agron. Crop Sci. 2007, 193, 387–397. [Google Scholar]
- Illes, A.; Bojtor, C.; Szeles, A.; Mousavi, S.M.N.; Toth, B.; Nagy, J. Analyzing the effect of intensive and low-input agrotechnical support for the physiological, phenometric, and yield parameters of different maize hybrids using multivariate statistical methods. Int. J. Agron. 2021, 2021. [Google Scholar] [CrossRef]
- Sudhakar, C.; Lakshmi, A.; Giridarakumar, S. Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci. 2001, 161, 613–619. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Janda, T.; Szalai, G.; Tari, I.; Paldi, E. Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta 1999, 208, 175–180. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Fisher, R.A. Statistical methods for research workers. In Breakthroughs in Statistics; Springer: New York, NY, USA, 1992; pp. 66–70. [Google Scholar]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef]
- Meyers, L.S.; Gamst, G.; Guarino, A.J. Applied Multivariate Research: Design and Interpretation; Sage: Thousand Oaks, CA, USA, 2016. [Google Scholar]
- Li, J.; Linear, R.R. Principal Component Analysis. Multivariate Statistics; Springer: Berlin, Germany, 2014; Volume 487, pp. 163–183. [Google Scholar]
- Gossett, D.R.; Millhollon, E.P.; Lucas, M.C. Antioxidant response to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Sci. 1994, 34, 706–714. [Google Scholar]
- Vranová, E.; Inzé, D.; Van Breusegem, F. Signal transduction during oxidative stress. J. Exp. Bot. 2002, 53, 1227–1236. [Google Scholar]
- Mao, S.; Islam, M.R.; Hu, Y.; Qian, X.; Chen, F.; Xue, X. Antioxidant enzyme activities and lipid peroxidation in corn (Zea mays L.) following soil application of superabsorbent polymer at different fertilizer regimes. Afr. J. Biotechnol. 2011, 10, 10000–10008. [Google Scholar]
- Liu, Y.; Wang, R.; Hua, L.; Xie, Z.; Chen, Y. Effects of N application rates on early senescence of spring maize. J. Anhui Agric. Univ. 2011, 42, 539–543. [Google Scholar]
- Mousavi, S.M.N.; Nagy, J. Evaluation of plant characteristics related to grain yield of FAO410 and FAO340 hybrids using regression models. Cereal Res. Commun. 2021, 49, 161–169. [Google Scholar]
- Liu, Y.; Wang, R.; Hua, L.; Xie, Z. Effects of N applied rate on leaf senescence and protective enzyme system at later growth stage of maize. J. Maize Sci. 2012, 20, 124–127. [Google Scholar]

| Control | APX (Units 1 μmol of Substrate/min) | LP (MDA × μmol −1 FW) | SOD (Units/mg FW) | |
|---|---|---|---|---|
| 2019 | V4 | 8.043 ± 1.664 B | 4.331 ± 0.731 B | 3.870 ± 0.475 B |
| V6 | 8.166 ± 1.599 B | 4.180 ± 0.922 A | 3.958 ± 0.534 A | |
| V8 | 5.315 ± 0.914 C | 4.150 ± 0.933 C | 3.940 ± 0.555 A | |
| V14 | 6.470 ± 2.281 B | 3.820 ± 0.961 C | 4.123 ± 0.588 A | |
| Silking | 13.738 ± 4.371 A | 4.493 ± 0.582 A | 3.799 ± 0.393 B | |
| 2020 | V4 | 15.040 ± 5.249 B | 2.206 ± 0.604 C | 4.313 ± 0.283 B |
| V6 | 13.440 ± 3.484 A | 4.191 ± 0.925 A | 4.258 ± 0.362 B | |
| V8 | 12.632 ± 2.701 A | 2.570 ± 0.396 B | 3.599 ± 0.592 C | |
| V14 | 12.109 ± 2.871 B | 4.133 ± 0.906 A | 4.270 ± 0.615 A | |
| Silking | 12.237 ± 2.858 B | 2.787 ± 0.998 C | 2.538 ± 0.540 D | |
| N5 | APX (Units 1 μmol of Substrate/min) | LP (MDA μmol × g−1 FW) | SOD (Units/mg FW) | |
|---|---|---|---|---|
| 2019 | V4 | 7.694 ± 1.850 B | 4.511 ± 0.748 A | 3.694 ± 0.480 B |
| V6 | 7.923 ± 0.830 B | 3.958 ± 0.739 C | 3.929 ± 0.522 B | |
| V8 | 5.617 ± 0.890 C | 4.499 ± 1.080 B | 3.722 ± 0.402 B | |
| V14 | 7.340 ± 2.765 C | 4.013 ± 0.797 C | 3.987 ± 0.563 B | |
| Silking | 12.385 ± 3.117 A | 4.131 ± 0.488 C | 3.725 ± 0.395 A | |
| 2020 | V4 | 17.356 ± 4.654 A | 2.111 ± 0.437 D | 4.199 ± 0.322 A |
| V6 | 13.427 ± 2.416 B | 4.680 ± 0.846 A | 4.153 ± 0.335 A | |
| V8 | 12.497 ± 2.816 B | 2.692 ± 0.512 C | 3.366 ± 0.603 B | |
| V14 | 13.183 ± 2.566 B | 4.339 ± 1.002 A | 4.228 ± 0.581 AB | |
| Silking | 12.452 ± 2.596 B | 3.079 ± 1.088 B | 2.464 ± 0.495 C | |
| N5 | APX (Units 1 μmol of Substrate/min) | LP (MDA × μmol g−1 FW) | SOD (Units/mg FW) | |
|---|---|---|---|---|
| 2019 | V4 | 8.558 ± 1.734 B | 4.323 ± 0.835 ABC | 3.851 ± 0.383 AB |
| V6 | 9.403 ± 2.166 BCB | 4.347 ± 0.902 BC | 3.495 ± 0.609 C | |
| V8 | 5.210 ± 1.152 D | 4.526 ± 0.743 AB | 3.870 ± 0.550 BC | |
| V14 | 7.201 ± 1.732 D | 3.601 ± 0.874 C | 3.929 ± 0.575 ABC | |
| Silking | 12.307 ± 4.122 A | 4.915 ± 0.554 A | 3.813 ± 0.354 A | |
| 2020 | V4 | 12.549 ± 2.273 AB | 2.328 ± 0.861 C | 4.492 ± 0.252 A |
| V6 | 14.650 ± 4.429 A | 4.254 ± 1.086 A | 4.070 ± 0.552 A | |
| V8 | 14.543 ± 2.221A B | 2.534 ± 0.259 C | 3.367 ± 0.744 AB | |
| V14 | 13.732 ± 3.020 A | 4.392 ± 0.964 A | 3.856 ± 0.880 | |
| Silking | 11.124 ± 3.643 B | 3.402 ± 1.123 B | 2.397 ± 0.580 C | |
| Indices | Source | Df | F-Value | Indices | Source | Df | F-Value |
|---|---|---|---|---|---|---|---|
| APX | Sampling times | 4 | 18.47 ** | SOD | Sampling times | 4 | 41.87 ** |
| years | 1 | 258.33 ** | years | 1 | 2.74 ns | ||
| hybrids | 1 | 1.54 ns | hybrids | 1 | 0.00 ns | ||
| fertilizer levels | 2 | 10.56 ** | fertilizer levels | 2 | 10.42 ** | ||
| years*hybrids | 1 | 9.49 ** | years*hybrids | 1 | 4.22 * | ||
| years*fertilizer levels | 2 | 25.47 ** | years*fertilizer levels | 2 | 1.03 ns | ||
| hybrids*fertilizer levels | 2 | 2.06 ns | hybrids*fertilizer levels | 2 | 1.56 ns | ||
| years*hybrids*fertilizer levels | 2 | 5.28 ** | years*hybrids*fertilizer levels | 2 | 1.52 ns | ||
| LP | Sampling times | 4 | 16.36 ** | Yield | Sampling times | 4 | 0.04 ns |
| years | 1 | 85.23 ** | years | 1 | 197.03 ** | ||
| hybrids | 1 | 0.02 ns | hybrids | 1 | 267.87 ** | ||
| fertilizer levels | 2 | 24.87 ** | fertilizer levels | 2 | 1555.13 ** | ||
| years*hybrids | 1 | 2.43 ns | years*hybrids | 1 | 2.18 ns | ||
| years*fertilizer levels | 2 | 8.39 ** | years*fertilizer levels | 2 | 135.69 ** | ||
| hybrids*fertilizer levels | 2 | 0.18 ns | hybrids*fertilizer levels | 2 | 8.88 ** | ||
| years*hybrids*fertilizer levels | 2 | 0.26 ns | years*hybrids*fertilizer levels | 2 | 4.86 ** |
| Sampling Time | N | Mean | Grouping | Sampling Time | N | Mean | Grouping | ||
|---|---|---|---|---|---|---|---|---|---|
| APX | Silking | 60 | 12.4743 | A | SOD | V14 | 60 | 4.18247 | A |
| V4 | 60 | 12.4412 | A | V6 | 60 | 4.11783 | A | ||
| V6 | 60 | 10.5225 | B | V4 | 60 | 3.97373 | A | ||
| V14 | 60 | 9.4396 | B | V8 | 60 | 3.65131 | B | ||
| V8 | 60 | 9.0024 | C | Silking | 60 | 3.10474 | C | ||
| LP | V6 | 60 | 4.27245 | A | Yield | Silking | 60 | 9.90354 | A |
| V14 | 60 | 4.04745 | A | V14 | 60 | 9.90354 | A | ||
| Silking | 60 | 3.55468 | B | V8 | 60 | 9.90354 | A | ||
| V8 | 60 | 3.38393 | B | V6 | 60 | 9.90354 | A | ||
| V4 | 60 | 3.22379 | B | V4 | 60 | 9.90354 | A |
| Indices | Source | Df | F-Value | Indices | Source | Df | F-Value |
|---|---|---|---|---|---|---|---|
| APX | Sampling times | 4 | 9.25 ** | SOD | Sampling times | 4 | 22.20 ** |
| years | 1 | 119.75 ** | years | 1 | 6.25 * | ||
| hybrids | 1 | 1.96 ns | hybrids | 1 | 14.01 ** | ||
| fertilizer levels | 2 | 10.78 ** | fertilizer levels | 2 | 10.98 ** | ||
| years*hybrids | 1 | 0.16 ns | years*hybrids | 1 | 1.16 ns | ||
| years*fertilizer levels | 2 | 10.61 ** | years*fertilizer levels | 2 | 3.87 * | ||
| hybrids*fertilizer levels | 2 | 6.86 ** | hybrids*fertilizer levels | 2 | 0.34 ns | ||
| years*hybrids*fertilizer levels | 2 | 5.15 ** | years*hybrids*fertilizer levels | 2 | 1.07 ns | ||
| LP | Sampling times | 4 | 9.33 ** | Yield | Sampling times | 4 | 0.004 ns |
| years | 1 | 112.65 ** | years | 1 | 13.60 ** | ||
| hybrids | 1 | 0.05 ns | hybrids | 1 | 53.28 ** | ||
| fertilizer levels | 2 | 13.22 ** | fertilizer levels | 2 | 2144.33 ** | ||
| years*hybrids | 1 | 2.00 ns | years*hybrids | 1 | 7.24 ** | ||
| years*fertilizer levels | 2 | 21.46 ** | years*fertilizer levels | 2 | 77.30 ** | ||
| hybrids*fertilizer levels | 2 | 0.36 ns | hybrids*fertilizer levels | 2 | 5.86 ** | ||
| years*hybrids*fertilizer levels | 2 | 0.35 ns | years*hybrids*fertilizer levels | 2 | 9.76 ** |
| Sampling Time | N | Mean | Grouping | Sampling Time | N | Mean | Grouping | ||
|---|---|---|---|---|---|---|---|---|---|
| APX | Silking | 60 | 12.0936 | A | SOD | V4 | 60 | 4.18261 | A |
| V6 | 60 | 11.4721 | B | V14 | 60 | 4.06520 | B | ||
| V4 | 60 | 10.2217 | C | V6 | 60 | 3.91775 | B | ||
| V14 | 60 | 9.5283 | C | V8 | 60 | 3.79509 | B | ||
| V8 | 60 | 9.3180 | C | Silking | 60 | 3.19803 | C | ||
| LP | V6 | 60 | 4.13320 | A | Yield | Silking | 60 | 8.91745 | A |
| Silking | 60 | 4.00025 | A | V14 | 60 | 8.91745 | A | ||
| V14 | 60 | 3.91477 | B | V8 | 60 | 8.91745 | A | ||
| V8 | 60 | 3.47665 | C | V6 | 60 | 8.91745 | A | ||
| V4 | 60 | 3.31037 | C | V4 | 60 | 8.91745 | A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illés, Á.; Bojtor, C.; Mousavi, S.M.N.; Széles, A.; Tóth, B.; Szabó, A.; Nagy, J. Evaluation of Complete Fertilizer in the Aspect of the Antioxidant Enzyme System of Maize Hybrids. Agronomy 2021, 11, 2129. https://doi.org/10.3390/agronomy11112129
Illés Á, Bojtor C, Mousavi SMN, Széles A, Tóth B, Szabó A, Nagy J. Evaluation of Complete Fertilizer in the Aspect of the Antioxidant Enzyme System of Maize Hybrids. Agronomy. 2021; 11(11):2129. https://doi.org/10.3390/agronomy11112129
Chicago/Turabian StyleIllés, Árpád, Csaba Bojtor, Seyed Mohammad Nasir Mousavi, Adrienn Széles, Brigitta Tóth, Atala Szabó, and János Nagy. 2021. "Evaluation of Complete Fertilizer in the Aspect of the Antioxidant Enzyme System of Maize Hybrids" Agronomy 11, no. 11: 2129. https://doi.org/10.3390/agronomy11112129
APA StyleIllés, Á., Bojtor, C., Mousavi, S. M. N., Széles, A., Tóth, B., Szabó, A., & Nagy, J. (2021). Evaluation of Complete Fertilizer in the Aspect of the Antioxidant Enzyme System of Maize Hybrids. Agronomy, 11(11), 2129. https://doi.org/10.3390/agronomy11112129












