Impact of Methanol and Glycine Betaine on Yield and Quality of Fodder Beet Genotypes (Beta vulgaris subsp. vulgaris)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
2.3. Plant Culture
2.4. Sample Collection and Analysis
- Root yield; Total yield (t/ha)
- Root DM yield; Shoot DM yield; Total DM yield (t/ha)
- Root length (cm)
- Root DM digestibility; Root crude protein (%)
- Sodium (Na+) content in roots (mg/100 g sugar)
- Catalase (CAT) content (u/mg pr)
2.5. Statistical Methods
3. Results and Discussion
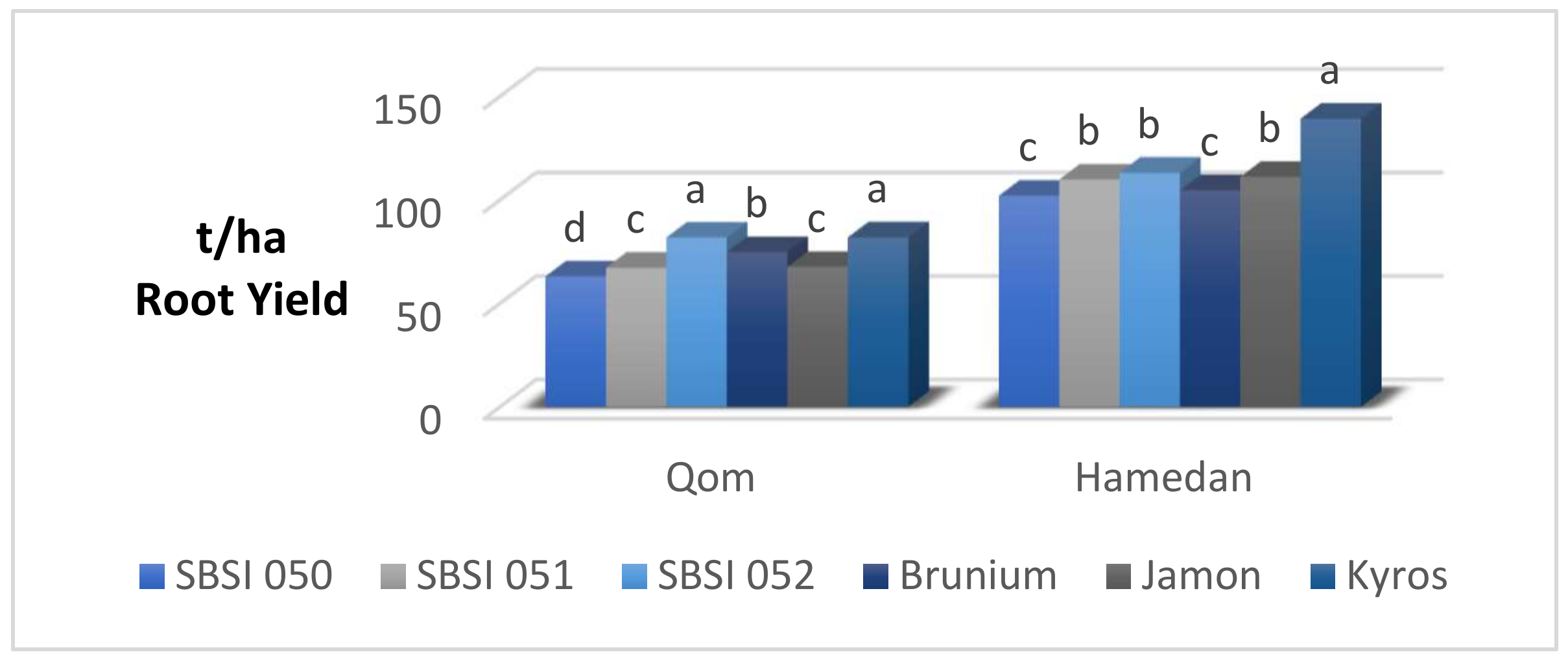
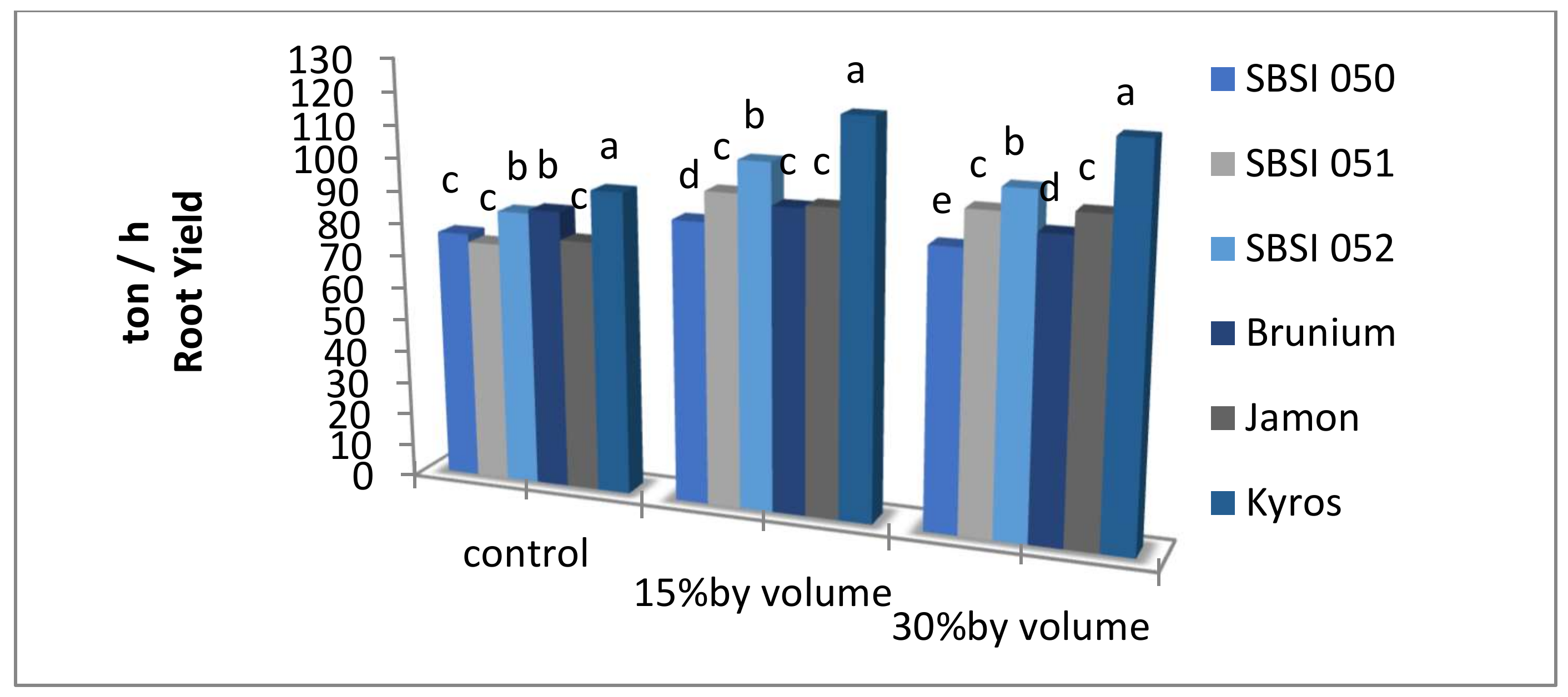
3.1. Root Yield
3.2. Root Dry Matter Yield
3.3. Shoot Dry Matter Yield
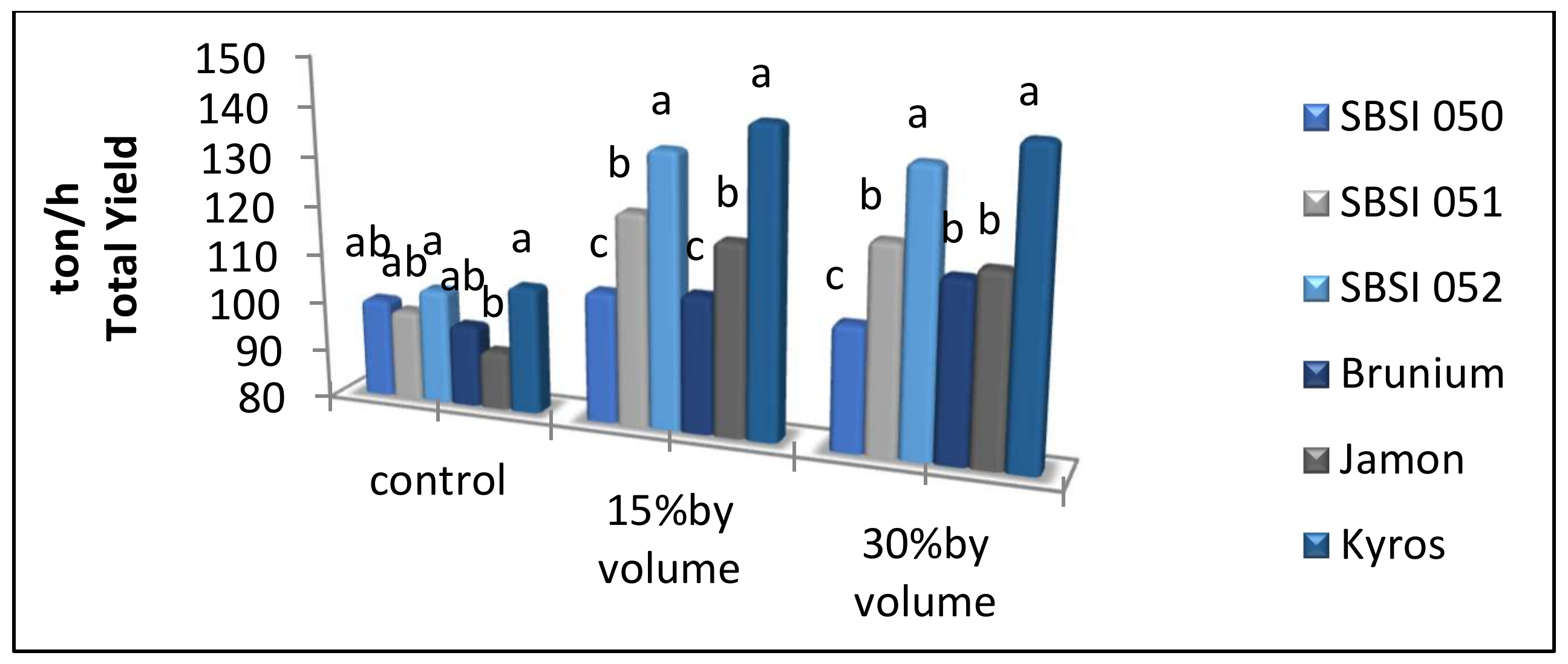
3.4. Total Yield
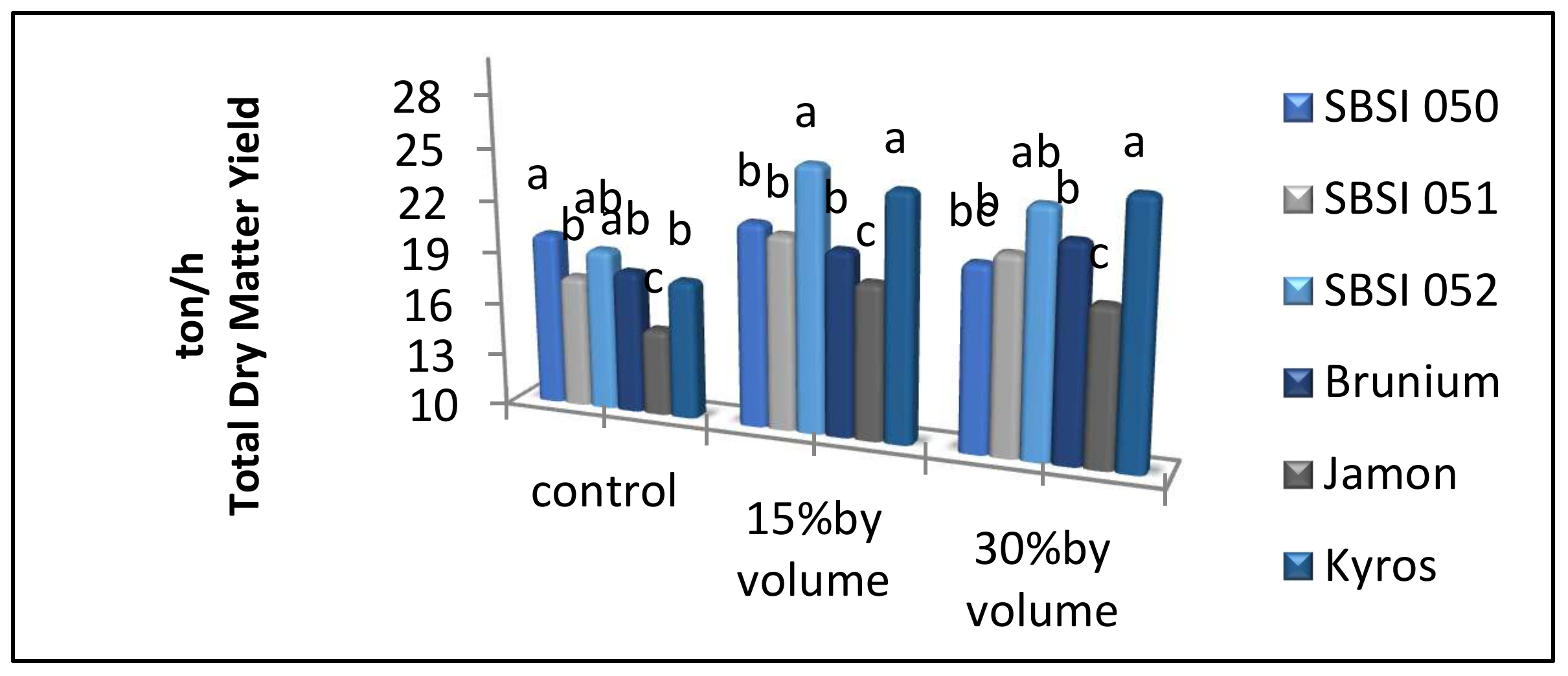
3.5. Total Dry Matter Yield
3.6. Root Dry Matter Digestibility Percentage
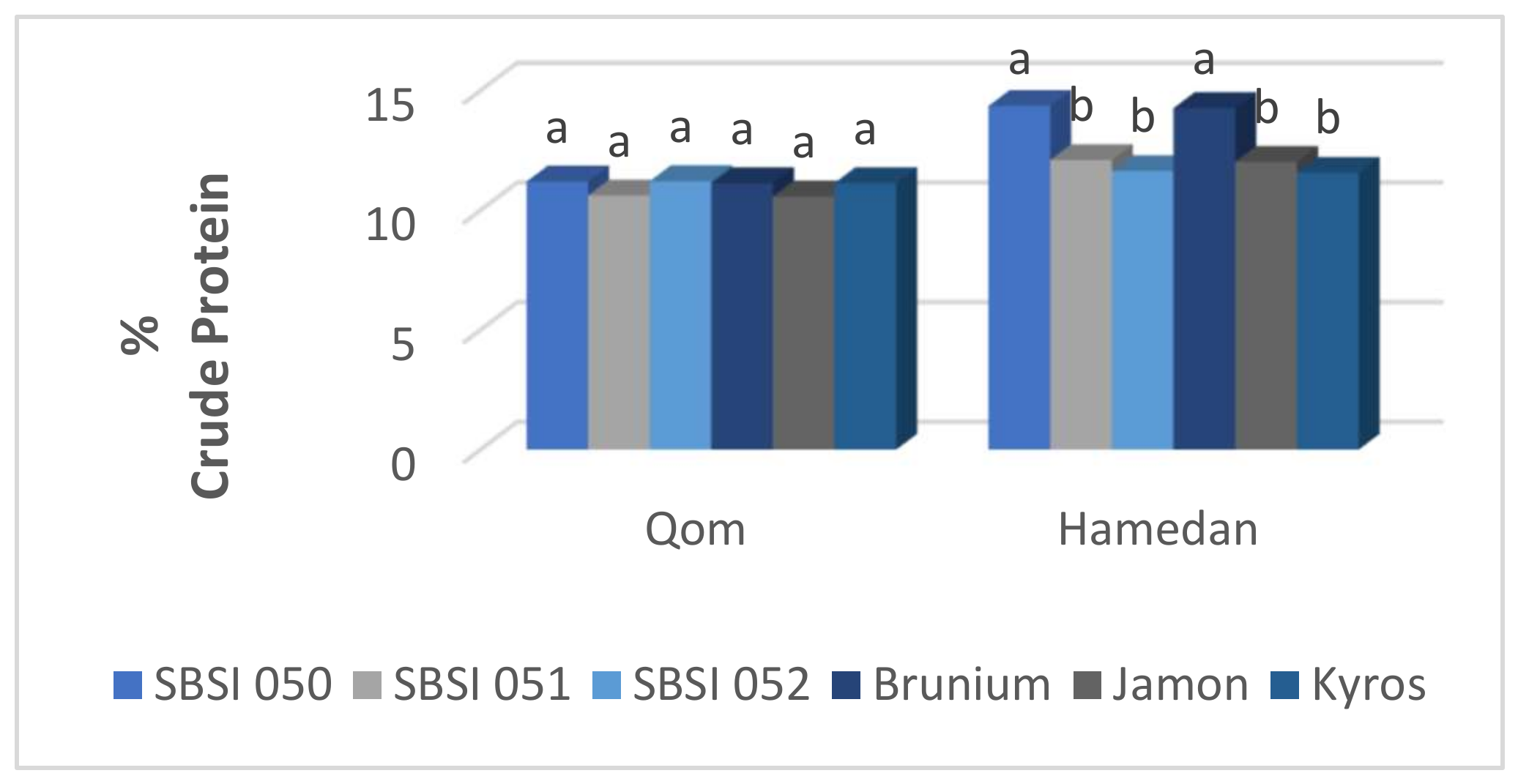
3.7. Root Crude Protein Percentage
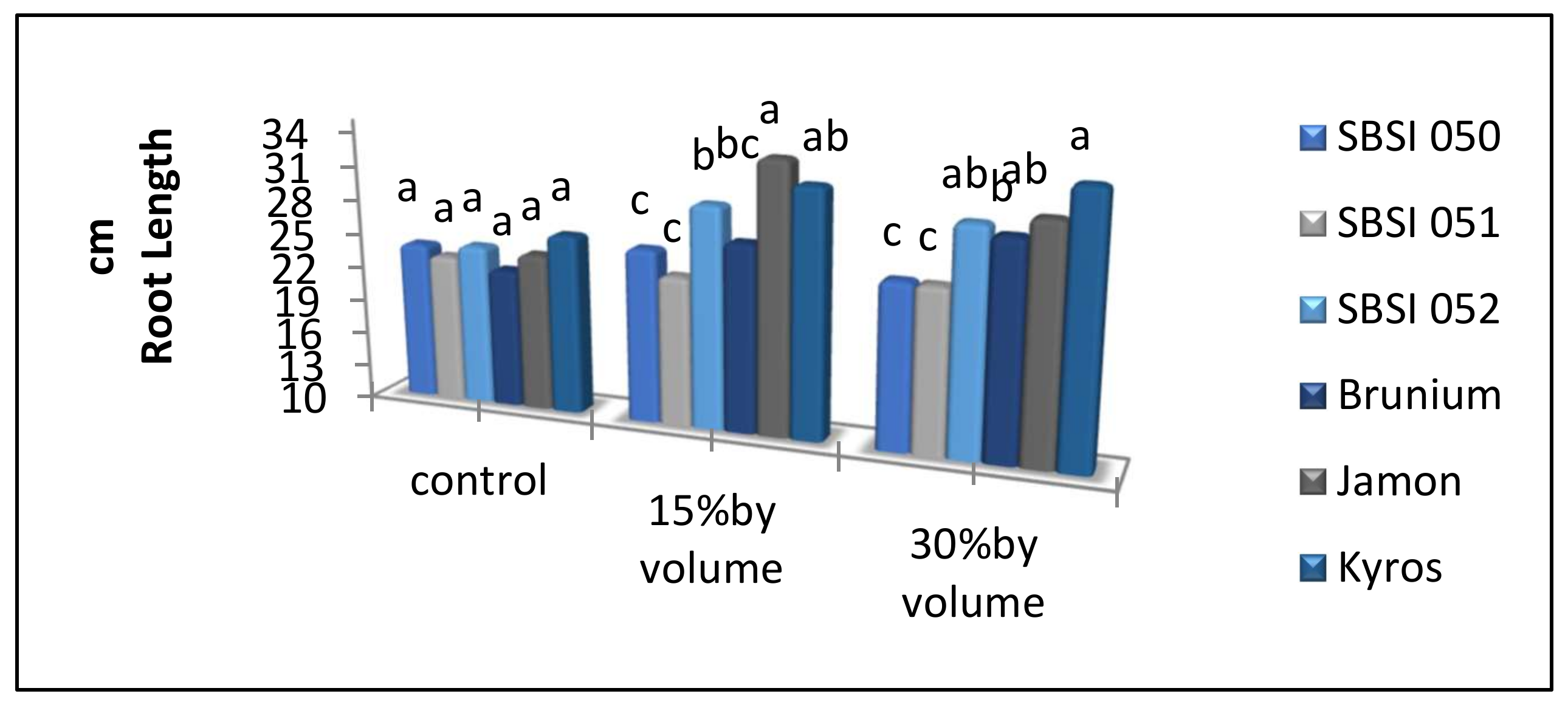
3.8. Root Length
3.9. Sodium Content
3.10. Catalase Activity
3.11. Sugar Content
4. Supplementary Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, M.; He, C.-Q.; Ding, N.-Z. Abiotic Stresses: General Defenses of Land Plants and Chances for Engineering Multistress Tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeandroz, S.; Lamotte, O. Plant responses to biotic and abiotic stresses: Lessons from cell signaling. Front. Plant Sci. 2017, 8, 1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldman, I.L.; Janick, J. Evolution of Root Morphology in Table Beet: Historical and Iconographic. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, A.; Andryka-Dudek, P.; Czerwiński, M.; Chołuj, D. Fodder beet is a reservoir of drought tolerance alleles for sugar beet breeding. Plant Physiol. Biochem. 2019, 145, 120–131. [Google Scholar] [CrossRef]
- SadeghiShoae, M.; Mirzaee, M.R.; Mahdikhani, M.; Jalilian, A.; Nadali, F.; Pedram, A.; Nemati, R.; Rezaei, J. Determination of Field Value of Fodder Beet Cultivars; Final Report of Sugar Beet Seed Institute (SBSI); Journal of Sugar Beet; Agricultural Research Education and Extension Organization (AREEO): Karaj, Iran, 2020; 28p. [Google Scholar]
- SadeghiShoae, M.; Aghaeezadeh, M.; Rahnamaeian, M. Purification and Preparation of New Fodder Beet Masses; Final Report of Sugar Beet Seed Institute, (SBSI); Journal of Sugar Beet; Agricultural Research, Education and Extension Organization (AREEO): Karaj, Iran, 2019; 17p. [Google Scholar]
- Islam, J.; Kim, J.; Begum, M.; Sohel, A.T.; Lim, Y.-S. Physiological and Biochemical Changes in Sugar Beet Seedlings to Confer Stress Adaptability under Drought Condition. Plants 2020, 9, 1511. [Google Scholar] [CrossRef]
- Hussain, S.; Shuxian, L.; Mumtaz, M.; Shafiq, I.; Iqbal, N.; Brestic, M.; Shoaib, M.; Sisi, Q.; Li, W.; Mei, X.; et al. Foliar application of silicon improves stem strength under low light stress by regulating lignin biosynthesis genes in soybean (Glycine max (L.) Merr.). J. Hazard. Mater. 2020, 401, 123256. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Elgawad, H.A.; Xiong, Y.; Macovei, A.; Brestic, M.; Skalicky, M.; Shaghaleh, H.; Hamoud, Y.A.; El-Sawah, A.M. Inoculation with Bacillus amyloliquefaciens and mycorrhiza confers tolerance to drought stress and improve seed yield and quality of soybean plant. Physiol. Plant. 2021. [Google Scholar] [CrossRef]
- Felix, J.D.; Roebuck, J.A.; Mead, R.N.; Willey, J.D.; Avery, G.B.; Kieber, R.J. Methanol and ethanol concentrations in a Greenland ice core. Atmos. Environ. 2019, 217, 116948. [Google Scholar] [CrossRef]
- Saneienejad, A.; Tohidi, M.; Khaniani, B.H.; Sadeghi, M. Effect of Foliar Application of Methanol on Changes of Antioxidant Enzymes of Vigna unguiculata L. in Water-Deficit Stress. Agric. Sci. Dig. A Res. J. 2019, 39, 296–300. [Google Scholar] [CrossRef] [Green Version]
- Dorokhov, Y.L.; Sheshukova, E.V.; Komarova, T.V. Methanol in plant life. Front. Plant Sci. 2018, 9, 1623. [Google Scholar] [CrossRef] [Green Version]
- Nonomura, A.M.; Benson, A.A. The path of carbon in photosynthesis: Improved crop yields with methanol. Proc. Natl. Acad. Sci. USA 1992, 89, 9794–9798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demmers-Derks, H.; Mitchel, R.A.C.; Mitchell, V.J.; Driscoll, S.P.; Gibbard, C.; Lawlor, D.W. Sugar beet under climatic change: Photosynthesis and production. Asp. Appl. Biol. 1996, 45, 163–170. [Google Scholar]
- Nadali, I.; Paknejad, F.; Moradi, F.; Vazan, S.; Tookalo, M.; Jami Al-Ahmadi, M.; Pazoki, A. Effect of foliar application of methanol on sugar beet (Beta vulgaris). Aust. J. Crop. Sci. 2010, 4, 398–401. [Google Scholar]
- Fall, R.; Benson, A.A. Leaf methanol—The simplest natural product from plants. Trends Plant Sci. 1996, 1, 296–301. [Google Scholar] [CrossRef]
- Galbally, E.; Kirstine, W. The Production of methanol by flowering plants and the global cycle of methanol. J. Atmos. Chem. 2002, 43, 195–229. [Google Scholar] [CrossRef]
- Nonomura, A.M. Method and composition for enhancing carbon fixation in plants. Proc. Natl. Acad. Sci. USA 1997, 91, 0094–0098. [Google Scholar]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [Green Version]
- AL-Aghabary, K.; Zhujun, Z.; Qinhua, S. Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. Plant Nutr. 2004, 27, 2101–2115. [Google Scholar] [CrossRef]
- Esfandiari, A.; Mahbob, S.; Shekari, F. Harmful Effects of Active Oxygen Types, Preservative Mechanisms of Plant And Necessity of Paying Attention To Them. In Proceedings of the of 10th Congress of Iran Agronomy and Plant Breeding, Karaj, Iran, 19–21 August 2008; pp. 1–8. [Google Scholar]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of Active Oxygens and dissipation of exess photons. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Guo, Z.; Tan, H.; Zhu, Z.; Lu, S.; Zhou, B. Effects of intermediates on ascorbic acid and oxalate. Plant Physiol. Biochem. 2005, 43, 955–962. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Ames, B.N.; Shigena, M.K.; Hegen, T.M. Oxidants, antioxidants and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [Green Version]
- Khatun, S.; Ali, M.B.; Hahn, E.J.; Paek, K.Y. Cooper toxicity in Withania somnifera: Growth and antioxidant enzymes responses of In Vitro grown plants. J. Exp. Bot. 2008, 47, 259–266. [Google Scholar]
- Gaetke, L.M.; Chow, C.K. Copper toxicity oxidative stress and antioxidant nutrients. Toxicology 2003, 189, 163–197. [Google Scholar] [CrossRef]
- Garnczarska, M.; Ratajczak, L. Metabolic responses of Lemna minor to lead ions II. Induction of antioxidant enzymes in roots. Acta Physiol. Plant. 2000, 22, 429–432. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Ludlow, M.M. Who taught plants thermodynamics? The unfulfilled potential of plant water potential. Aust. J. Plant Biol. 1985, 12, 213–217. [Google Scholar] [CrossRef]
- Nemecek-Marshall, M.; MacDonald, R.C.; Franzen, J.J.; Wojciechowski, C.L.; Fall, R. Methanol emission from leaves: Enzymatic detection of gas-phase methanol and relation of methanol fluxes to stomatal conductance and leaf development. Plant Physiol. 1995, 108, 1359–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valizadeh-Kamran, R.; Mehrabani, L.V.; Pessarakli, M. Effects of foliar application of methanol on some physiological characteristics of Lavandula stoechas L. under NaCl salinity conditions. J. Plant Nutr. 2019, 42, 261–268. [Google Scholar] [CrossRef]
- Safarzade Vishkaei, M. Effect of Methanol on Growth and Yield of Peanut. Ph.D. Thesis, Islamic Azad University, Science and Research Branch. Tehran, Iran, 2007; 270p. [Google Scholar]
- Lee, H.S.; Madhaiyan, M.; Kim, C.W.; Choi, S.J.; Chung, K.Y.; Sa, T.M. Physiological enhancement of early growth of rice seedlings (Oryza sativa L.) by production of phytohormone of N 2-fixing methylotrophic isolates. Biol. Fertil. Soils 2006, 42, 402–408. [Google Scholar] [CrossRef]
- Staler, S.O.; Thimann, K.V. The Influence of aliphatic alcohols on leaf senescence. Plant Physiol. 1980, 66, 395–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramberg, H.A.; Bradley, J.C.; Olson, J.S.C.; Nishio, J.N.; Markwell, J.; Osterman, J.C. The role of methanol in promoting plant growth: An update. Rev. Plant Biochem. Biotechnol. 2002, 1, 113–126. [Google Scholar]
- Ramírez, I.; Dorta, F.; Espinoza, V.; Jiménez, E.; Mercado, A.; Peña-Cortés, H. Effects of Foliar and Root Applications of Methanol on the Growth of Arabidopsis, Tobacco, and Tomato Plants. J. Plant Growth Regul. 2006, 25, 30–44. [Google Scholar] [CrossRef]
- Devlin, M.; Bhowmik, P.C.; Karczmarczyk, S.J. Influence of methanol on plant germination and growth. Plant Growth Regulation. Soc. Am. Q. 1994, 22, 102–108. [Google Scholar]
- Faver, K.L.; Gerik, T.J. Foliar-applied methanol effects on cotton (Gossypium hirsutum L.) gas exchange and growth. Field Crops Res. 1996, 47, 227–234. [Google Scholar] [CrossRef]
- Sadeghi-Shoae, M.; Paknejad, F.; Kashani, A.; Vazan, S.; Nooralvandi, T. Methanol and its period of foliar application on sugar beet in different available water. In Proceedings of the Conference on International Research on Food Security, Natural Resource Management and Rural Development, Göttingen, Germany, 19–21 September 2012. [Google Scholar]
- Paknejad, F.; Mirakhori, M.; Jami Al-Ahmadi, M.; Tookalo, M.R.; Pazoki, A.R.; Nazeri, P. Physiological Response of Soybean (Glycine max) to Foliar Application of Methanol under Different Soil Moistures. Am. J. Agric. Biol. Sci. 2009, 4, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Paknejad, F.; Bayat, V.; Ardakani, M.R.; Vazan, S. Effect of Methanol Foliar Application on Seed Yield and the Quality of Soybean (Glycine max L.) under Water Deficit Conditions. Ann. Biol. Res. 2012, 3, 2108–2117. [Google Scholar]
- Preedy, V. Betaine: Chemistry, Analysis, Function and Effects; Royal Society of Chemistry: Cambridge, UK, 2015. [Google Scholar]
- Park, E.J.; Jeknic, Z.; Chen, T.H. Exogenous application of glycine betaine increases chilling tolerance in tomato plants. Plant Cell Physiol. 2006, 47, 706–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashraf, M.; Foolad, M. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Mäkelä, P.; Kärkkäinen, J.; Somersalo, S. Effect of Glycinebetaine on Chloroplast Ultrastructure, Chlorophyll and Protein Content, and RuBPCO Activities in Tomato Grown under Drought or Salinity. Biol. Plant. 2000, 43, 471–475. [Google Scholar] [CrossRef]
- Mäkelä, P.; Peltonen-Sainio, P.; Jokinen, K.; Pehu, E.; Setälä, H.; Hinkkanen, R.; Somersalo, S. Uptake and translocation of foliar-applied glycine betaine in crop plants. Plant Sci. 1996, 121, 221–230. [Google Scholar] [CrossRef]
- Dini-Andreote, F. Endophytes: The second layer of plant defense. Trends Plant Sci. 2020, 25, 4. [Google Scholar] [CrossRef] [PubMed]
- Skalicky, M.; Kubes, J.; Shokoofeh, H.; Arif, T.U.; Vachova, P.; Hejnak, V. Betacyanins and Betaxanthins in Cultivated Varieties of Beta vulgaris L. Compared to Weed Beets. Molecules 2020, 25, 5395. [Google Scholar] [CrossRef] [PubMed]
- Tilley, J.M.A.; Terry, R.A. A Two-Stage Technique for the In Vitro Digestion of Forage Crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Clover, G.; Smith, H.; Jaggard, K. The crop under stress. Br. Sugar Beet Rev. 1998, 66, 17–19. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- McGiffen, M.; Manthey, J.A. The role of methanol in promoting plant growth: A current evaluation. HortScience 1996, 31, 1092–1096. [Google Scholar] [CrossRef]
- Downie, A.; Miyazaki, S.; Bohnert, H.; John, P.; Coleman, J.; Parry, M.; Haslam, R. Expression profiling of the response of Arabidopsis thaliana to methanol stimulation. Phytochemistry 2004, 65, 2305–2316. [Google Scholar] [CrossRef]
- Hemming, D.J.; Criddle, R.S.; Hansen, L.D. Effects of Methanol on Plant Respiration. J. Plant Physiol. 1995, 146, 193–198. [Google Scholar] [CrossRef]
- Andres, R.J.; Lazaro, A.; Chueca, R.; Hermoso, L.; Gorge, L. Effect of alcohols on the association of photosynthetic fructose-1, 6-bisphosphatase to thylakoid membranes. Plant Physiol. 1990, 78, 409–413. [Google Scholar] [CrossRef]
- Nadali, I.; Paknejad, F.; Soghani, M.; Elahipanah, F.; Ghafari, M. Effect of methanol on yield, yield component and growth indices in chick pea. J. Crop. Ecophysiol. 2010, 2, 176–184. [Google Scholar] [CrossRef]
- Farshpour, A.; Sadeghi Shoae, M.; Habibi, D. Quantitative and Qualitative Yield of Fodder Beet Cultivars during Spring and Summer Cultivation in Karaj Region. Master’s Thesis, Islamic Azad University, Karaj, Iran, 2017; 109p. [Google Scholar]
- Zbieć, I.I.; Karczmarczyk, S.; Koszański, Z. Influence of methanol on some cultivated plants. Folia Univ. Agric. Stetin. Agric. 1999, 73, 217–220. [Google Scholar]
- Rowe, R.N.; Farr, D.J.; Richards, B.A.J. Effects of foliar and root applications of methanol or ethanol on the growth of tomato plants (Lycopersicon esculentum Mill.). N. Zealand J. Crop. Hortic. Sci. 1994, 22, 335–337. [Google Scholar] [CrossRef]
- Omer, Z.S.; Tombolini, R.; Gerhardson, B. Plant colonization by pink-pigmented facultative methylotrophic bacteria (PPFMS). FEMS Microbiol. Ecol. 2004, 46, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, E.G.; Doronina, N.V.; Trotsenko, Y.A. Aerobic methyl bacteria are capable of synthesizing auxins. Microbiology 2001, 70, 392–397. [Google Scholar] [CrossRef]
- Makhdum, M.I.; Malik, M.N.A.; Din, S.U.; Ahmad, F.; Chaudhry, F.I. Physiology response of cotton to methanol foliar application. J. Res. Sci. 2002, 13, 37–43. [Google Scholar]
- Khani Chegeni, A.; Sadeghi Shoae, M.; Habibi, D. Comparison of Quantitative and Qualitative Yield Response and Water Use Efficiency of Fodder Beet Cultivars under Different Irrigation Conditions. Master’s Thesis, Islamic Azad University, Karaj, Iran, 2017; 100p. [Google Scholar]
- Zbiec, L.; Karczmarczyk, S.; Podsiadlo, C. Response of some cultivated plants to methanol as compared to supplemental irrigation. Electron. J. Pol. Agric. Univ. 2003, 6, 1–7. [Google Scholar]
- Li, Y.; Gupta, G.; Joshi, J.M.; Siyumbano, A.K. Effect of methanol on soybean photosynthesis and chlorophyll. J. Plant Nutr. 1995, 18, 1875–1880. [Google Scholar] [CrossRef]
- Kotzabasis, K.; Hatziathanasiou, A.; Bengoa-Ruigomez, M.V.; Kentouri, M.; Divanach, P. Methanol as alternative carbon source for quicker efficient production of the microalgae Chlorella minutissima: Role of the concentration and frequence of administraion. J. Biotechnol. 1999, 70, 357–362. [Google Scholar] [CrossRef]
- Ghafari, S.; Sadeghi Shoae, M.; Mirhadi, M.J.; Ghasemkhan Ghajar, F. Evaluation of the Effect of Nano-Micronutrients and Growth-Promoting Bacteria on Quantitative and Qualitative Yield of Different Forage Beet Cultivars. Master’s Thesis, Islamic Azad University, Research Sciences Branch. Tehran, Iran, 2018; 140p. [Google Scholar]
- Koochaki, A.; Rashed Mohassel, M.H.; Nasiri, M.; Sadrabadi, R. The Physiological Basis of Crop Plants Growth and Development; Razavi Publications: Teheran, Iran, 1996. [Google Scholar]
- Loilier, M. Improvement of the quality of sugar root. Sucre Fr. 1981, 122, 131–140. [Google Scholar]
- Raza, M.A.S.; Saleem, M.F.; Shah, G.M.; Khan, I.H.; Raza, A. The exogenous application of glycine betaine and potassium for improving water relations and grain yield of wheat under drought. J. Soil Sci. Plant Nutr. 2014, 14, 348–364. [Google Scholar]
- Tohidi-Moghadam, H.; Shirani-Rad, A.; Nour-Mohammadi, G.; Habibi, D.; Mash-hadi-Akbar-Boojar, M. Effect of Super Absorbent Application on Antioxidant Enzyme Activities in Canola (Brassica napus L.) Cultivars under Water Stress Conditions. Am. J. Agric. Biol. Sci. 2009, 4, 215–223. [Google Scholar] [CrossRef]
- Amraei, B.; Paknejad, F.; Ebrahimi, M.; Sobhanian, H. Effects of methanol spraying on some biochemical and physiological characteristics of soybean (Glycine betaine max L.) under drought stress. J. Iran. Plant Eco Physiol. Res. 2017, 12, 81–94. [Google Scholar]
- Keles, Y.; Öncel, I. Growth and Solute Composition in Two Wheat Species Experiencing Combined Influence of Stress Conditions. Russ. J. Plant Physiol. 2004, 51, 203–209. [Google Scholar] [CrossRef]
- Cruz, F.J.R.; de Castro, G.L.S.; Junior, D.D.D.S.; Festucci-Buselli, R.A.; Pinheiro, H.A. Exogenous glycine betaine modulates ascorbate peroxidase and catalase activities and prevent lipid peroxidation in mild water-stressed Carapa guianensis plants. Photosynthetica 2013, 51, 102–108. [Google Scholar] [CrossRef]
- Prajapat, P.; Singh, D.; Tripathi, S.; Patel, K.; Abbas, H.; Patel, A. Effect of water stress on antioxidative enzymes and glycine betaine content in drought tolerant and drought susceptible cotton (Gossypium hirsutum L.) genotypes. Indian J. Biochem. Biophys. 2018, 55, 198–204. Available online: http://nopr.niscair.res.in/handle/123456789/44627 (accessed on 28 September 2021).
- Fu, J.; Huang, B. Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ. Exp. Bot. 2001, 45, 105–114. [Google Scholar] [CrossRef]
- Gente, R.; Busch, S.F.; Stubling, E.-M.; Schneider, L.M.; Hirschmann, C.B.; Balzer, J.C.; Koch, M. Quality Control of Sugar Beet Seeds with THz Time-Domain Spectroscopy. IEEE Trans. Terahertz Sci. Technol. 2016, 6, 1–3. [Google Scholar] [CrossRef]
- Hoffmann, C.M. Sucrose Accumulation in Sugar Beet Under Drought Stress. J. Agron. Crop. Sci. 2010, 196, 243–252. [Google Scholar] [CrossRef]
- Sadeghi-Shoae, M.; Paknejad, F.; Kashani, A.; Nooralvandi, T.; Tookalloo, M.R. Can foliar application with methanol improve the yield, yield components and physiological performance of mung bean (Vigna radiata L.). Ann. Biol. Res. 2012, 3, 4780–4785. [Google Scholar]
- Ali, Q.; Ahraf, M. Exogenously applied glycine betaine enhances seed and seed oil quality of maize (Zea mays L.) under water deficit conditions. Environ. Exp. Bot. 2011, 71, 249–259. [Google Scholar] [CrossRef]
- Kurpein, L.V.; Ivanov, A.G.; Zaman, M.; Pharis, R.P.; Hurry, V.; Huner, N.P. Interaction of glycine betaine and plant hormones: Protection of the photosynthetic apparatus during abiotic stress. In Photosynthesis: Structures, Mechanisms, and Applications; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Outlaw, W.H. An introduction to carbon metabolism in guard cells. In Stomatal Function; Zeiger, E., Farquhar, G.D., Cowan, L.R., Eds.; Stanford University Press: Stanford, CA, USA, 1987; pp. 115–123. [Google Scholar]



| P | EC | Na | P | K | NH4 | NO3 | O.C | pH | Soil Texture |
|---|---|---|---|---|---|---|---|---|---|
| U | ds∙m−1 | meq∙l−1 | mg∙kg−1 | mg∙kg−1 | mg∙kg−1 | mg∙kg−1 | % | -- | |
| H | 1.03 | 6.36 | 12.06 | 568 | 5.95 | 14.63 | 1.03 | 7.84 | Clay-Loam |
| Q | 1.83 | 8.92 | 8.46 | 689 | 6.30 | 15.82 | 0.21 | 7.82 | Clay |
| S.O.V | DF | T 1 | T 2 | T 3 | T 4 | T 5 |
|---|---|---|---|---|---|---|
| L | 1 | 88973.28 ** | 2491.79 ** | 39.01 ** | 111,966 ** | 3154.40 ** |
| Block | 4 | 286.84 | 10.35 | 7.77 | 466.37 | 18.64 |
| A | 2 | 6724.13 ** | 187.82 ** | 32.00 ** | 13,072 ** | 373.39 ** |
| B | 1 | 9434.05 ** | 235.54 ** | 23.93 * | 16,480 ** | 409.64 ** |
| A ⨯ B | 2 | 1526.84 ns | 48.76 ns | 2.58 ns | 1065.69 ns | 31.84 ns |
| L ⨯ A | 2 | 670.60 ns | 11.21 ns | 18.98 * | 1744.62 * | 49.55 * |
| L ⨯ B | 1 | 3182.89 ns | 92.51ns | 2.84 ns | 2443.32 ns | 62.92 ns |
| L ⨯ A ⨯ B | 2 | 567.31 ns | 20.23 ns | 13.21 ns | 226.71 ns | 6.07 ns |
| Block(A ⨯ B) | 10 | 422.09 | 13.64 | 3.03 | 420.63 | 13.83 |
| C | 5 | 3465.98 ** | 100.91 ** | 9.63 * | 3900.65 ** | 123.82 ** |
| L ⨯ C | 5 | 899.52 ** | 16.46 ns | 7.10 ns | 661.25 ** | 11.28 * |
| A ⨯ C | 10 | 255.04 ** | 7.81 ** | 7.29 * | 545.95 ** | 17.18 ** |
| L ⨯ A ⨯ C | 10 | 205.89 ns | 7.20 ns | 6.61 ns | 447.14 ns | 13.70 ns |
| B ⨯ C | 5 | 162.40 ns | 4.48 ns | 7.19 ns | 230.00 ns | 11.06 ns |
| L ⨯ B ⨯ C | 5 | 164.69 ns | 3.24 ns | 9.32 ns | 290.64 ns | 12.99 ns |
| A ⨯ B ⨯ C | 10 | 64.34 ns | 1.95 ns | 5.58 ns | 138.28 ns | 4.39 ns |
| L ⨯ A ⨯ B ⨯ C | 10 | 48.64 ns | 2.36 ns | 1.59 ns | 50.61 ns | 3.40 ns |
| (error) | 130 | 21.78 | 1.14 | 3.96 | 140.52 | 5.19 |
| T 1 | T 2 | T 3 | T 4 | T 5 | |
|---|---|---|---|---|---|
| L | |||||
| Qom | 72.71 b | 13.07 b | 3.72 b | 92.11 b | 16.80 b |
| Hamedan | 113.30 a | 19.86 a | 4.57 a | 137.64 a | 24.44 a |
| A | |||||
| Control | 81.85 b | 14.60 b | 3.38 b | 99.33 b | 17.99 b |
| 15% V.V | 98.76 a | 17.45 a | 4.46 a | 122.19 a | 21.92 a |
| 30% V.V | 98.41 a | 17.34 a | 4.60 a | 123.11 a | 21.95 a |
| B | |||||
| Control | 86.40 b | 15.42 b | 3.82 b | 106.14 b | 19.24 b |
| 4 gram | 99.62 a | 17.51 a | 4.48a | 123.61 a | 22.00 a |
| C | |||||
| SBSI 050 | 82.54 d | 16.50 b | 4.21 ab | 103.91 c | 20.71 b |
| SBSI 051 | 88.52 c | 15.48 c | 4.49 ab | 114.19 b | 19.98 b |
| SBSI 052 | 97.44 b | 17.80 a | 4.98 a | 125.06 a | 22.80 a |
| Brunium | 89.68 c | 16.73 b | 3.59 b | 106.77 bc | 20.32 b |
| Jamon | 89.32 c | 13.80 d | 3.80 ab | 109.53 bc | 17.60 c |
| Kyros | 110.54 a | 18.47 a | 3.83 ab | 129.81 a | 22.31 a |
| S.O.V | DF | T 6 | T 7 | T 8 | T 9 | T 10 | T11 |
|---|---|---|---|---|---|---|---|
| L | 1 | 2006.71 ** | 152.67 ** | 30.88 * | 13422.84 ** | 23.89 ** | 2,084,425.09 ** |
| Block | 4 | 21.94 | 4.34 | 7.62 ns | 13.42 | 0.11 | 39,542.03 * |
| A | 2 | 468.65 ** | 3.68 ns | 8.21 ns | 126.16 ** | 2.11 ** | 552,236.82 ** |
| B | 1 | 401.55 ** | 0.29 ns | 33.50 ns | 151.22 ** | 3.56 ** | 58,666.62 * |
| A ⨯ B | 2 | 17.18 ns | 2.93 ns | 4.94 ns | 16.64 * | 0.16 ** | 5939 * |
| L ⨯ A | 2 | 303.53 ** | 0.11 ns | 0.91 ns | 56.22 ** | 0.47 ** | 626,817.64 ** |
| L ⨯ B | 1 | 88.12 ns | 0.001 ns | 0.0004 ns | 31.14 * | 2.84 * | 46,372.51 * |
| L ⨯ A ⨯ B | 2 | 70.06 ns | 0.35 ns | 2.73 ns | 36.66 ** | 0.27 * | 33,186.76 * |
| Block (A ⨯ B) | 10 | 18.31 | 1.44 | 5.97 | 6.89 | 0.07 | 9487.34 |
| C | 5 | 253.10 ** | 18.39 ** | 22.54 * | 63.73 ** | 0.79 ** | 204,233.34 ** |
| L ⨯ C | 5 | 58.00 * | 13.56 ** | 17.54 ns | 18.98 ** | 0.73 ** | 12,957.42 * |
| A ⨯ C | 10 | 48.93 * | 1.11 ns | 5.02 ns | 10.92 * | 0.09 ns | 25,040.29 * |
| L ⨯ A ⨯ C | 10 | 35.78 ns | 0.27 ns | 3.34 ns | 11.13 * | 0.26 * | 15,196.35 * |
| B ⨯ C | 5 | 23.96 ns | 0.0003 ns | 0.0002 ns | 4.85 ns | 0.05 ns | 10,650.56 * |
| L ⨯ B ⨯ C | 5 | 14.03 ns | 0.0001 ns | 0.0003 ns | 10.73 * | 0.12 * | 18,881.84 * |
| A ⨯ B ⨯ C | 10 | 41.42 ns | 3.30 ns | 15.11 ns | 4.1 ns | 0.04 ns | 10,101.9 * |
| L ⨯ A ⨯ B ⨯ C | 10 | 27.63 ns | 0.83 ns | 10.03 ns | 7.31 * | 0.1 * | 2,084,425.09 ** |
| (error) | 130 | 20.90 | 2.22 | 8.44 | 3.56 | 0.05 | 39,542.03 * |
| T 6 | T 7 | T 8 | T 9 | T 10 | T 11 | |
|---|---|---|---|---|---|---|
| L | ||||||
| Qom | 23.71 b | 88.17 a | 10.98 b | 5.23 b | 2.25 a | 595.27 b |
| Hamedan | 29.81 a | 88.15 a | 12.66 a | 15.68 a | 1.58 b | 791.74 a |
| A | ||||||
| Control | 23.81 b | 88.38 a | 11.97 a | 11.74 c | 1.72 b | 592.64 b |
| 15% V.V | 28.17 a | 88.33 a | 11.93 a | 14.39 a | 2.02 a | 737.61 a |
| 30% V.V | 28.29 a | 87.77 a | 11.56 a | 13.21 b | 2.01 a | 750.26 a |
| B | ||||||
| Control | 25.40 b | 88.55 a | 11.86 a | 12.28 b | 1.79 b | 639.63 b |
| 4 grams | 28.12 a | 87.76 a | 11.78 a | 13.95 a | 2.04 a | 747.37 a |
| C | ||||||
| SBSI 050 | 24.47 c | 89.04 a | 12.78 a | 13.29 c | 1.91 c | 697.97 c |
| SBSI 051 | 23.42 c | 87.50 ab | 11.36 b | 12.76 c | 1.89 c | 662.74 c |
| SBSI 052 | 27.64 ab | 88.77 ab | 11.43 b | 14.72 b | 2.1 b | 795.09 a |
| Brunium | 25.75 bc | 88.48 ab | 12.70 a | 12.79 c | 1.85 c | 683.86 c |
| Jamon | 29.18 a | 86.96 b | 11.30 b | 10.92 d | 1.69 d | 574.56 d |
| Kyros | 30.09 a | 88.20 ab | 11.35 b | 14.20 a | 2.05 a | 746.79 b |
| Treatments | T1 | T 2 | T 3 | T 4 | T 5 | T 6 | T 7 | T 8 | T 9 | T 10 | T 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hamedan Met 0 Gly 0 | 98.31 | 17.27 | 3.97 | 119.76 | 21.26 | 25.93 | 93.44 | 14.17 | 23.56 | 1.33 | 673.31 |
| Hamedan Met 0 Gly 4 | 105.19 | 18.46 | 4.25 | 128.05 | 22.71 | 27.72 | 96.95 | 13.54 | 24.08 | 1.4 | 747.90 |
| Hamedan Met 15 Gly 0 | 107.35 | 18.85 | 4.34 | 129.94 | 23.23 | 28.34 | 87.26 | 12.03 | 18.88 | 1.56 | 758.91 |
| Hamedan Met 15 Gly 4 | 135.67 | 23.86 | 5.48 | 165.16 | 29.37 | 35.76 | 85.50 | 12.42 | 10.93 | 1.67 | 938.78 |
| Hamedan Met 30 Gly 0 | 101.97 | 17.84 | 4.12 | 123.87 | 21.98 | 26.87 | 81.97 | 12.15 | 14.60 | 1.65 | 721.93 |
| Hamedan Met 30 Gly 4 | 129.95 | 22.83 | 5.25 | 158.25 | 28.14 | 34.25 | 83.74 | 11.64 | 12.78 | 1.89 | 909.59 |
| Qom Met 0 Gly 0 | 56.71 | 10.19 | 2.91 | 71.84 | 13.11 | 18.49 | 99.64 | 12.07 | 4.10 | 1.95 | 459.38 |
| Qom Met 0 Gly 4 | 60.34 | 10.84 | 3.11 | 76.45 | 13.97 | 19.67 | 94.35 | 11.63 | 4.39 | 2.19 | 489.97 |
| Qom Met 15 Gly 0 | 73.52 | 13.22 | 3.76 | 93.03 | 16.95 | 23.96 | 86.41 | 10.65 | 5.27 | 2.19 | 598.18 |
| Qom Met 15 Gly 4 | 79.97 | 14.37 | 4.07 | 101.32 | 18.44 | 26.17 | 84.65 | 10.87 | 5.71 | 2.64 | 654.58 |
| Qom Met 30 Gly 0 | 75.61 | 13.59 | 3.86 | 95.79 | 17.49 | 24.35 | 83.77 | 10.43 | 5.45 | 2.04 | 626.08 |
| Qom Met 30 Gly 4 | 90.16 | 16.24 | 4.62 | 114.21 | 20.83 | 29.41 | 81.13 | 10.21 | 6.45 | 2.47 | 743.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haghighi, P.; Habibi, D.; Mozafari, H.; Sani, B.; Sadeghishoae, M. Impact of Methanol and Glycine Betaine on Yield and Quality of Fodder Beet Genotypes (Beta vulgaris subsp. vulgaris). Agronomy 2021, 11, 2122. https://doi.org/10.3390/agronomy11112122
Haghighi P, Habibi D, Mozafari H, Sani B, Sadeghishoae M. Impact of Methanol and Glycine Betaine on Yield and Quality of Fodder Beet Genotypes (Beta vulgaris subsp. vulgaris). Agronomy. 2021; 11(11):2122. https://doi.org/10.3390/agronomy11112122
Chicago/Turabian StyleHaghighi, Pezhman, Davood Habibi, Hamid Mozafari, Behzad Sani, and Mehdi Sadeghishoae. 2021. "Impact of Methanol and Glycine Betaine on Yield and Quality of Fodder Beet Genotypes (Beta vulgaris subsp. vulgaris)" Agronomy 11, no. 11: 2122. https://doi.org/10.3390/agronomy11112122
APA StyleHaghighi, P., Habibi, D., Mozafari, H., Sani, B., & Sadeghishoae, M. (2021). Impact of Methanol and Glycine Betaine on Yield and Quality of Fodder Beet Genotypes (Beta vulgaris subsp. vulgaris). Agronomy, 11(11), 2122. https://doi.org/10.3390/agronomy11112122







