Interactive Effects of Drought–Flood Abrupt Alternation on Morpho-Agronomic and Nutrient Use Traits in Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Pot Establishment and Plant Cultivation
2.3. Experimental Design and Management
- (1)
- Control treatment (without water stress), keeping 2–3 cm water level above the soil surface;
- (2)
- Nine DFAA treatments, combined with drought degree (50%, 60% and 70% of field capacity (FC)), drought duration (5, 10 and 15 days), flooding degree (1/2, 3/4 and 1/1 of plant height) and flooding duration (5, 7 and 9 days) according to the standard orthogonal table (L9 (34));
- (3)
- Nine drought alone treatments, drought stage following the same as DFAA treatments and the other stages following the same as the control treatment;
- (4)
- Nine flooding alone treatments, flooding stage following the same as DFAA treatments and the other stages following the same as the control treatment.
2.4. Sampling and Measurements
2.4.1. Shoot and Root Sampling
2.4.2. Chemical Analysis and Calculation
2.5. Statistical Analysis
3. Results
3.1. Morpho-Agronomic Traits
3.1.1. Biological Yield
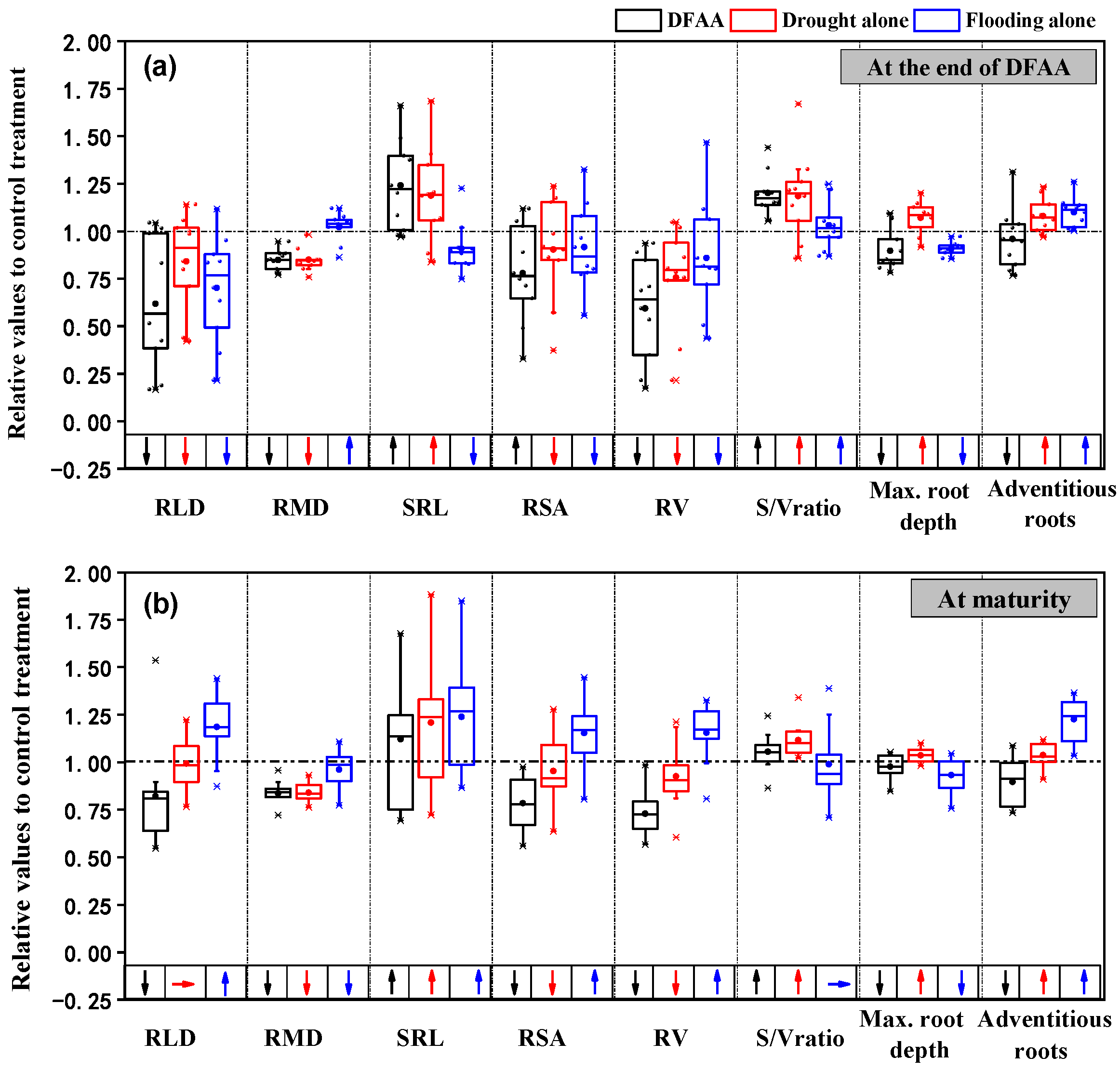
3.1.2. Root Morphology
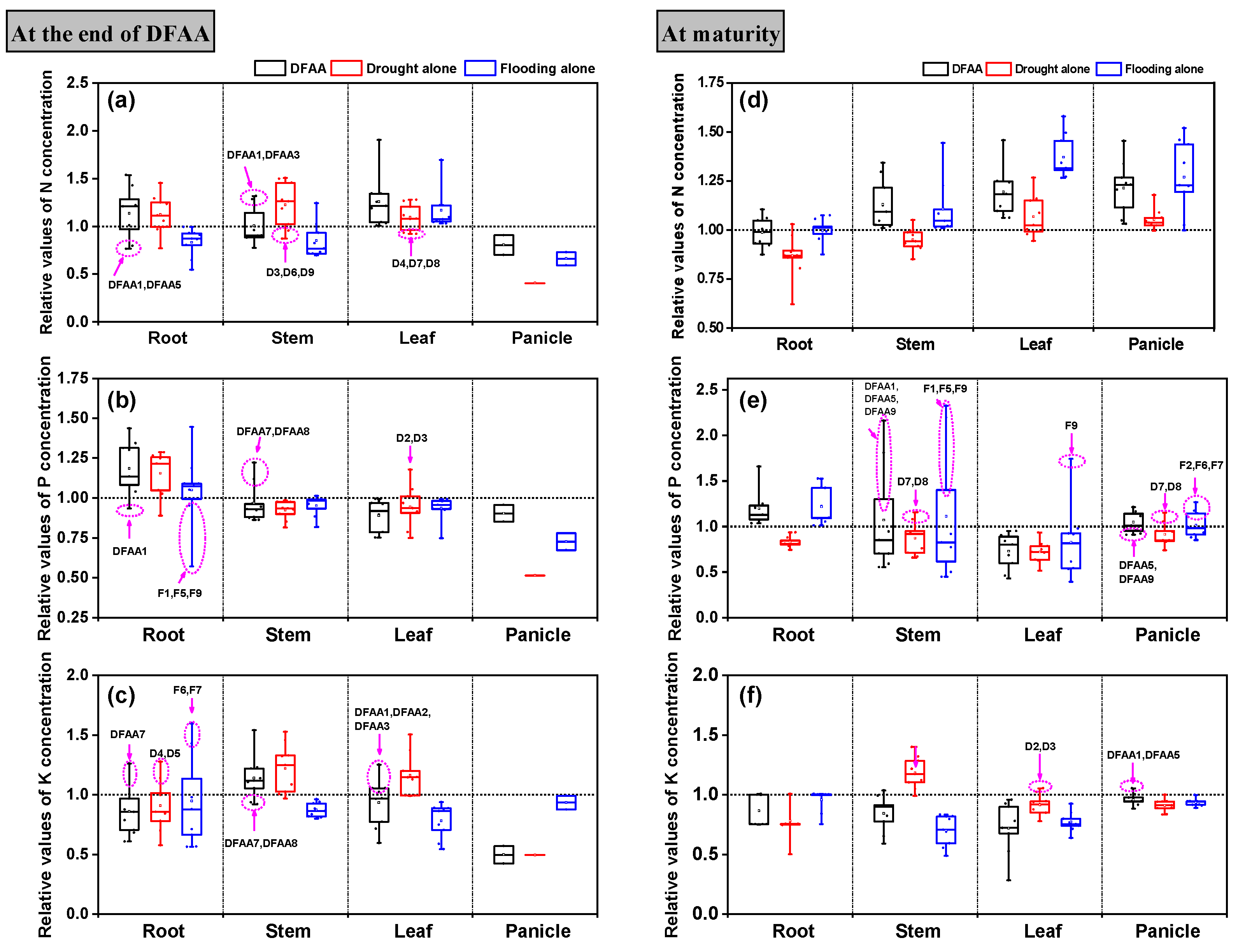
3.2. Accumulation and Partitioning of N, P and K
3.3. N, P and K Uptake and Use Efficiencies
4. Discussion
4.1. Co-Occurring Drought and Flooding Altered Biomass Accumulation and Partitioning
4.2. Co-Occurring Drought and Flooding Altered N, P and K Uptake and Utilization
4.3. Influence of Root Plasticity on N, P and K Uptake and Utilization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DFAA | drought–flood abrupt alternation or drought followed by flood |
| D | drought followed by no flood |
| F | no drought followed by flood |
| FC | field capacity |
| N | nitrogen |
| P | phosphorus |
| K | potassium |
| DW | dry weight |
| GY | grain yield |
| RLD | root length density |
| SRL | specific root length |
| RMD | root mean diameter |
| RSA | root surface area |
| RV | root volume |
| S/V | ratio the ratio of root surface area and root volume |
| UE | use efficiency |
| SAR | specific absorption rate |
References
- Lehner, B.; Döll, P.; Alcamo, J.; Henrichs, T.; Kaspar, F. Estimating the Impact of Global Change on Flood and Drought Risks in Europe: A Continental, Integrated Analysis. Clim. Chang. 2006, 75, 273–299. [Google Scholar] [CrossRef]
- Easterling, D.R.; Evans, J.L.; Groisman, P.Y.; Karl, T.R.; Kunkel, K.E.; Ambenje, P. Observed variability and trends in extreme climate events: A brief review. Bull. Am. Meteorol. Soc. 2000, 81, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Miao, C.; Duan, Q. Extreme climate events and agricultural climate indices in China: CMIP5 model evaluation and projections. Int. J. Clim. 2016, 36, 43–61. [Google Scholar] [CrossRef]
- Watanabe, T.; Cullmann, J.; Pathak, C.S.; Turunen, M.; Emami, K.; Ghinassi, G.; Siddiqi, Y. Management of Climatic Extremes with Focus on Floods and Droughts in Agriculture. Irrig. Drain. 2018, 67, 29–42. [Google Scholar] [CrossRef]
- Tao, F.; Zhang, S.; Zhang, Z. Changes in rice disasters across China in recent decades and the meteorological and agronomic causes. Reg. Environ. Chang. 2013, 13, 743–759. [Google Scholar] [CrossRef]
- Sultan, S.E. Phenotypic plasticity for plant development, function and life history. Trends Plant Sci. 2000, 5, 537–542. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Han, A.R.; Kim, H.S. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Nishiuchi, S.; Yamauchi, T.; Takahashi, H.; Kotula, L.; Nakazono, M. Mechanisms for coping with submergence and waterlogging in rice. Rice 2012, 5, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradhan, C.; Mohanty, M. Submergence stress: Responses and adaptations in crop plants. In Molecular Stress Physiology Plants; Springer: Utkal University, BhubaneswarIndia, India, 2013; pp. 31–357. [Google Scholar]
- Steffens, B.; Wang, J.; Sauter, M. Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deep water rice. Planta 2006, 223, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, A.; Pardales, J.R., Jr.; Kono, Y. Root system structure and its relation to stress tolerance. In Roots and Nitrogen in Cropping Systems of the Semi-Arid Tropics; Ito, O., Katayama, K., Johansen, C., Kumar Rao, J.V.D.K., Adu-Gyamfi, J.J., Rego, T.J., Eds.; JIRCAS Publication: Tsukuba, Japan, 1996; pp. 211–234. [Google Scholar]
- Zhang, H.; Xue, Y.; Wang, Z.; Yang, J.; Zhang, J. An alternate wetting and moderate soil drying regime improves root and shoot growth in rice. Crop. Sci. 2009, 49, 2246–2260. [Google Scholar] [CrossRef]
- Chu, G.; Chen, T.; Wang, Z.; Yang, J.; Zhang, J. Reprint of “Morphological and physiological traits of roots and their relationships with water productivity in water-saving and drought-resistant rice”. Field Crop. Res. 2014, 165, 36–48. [Google Scholar] [CrossRef]
- Xu, G.-W.; Lyu, Q.; Lu, D.-K.; Wang, H.-Z.; Chen, M.-C. Effect of Wetting and Drying Alternative Irrigation Coupling with Nitrogen Application on Root Characteristic and Grain-sink Activity. Acta Agron. Sin. 2016, 42, 1495–1505. [Google Scholar] [CrossRef]
- Mehrabi, F.; Sepaskhah, A.R.; Ahmadi, S.H. Winter wheat root distribution with irrigation, planting methods, and nitrogen application. Nutr. Cycl. Agroecosys. 2021, 119, 231–245. [Google Scholar] [CrossRef]
- Wang, J.; Niu, W.; Zhang, M.; Li, Y. Effect of alternate partial root-zone drip irrigation on soil bacterial communities and tomato yield. Appl. Soil Ecol. 2017, 119, 250–259. [Google Scholar] [CrossRef]
- Du, S.; Tong, L.; Kang, S.; Li, F.; Du, T.; Li, S.; Ding, R. Alternate partial root-zone irrigation with high irrigation frequency improves root growth and reduces unproductive water loss by apple trees in arid north-west China. Front. Agric. Sci. Eng. 2018, 5, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Suralta, R.R.; Kano-Nakata, M.; Niones, J.M.; Inukai, Y.; Kameoka, E.; Tran, T.T.; Menge, D.; Mitsuya, S.; Yamauchi, A. Root plasticity for maintenance of productivity under abiotic stressed soil environments in rice: Progress and prospects. Field Crop. Res. 2016, 220, 57–66. [Google Scholar] [CrossRef]
- Shao, G.-C.; Deng, S.; Liu, N.; Yu, S.-E.; Wang, M.-H.; She, D.-L. Effects of controlled irrigation and drainage on growth, grain yield and water use in paddy rice. Eur. J. Agron. 2014, 53, 1–9. [Google Scholar] [CrossRef]
- Shekhar, S.; Mailapalli, D.R.; Raghuwanshi, N.S. Simulating nitrogen transport in paddy crop irrigated with alternate wetting and drying practice. Paddy Water Environ. 2021, 19, 499–513. [Google Scholar] [CrossRef]
- Shao, G.-C.; Wang, M.-H.; Yu, S.-E.; Liu, N.; Xiao, M.-H.; Yuan, M. Potential of Controlled Irrigation and Drainage for Reducing Nitrogen Emission from Rice Paddies in Southern China. J. Chem. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Suriyagoda, L.; De Costa, W.A.J.M.; Lambers, H. Growth and phosphorus nutrition of rice when inorganic fertiliser application is partly replaced by straw under varying moisture availability in sandy and clay soils. Plant Soil 2014, 384, 53–68. [Google Scholar] [CrossRef]
- Kato, Y.; Tajima, R.; Toriumi, A.; Homma, K.; Moritsuka, N.; Shiraiwa, T.; Yamagishi, J.; Mekwatanakern, P.; Chamarerk, V.; Jongdee, B. Grain yield and phosphorus uptake of rainfed lowland rice under unsubmerged soil stress. Field Crop. Res. 2016, 190, 54–59. [Google Scholar] [CrossRef]
- Ichsan, C.N.; Basyah, B.; Zakaria, S.; Efendi, E. Alteration of dry matter accumulation under soil moisture fluctuation stress in rice (Oryza sativa L.). Aust. J. Crop. Sci. 2021, 15, 757–763. [Google Scholar] [CrossRef]
- Singh, S.P.; Mahapatra, B.S.; Pramanick, B.; Yadav, V.R. Effect of irrigation levels, planting methods and mulching on nutrient uptake, yield, quality, water and fertilizer productivity of field mustard (Brassica rapa L.) under sandy loam soil. Agric. Water Manag. 2021, 244, 106539. [Google Scholar] [CrossRef]
- Suralta, R.R. Plastic root system development responses to drought-enhanced nitrogen uptake during progressive soil drying conditions in rice. Philipp. Agric. 2011, 93, 458–462. [Google Scholar]
- He, M.; Dijkstra, F.A. Drought effect on plant nitrogen and phosphorus: A meta-analysis. New Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.C.; Ni, M.J.; Wang, J.J. Effects of water stress on rice yields and nutrients absorption under aerobic condition. Trans. Chin. Soc. Agric. Eng. 2007, 23, 101–104. [Google Scholar]
- Reddy, M.D.; Mittra, B.N. Effect of complete plant submergence at different growth stages on grain yield, yield components and nutrient content of rice. Plant Soil 1985, 86, 379–386. [Google Scholar] [CrossRef]
- Reddy, M.D.; Mittra, B.N. Effects of complete plant submergence on vegetative growth, grain yield and some biochemical changes in rice plants. Plant Soil 1985, 87, 365–374. [Google Scholar] [CrossRef]
- Setter, T.; Greenway, H.; Kupkanchanakul, T. Submergence of Rice. II. Adverse Effects of Low CO2 Concentrations. Funct. Plant Biol. 1989, 16, 265–278. [Google Scholar] [CrossRef]
- Somaweera, K.A.T.N.; Suriyagoda, L.D.B.; Sirisena, D.N.; De Costa, W.A.J.M. Accumulation and partitioning of biomass, nitrogen, phosphorus and potassium among different tissues during the life cycle of rice grown under different water management regimes. Plant Soil 2016, 401, 169–183. [Google Scholar] [CrossRef]
- Cabangon, R.J.; Tuong, T.P.; Castillo, E.G.; Bao, L.X.; Lu, G.; Wang, G.; Cui, Y.; Bouman, B.A.M.; Li, Y.; Chen, C.; et al. Effect of irrigation method and N-fertilizer management on rice yield, water productivity and nutrient-use efficiencies in typical lowland rice conditions in China. Paddy Water Environ. 2004, 2, 195–206. [Google Scholar] [CrossRef]
- Li, Z.J.; Zhang, F.C.; Kang, S.Z. Impacts of the controlled roots-divided alternative irrigation on water and nutrient use of winter wheat. Trans. Chin. Soc. Agric. Eng. 2005, 21, 17–21. [Google Scholar]
- Gao, S.-K.; Yu, S.-E.; Shao, G.-C.; She, D.-L.; Wang, M.; Guo, R.; Cao, R.-Z.; Yan, S.-F.; Ding, J.-H. Effects of Controlled Irrigation and Drainage on Nitrogen and Phosphorus Concentrations in Paddy Water. J. Chem. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Haefele, S.; Wopereis, M.; Ndiaye, M.; Barro, S.; Isselmou, M.O. Internal nutrient efficiencies, fertilizer recovery rates and indigenous nutrient supply of irrigated lowland rice in Sahelian West Africa. Field Crop. Res. 2003, 80, 19–32. [Google Scholar] [CrossRef]
- Sattari, S.Z.; Ittersum, M.K.V.; Bouwman, A.F.; Smit, A.L.; Janssen, B.H. Crop yield response to soil fertility and N, P, K inputs in different environments: Testing and improving the quefts model. Field Crop. Res. 2014, 157, 35–46. [Google Scholar] [CrossRef]
- Mao, Z. Study on evaluation of irrigation performance in China. In Maintenance and Operation of Irrigation/Drainage Schemes for Improved Performance, Proceedings of the Asian Regional Symposium, Beijing, China, 24–27 May 1993; HR Wallingford: Oxford, UK, 1993; pp. 6.35–6.49. [Google Scholar]
- Devkota, K.P.; Manschadi, A.; Lamers, J.P.; Devkota, M.; Vlek, P.L. Mineral nitrogen dynamics in irrigated rice—Wheat system under different irrigation and establishment methods and residue levels in arid drylands of Central Asia. Eur. J. Agron. 2013, 47, 65–76. [Google Scholar] [CrossRef]
- Goswami, N.N.; Banerjee, M.K. Phosphorus, Potassium and Other Macro Elements in Soils and Rice; IRRI: Los Banos, Philippines, 1978; pp. 561–580. [Google Scholar]
- Somaweera, K.A.T.N.; Suriyagoda, L.D.B.; Sirisena, D.N.; De Costa, W.A.J.M. Growth, root adaptations, phosphorus and potassium nutrition of rice when grown under the co-limitations of phosphorus, potassium and moisture. J. Plant Nutr. 2017, 40, 18. [Google Scholar] [CrossRef]
- Guo, X.P.; Yuan, J.; Guo, F.; Chen, Z.P. Effects of rapid shift from drought to waterlogging stress on physiological characteristics of rice in late tillering stage. J. Hohai Univ. 2008, 36, 516–519. [Google Scholar]
- Ye, Y.; Liang, X.; Chen, Y.; Liu, J.; Gu, J.; Guo, R.; Li, L. Alternate wetting and drying irrigation and controlled-release nitrogen fertilizer in late-season rice. Effects on dry matter accumulation, yield, water and nitrogen use. Field Crop. Res. 2013, 144, 212–224. [Google Scholar] [CrossRef]
- Zhen, B.; Guo, X.; Lu, H. Effects of alternative stress of drought and waterlogging at tillering stage on rice root anatomical structure. Trans. Chin. Soc. Agric. Eng. 2015, 31, 107–113. [Google Scholar]
- Zhu, R.; Wu, F.; Zhou, S.; Hu, T.; Huang, J.; Gao, Y. Cumulative effects of drought–flood abrupt alternation on the photosynthetic characteristics of rice. Environ. Exp. Bot. 2020, 169, 103901. [Google Scholar] [CrossRef]
- Cheng, Z.; Xu, M.; Luo, L.S.; Ding, X.J. Climate characteristics of drought-flood abrupt change events in Huaihe river basin. J. China Hydrol. 2012, 32, 73–79. [Google Scholar]
- Huang, R.; Yan, D.H. Spatiotemporal Evolution of the Drought and Flood in Northeast China. Adv. Mater. Res. 2014, 1010–1012, 1075–1083. [Google Scholar] [CrossRef]
- Li, Y.S.; Peng, F.Y.; Li, D.M.; Li, Z.S. Relationship between hybrids and their parents on submergence tolerance at seedling stage. Hybrid Rice 2001, 16, 50–53. [Google Scholar]
- Cui, Y.; Mao, Z.; Li, Y. Study on temporal and spatial variation of rice water production function. Adv. Water Sci. 2002, 13, 484–491. [Google Scholar]
- Schuurman, J.J.; Goedewaagen, M.A.J. Methods for the Examination of Root Systems and Roots; Centre for Agricultural Publications and Documentation: Wageningen, The Netherlands, 1971. [Google Scholar]
- Bao, S.D. Soil and Agriculture Chemistry Analysis; Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Determination of Total Nitrogen in Plant Material 1. Agron. J. 1973, 65, 109–112. [Google Scholar] [CrossRef]
- Kitson, R.E.; Mellon, M.G. Colorimetric Determination of Phosphorus as Molybdivanadophosphoric Acid. Ind. Eng. Chem. Anal. Ed. 1944, 16, 379–383. [Google Scholar] [CrossRef]
- Ranst, E.V.; Verloo, M.; Demeyer, A. Manual for the Soil Chemistry and Fertility Laboratory-Analytical Methods for Soils and Plants, Equipment, and Management of Consumables; NUGI 835: Ghent, Belgium, 1999; 243p, ISBN 90-76603-01-4. [Google Scholar]
- Romero, J.M.R.; Marañón, T.; Murillo, J.M. Long-term responses of Melilotus segetalis to salinity. II. Nutrient absorption and utilization. Plant Cell Environ. 1994, 17, 1249–1255. [Google Scholar] [CrossRef]
- Ju, C.; Buresh, R.J.; Wang, Z.; Zhang, H.; Liu, L.; Yang, J.; Zhang, J. Root and shoot traits for rice varieties with higher grain yield and higher nitrogen use efficiency at lower nitrogen rates application. Field Crop. Res. 2015, 175, 47–55. [Google Scholar] [CrossRef]
- Blum, A.; Jordan, W.R. Breeding crop varieties for stress environments. Crit. Rev. Plant Sci. 1985, 2, 199–238. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Lee, H.; Adhikari, A.; Han, A.R.; Kim, H.S. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. Sci. Total Environ. 2021, 779, 146466. [Google Scholar] [CrossRef]
- Xu, G.-W.; Lu, D.-K.; Wang, H.-Z.; Li, Y. Morphological and physiological traits of rice roots and their relationships to yield and nitrogen utilization as influenced by irrigation regime and nitrogen rate. Agric. Water Manag. 2018, 203, 385–394. [Google Scholar] [CrossRef]
- Xie, X.; Quintana, M.R.; Sandhu, N.; Subedi, S.R.; Zou, Y.; Rutkoski, J.E.; Henry, A. Approaches to relating rice root plasticity with yield stability across different drought stress and establishment conditions. J. Exp. Bot. 2021, 72, 5208–5220. [Google Scholar] [CrossRef] [PubMed]
- Bleecker, A.B.; Schuette, J.L.; Kende, H. Anatomical analysis of growth and developmental patterns in the internode of deep-water rice. Planta 1986, 169, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Kende, H.; van der Knaap, E.; Cho, H.-T. Deepwater Rice: A Model Plant to Study Stem Elongation. Plant Physiol. 1998, 118, 1105–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phukan, U.J.; Mishra, S.; Shukla, R.K. Waterlogging and submergence stress: Affects and acclimation. Crit. Rev. Biotechnol. 2015, 36, 956–966. [Google Scholar] [CrossRef]
- Bhusal, N.; Kim, H.S.; Han, S.-G.; Yoon, T.-M. Photosynthetic traits and plant–water relations of two apple cultivars grown as bi-leader trees under long-term waterlogging conditions. Environ. Exp. Bot. 2020, 176, 104111. [Google Scholar] [CrossRef]
- Suralta, R.R.; Inukai, Y.; Yamauchi, A. Genotypic Variations in Responses of Lateral Root Development to Transient Moisture Stresses in Rice Cultivars. Plant Prod. Sci. 2008, 11, 324–335. [Google Scholar] [CrossRef]
- Suralta, R.R.; Inukai, Y.; Yamauchi, A. Utilizing Chromosome Segment Substitution Lines (CSSLs) for Evaluation of Root Responses to Transient Moisture Stresses in Rice. Plant Prod. Sci. 2008, 11, 457–465. [Google Scholar] [CrossRef] [Green Version]
- Niones, J.M.; Suralta, R.R.; Inukai, Y.; Yamauchi, A. Field evaluation on functional roles of root plastic responses on dry matter production and grain yield of rice under cycles of transient soil moisture stresses using chromosome segment substitution lines. Plant Soil 2012, 359, 107–120. [Google Scholar] [CrossRef]
- Feng, G.; Luo, Y. Simulation on functional equilibrium of winter wheat root and shoot under different soil water regimes. Acta Ecol. Sin. 1999, 19, 96–103. [Google Scholar]
- Guo, X.; Xiaopu, L.I.; Hongfei, L.U.; Wang, Z.; Yang, S. Study on dry matter accumulation and nitrogen uptake under alternating drought and flooding stress in rice tillering stage. J. Irrig. Drain. 2015, 34, 20–24. [Google Scholar]
- Alam, S.M. Nutrient uptake by plants under stress conditions. In Handbook of Plant and Crop Stress; Pessarakli, M., Ed.; Marcel Dekker: New York, NY, USA, 1999; pp. 285–313. [Google Scholar]
- Bhuiyan, M.K.A.; Bhuiya, S.U.; Saleque, M.A.; Khatun, A. Nitrogen application in direct wet seeded rice under alternate wetting and drying irrigation condition: Effects on grain yield dry matter production, nitrogen uptake and nitrogen use efficiencies. J. Plant Nutr. 2017, 40, 2477–2493. [Google Scholar] [CrossRef]
- Kronzucker, H.; Siddiqi, M.Y.; Glass, A.D.; Kirk, G. Nitrate-Ammonium Synergism in Rice. A Subcellular Flux Analysis. Plant Physiol. 1999, 119, 1041–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osaki, M.; Shinano, T.; Matsumoto, M.; Zheng, T.; Tadano, T. A root-shoot interaction hypothesis for high productivity of root crops. Soil Sci. Plant Nutr. 1997, 43, 289–301. [Google Scholar] [CrossRef] [Green Version]
- Ruberti, I.; Sessa, G.; Ciolfi, A.; Possenti, M.; Carabelli, M.; Morelli, G. Plant adaptation to dynamically changing environment: The shade avoidance response. Biotechnol. Adv. 2012, 30, 1047–1058. [Google Scholar] [CrossRef]
- Huang, J.; Hu, T.; Yasir, M.; Gao, Y.; Chen, C.; Zhu, R.; Wang, X.; Yuan, H.; Yang, J. Root growth dynamics and yield responses of rice (Oryza sativa L.) under drought—Flood abrupt alternating conditions. Environ. Exp. Bot. 2019, 157, 11–25. [Google Scholar] [CrossRef]
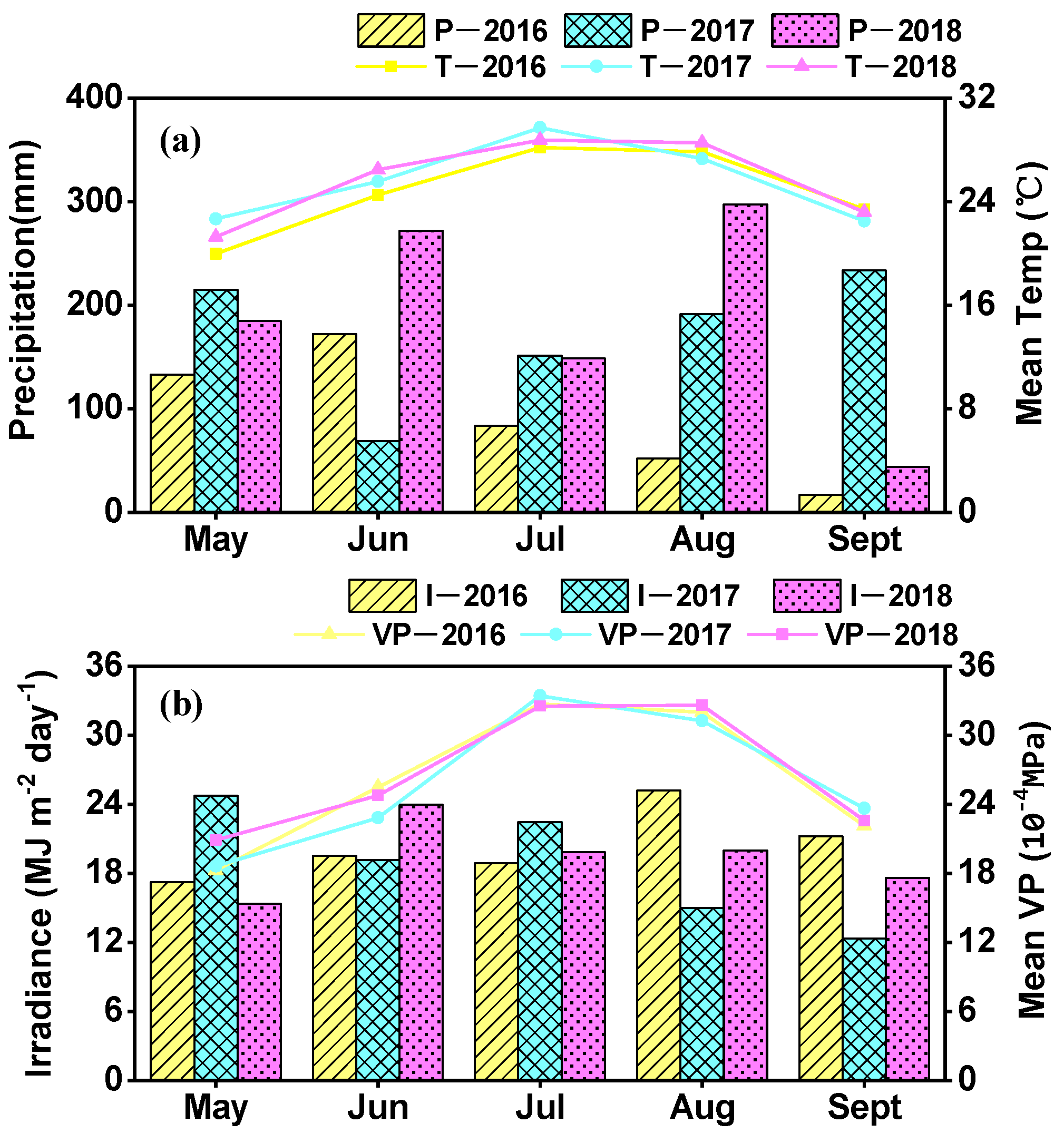



| Treatment | Drought Phase (Stage I) | Flooding Phase (Stage II) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drought Degree (% FC) | Duration (/D) | Beginning and Ending Dates (Mon/D) | Flooding Degree (/Plant Height) | Duration (/D) | Beginning and Ending Dates (Mon/D) | |||||
| 2016 | 2017 | 2018 | 2016 | 2017 | 2018 | |||||
| DFAA1 | 50(severe) | 5 | 7/25–7/29 | 7/30–8/3 | 7/28–8/1 | 1/1(heavy) | 7 | 7/30–8/5 | 8/4–8/10 | 8/2–8/8 |
| DFAA2 | 50(severe) | 10 | 7/25–8/3 | 7/25–8/3 | 7/23–8/1 | 1/2 | 9(heavy) | 8/4–8/12 | 8/4–8/12 | 8/2–8/10 |
| DFAA3 | 50(severe) | 15 | 7/25–8/8 | 7/20–8/3 | 7/18–8/1 | 3/4 | 5(light) | 8/9–8/13 | 8/4–8/8 | 8/2–8/6 |
| DFAA4 | 60(middle) | 5 | 7/25–7/29 | 7/30–8/3 | 7/28–8/1 | 3/4 | 9(heavy) | 7/30–8/7 | 8/4–8/12 | 8/2–8/10 |
| DFAA5 | 60(middle) | 10 | 7/25–8/3 | 7/25–8/3 | 7/23–8/1 | 1/1(heavy) | 5 | 8/4–8/8 | 8/4–8/8 | 8/2–8/6 |
| DFAA6 | 60(middle) | 15 | 7/25–8/8 | 7/20–8/3 | 7/18–8/1 | 1/2(light) | 7 | 8/9–8/15 | 8/4–8/10 | 8/2–8/8 |
| DFAA7 | 70(mild) | 5 | 7/25–7/29 | 7/30–8/3 | 7/28–8/1 | 1/2 | 5(light) | 7/30–8/3 | 8/4–8/8 | 8/2–8/6 |
| DFAA8 | 70(mild) | 10 | 7/25–8/3 | 7/25–8/3 | 7/23–8/1 | 3/4 | 7(light) | 8/4–8/10 | 8/4–8/10 | 8/2–8/8 |
| DFAA9 | 70(mild) | 15 | 7/25–8/8 | 7/20–8/3 | 7/18–8/1 | 1/1(heavy) | 9 | 8/9–8/17 | 8/4–8/12 | 8/2–8/10 |
| Drought alone | D1~D3, D7~D9 (2016) D1~D9 (2017, 2018) | Continuous flooding (2~3 cm) | ||||||||
| Flooding alone | Continuous flooding (2~3 cm) | F1~F3, F7~F9 (2016) F1~F9 (2017, 2018) | ||||||||
| Control | Continuous flooding (2~3 cm) | |||||||||
| Treatment | Biomass (Partitioning) | Yield and Its Components | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Root DW | Stem DW | Leaf DW | Panicle DW | Root/Shoot | Panicles | Spikelets | Filled Grains | Yield | |
| g/pot (%) | g/pot (%) | g/pot (%) | g/pot (%) | g/g | /pot | /Panicle | % | g/pot | |
| Control | 18.6 (5.6) A | 126.7 (38.2) C | 42.3 (12.7) B | 144.2 (43.5) A | 0.0592 A | 30.3 A | 241.9 A | 76.8 C | 137.9 A |
| DFAA | 13.0 (4.3) D | 139.6 (46.2) B | 46.4 (15.4) A | 103.0 (34.1) C | 0.0447 C | 31.0 A | 166.7 C | 79.0 B | 96.8 C |
| Drought alone | 14.8 (4.9) C | 111.2 (37.2) D | 42.4 (14.2) B | 130.4 (43.7) B | 0.0520 B | 30.9 A | 186.9 B | 85.2 A | 124.4 B |
| Flooding alone | 15.5 (4.9) B | 167.9 (53.4) A | 45.4 (14.5) A | 85.4 (27.2) D | 0.0514 B | 30.2 A | 156.6 D | 65.2 D | 78.8 D |
| Treatment | Specific Absorption Rates | Use Efficiencies | Nutrient’s Ratios | ||||||
|---|---|---|---|---|---|---|---|---|---|
| mg Nutrient/g Root DW | g Grain/g Nutrient | mg/mg | |||||||
| SAR-N | SAR-P | SAR-K | NUE | PUE | KUE | N:P | N:K | K:P | |
| Control | 225.3 C | 28.4 C | 162.0 D | 33.5 A | 140.3 B | 46.5 A | 4.12 D | 1.39 B | 3.12 D |
| DFAA | 334.4 A | 34.7 A | 220.1 A | 24.0 B | 121.4 c | 36.7 C | 5.13 A | 1.54 A | 3.36 B |
| Drought alone | 266.7 C | 29.2 B | 211.6 B | 32.5 A | 162.7 A | 42.2 B | 5.04 B | 1.30 B | 3.98 A |
| Flooding alone | 272.7 B | 24.9 D | 178.5 C | 19.5 C | 89.2 D | 29.4 D | 4.57 C | 1.55 A | 2.89 C |
| Traits a | Grain Yield | Total N | Total P | Total K | SAR-N | SAR-P | SAR-K | NUE | PUE | KUE |
|---|---|---|---|---|---|---|---|---|---|---|
| Grain Yield | 1.000 | 0.268 | 0.397 | 0.423 * | 0.108 | 0.240 | 0.348 | 0.926 ** | 0.826 ** | 0.801 ** |
| Stem DW | −0.856 ** | 0.056 | −0.154 | −0.352 | −0.046 | −0.138 | −0.300 | −0.860 ** | −0.792 ** | −0.847 ** |
| Leaf DW | −0.623 ** | 0.226 | 0.351 | −0.226 | 0.200 | 0.345 | −0.024 | −0.617 ** | −0.822 ** | −0.622 ** |
| Root DW | 0.058 | −0.130 | 0.031 | −0.087 | −0.894 ** | −0.704 ** | −0.720 ** | −0.156 | −0.234 | −0.222 |
| RLD | −0.254 | 0.303 | 0.459 * | 0.307 | −0.700 ** | −0.734 ** | −0.620 ** | 0.378 * | 0.437 * | 0.232 |
| SRL | −0.222 | 0.263 | 0.089 | 0.342 | 0.098 | 0.011 | 0.129 | 0.278 | 0.387 * | 0.277 |
| RMD | −0.139 | −0.507 ** | −0.403 * | −0.453 * | −0.038 | −0.143 | −0.123 | −0.380 * | −0.542 ** | −0.383 * |
| RSA | 0.180 | 0.203 | 0.232 | 0.110 | −0.676 ** | −0.702 ** | −0.592 ** | 0.287 | 0.251 | 0.305 |
| Max. root depth | 0.771 ** | 0.391 * | 0.568 ** | 0.566 ** | −0.140 | 0.017 | 0.234 | 0.483 ** | 0.530 ** | 0.202 |
| Adventitious roots | 0.048 | −0.325 | −0.022 | −0.055 | −0.075 | −0.260 | −0.085 | −0.158 | −0.253 | −0.277 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Wu, F.; Hu, T.; Liu, L.; Wang, J.; Wang, X.; Liang, C.; Liu, J. Interactive Effects of Drought–Flood Abrupt Alternation on Morpho-Agronomic and Nutrient Use Traits in Rice. Agronomy 2021, 11, 2103. https://doi.org/10.3390/agronomy11112103
Huang J, Wu F, Hu T, Liu L, Wang J, Wang X, Liang C, Liu J. Interactive Effects of Drought–Flood Abrupt Alternation on Morpho-Agronomic and Nutrient Use Traits in Rice. Agronomy. 2021; 11(11):2103. https://doi.org/10.3390/agronomy11112103
Chicago/Turabian StyleHuang, Jie, Fengyan Wu, Tiesong Hu, Luguang Liu, Jing Wang, Xin Wang, Changmei Liang, and Jia Liu. 2021. "Interactive Effects of Drought–Flood Abrupt Alternation on Morpho-Agronomic and Nutrient Use Traits in Rice" Agronomy 11, no. 11: 2103. https://doi.org/10.3390/agronomy11112103
APA StyleHuang, J., Wu, F., Hu, T., Liu, L., Wang, J., Wang, X., Liang, C., & Liu, J. (2021). Interactive Effects of Drought–Flood Abrupt Alternation on Morpho-Agronomic and Nutrient Use Traits in Rice. Agronomy, 11(11), 2103. https://doi.org/10.3390/agronomy11112103







