The Role of Water Content in the Casing Layer for Mushroom Crop Production and the Occurrence of Fungal Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Agronomic Evaluation of the Crop
2.2. Evaluation of the Disease Incidence
2.3. Statistical Analyses
3. Results
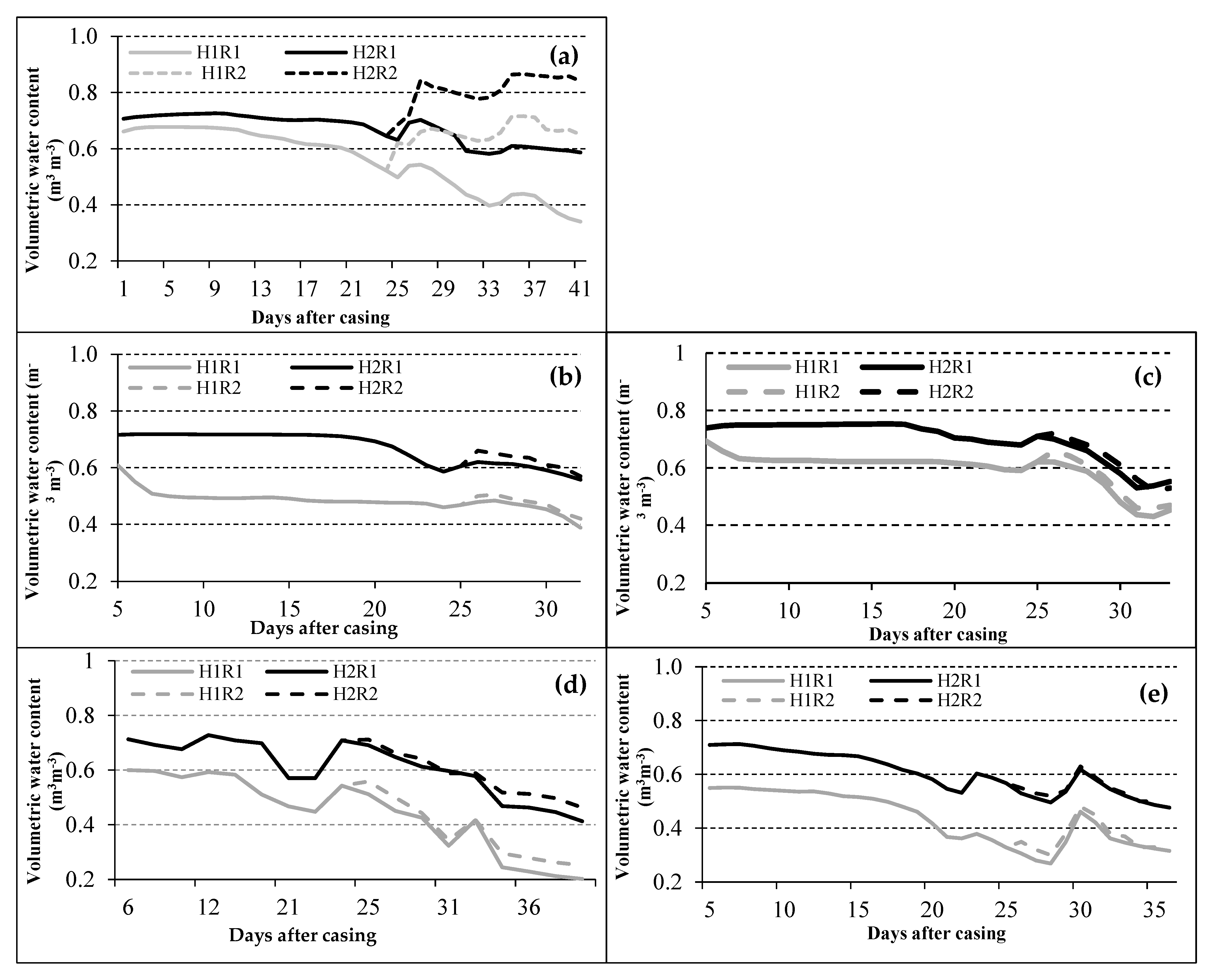
3.1. Agronomic Evaluation of the Crop
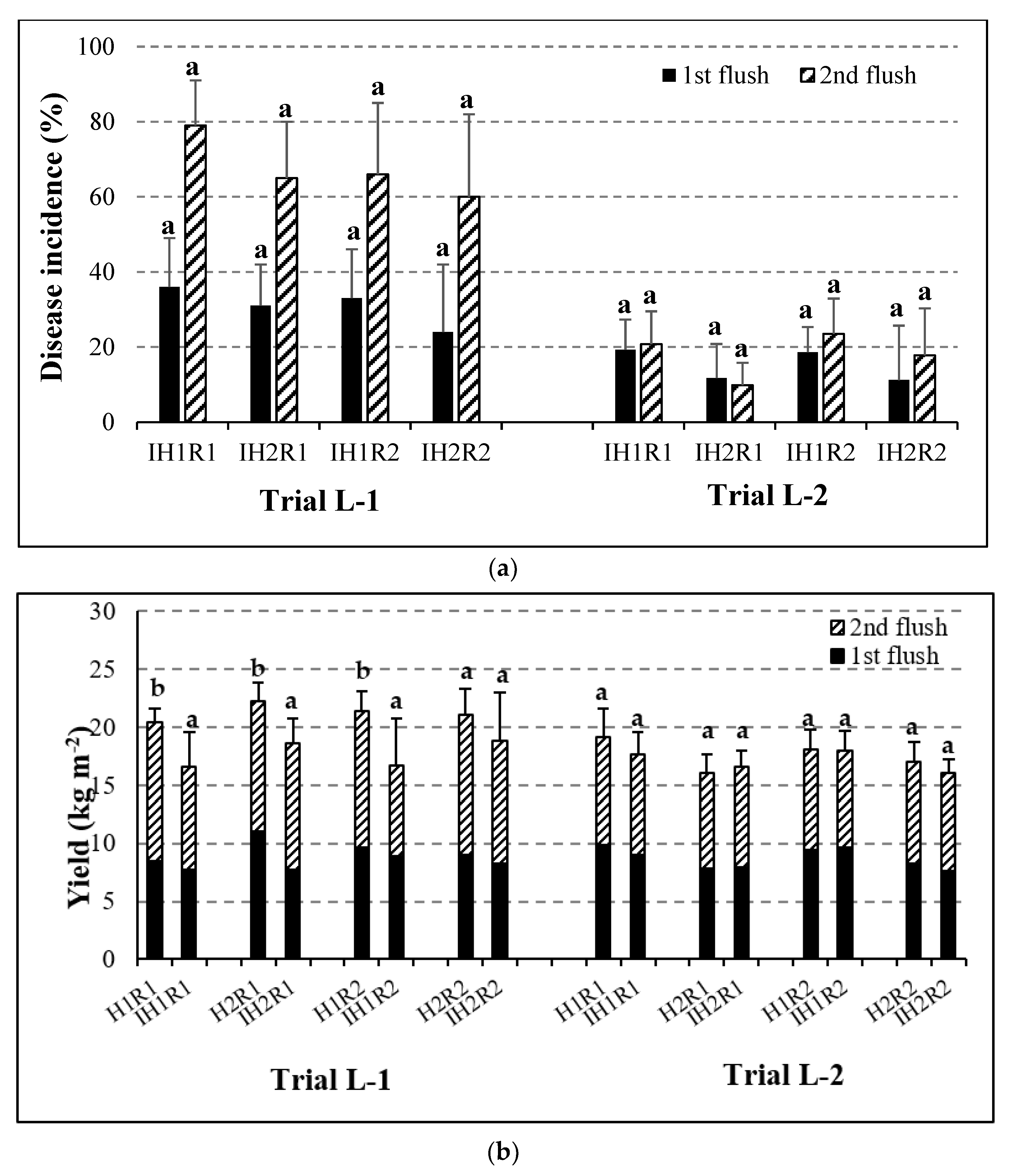
3.2. Evaluation of the Dry Bubble Disease Incidence
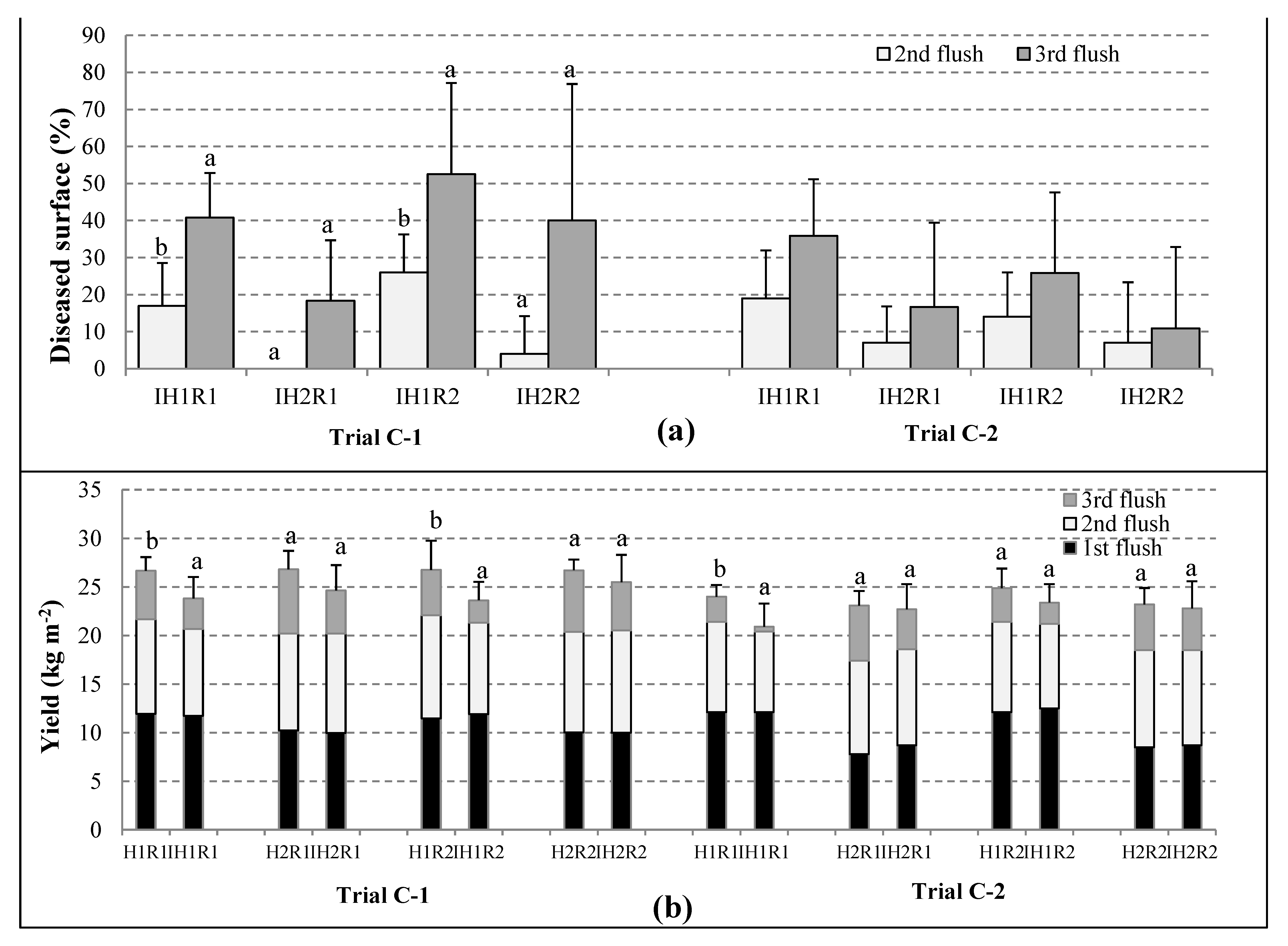
3.3. Evaluation of the Cobweb Disease Incidence
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pardo-Giménez, A.; Pardo, J.E.; Zied, D.C. Casing materials and techniques in Agaricus bisporus cultivation. In Edible and Medicinal Mushrooms: Technology and Applications; Zied, D.C., Pardo-Giménez, A., Eds.; Wiley-Blackwell: Chichester, UK, 2017; pp. 385–413. [Google Scholar]
- Pardo, A.; Pardo, J.E.; de Juan, J.A.; Zied, D.C. Modelling the effect of the physical and chemical characteristics of the materials used as casing layers on the production parameters of Agaricus bisporus. Arch. Microbiol. 2010, 192, 1023–1030. [Google Scholar] [CrossRef]
- Vieira, F.R.; Pecchia, J.A. An exploration into the bacterial community under different pasteurization conditions during substrate preparation (composting-Phase II) for Agaricus bisporus cultivation. Microb. Ecol. 2018, 75, 318–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGee, C.F. Microbial ecology of the Agaricus bisporus mushroom cropping process. Appl. Microbiol. Biotechnol. 2018, 102, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Siyoum, N.A.; Surridge, K.; van der Linde, E.J.; Korsten, L. Microbial succession in white button mushroom production systems from compost and casing to a marketable packed product. Ann. Microbiol. 2016, 66, 151–164. [Google Scholar] [CrossRef]
- Carrasco, J.; Tello, M.L.; de Toro, M.; Tkacz, A.; Poole, P.; Pérez-Clavijo, M. Casing microbiome dynamics during button mushroom cultivation: Implications for dry and wet bubble diseases. Microbiology 2019, 165, 611–624. [Google Scholar] [CrossRef]
- Carrasco, J.; García-Delgado, C.; Lavega, R.; Tello, M.L.; De Toro, M.; Barba-Vicente, V.; Rodr ıguez-Cruz, M.S.; Sanchez-Martın, M.-J.; Perez, M.; Preston, G.M. Holistic assessment of the microbiome dynamics in the substrates used for commercial champignon (Agaricus bisporus) cultivation. Microb. Biotechnol. 2020, 13, 1933–1947. [Google Scholar] [CrossRef]
- Colauto, N.B.; Fermor, T.R.; Eira, A.F.; Linde, G.A. Pseudomonas putida stimulates primordia on Agaricus bitorquis. Curr. Microbiol. 2016, 72, 482–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berendsen, R.L.; Kalkhove, S.I.; Lugones, L.G.; Wösten, H.A.; Bakker, P.A. Germination of Lecanicillium fungicola in the mycosphere of Agaricus bisporus. Environ. Microbiol. Rep. 2012, 4, 227–233. [Google Scholar] [CrossRef]
- Largeteau, M.L.; Savoie, J.M. Microbially induced diseases of Agaricusbisporus: Biochemical mechanisms and impact on commercial mushroom production. Appl. Microbiol. Biotechnol. 2010, 86, 63–73. [Google Scholar] [CrossRef]
- Gea, F.J.; Carrasco, J.; Santos, M.; Diánez, F.; Navarro, M.J. Incidence of Lecanicillium fungicola in white-button mushroom (Agaricus bisporus) cultivated with two types of casing soil. J. Plant Pathol. 2013, 95, 161–164. [Google Scholar] [CrossRef]
- Carrasco, J.; Navarro, M.J.; Santos, M.; Diánez, F.; Gea, F.J. Incidence, identification and pathogenicity of Cladobotryum mycophilum, causal agent of cobweb disease on Agaricus bisporus mushroom crops in Spain. Ann. Appl. Biol. 2016, 168, 214–224. [Google Scholar] [CrossRef]
- Ekardt, F.; Jacobs, B.; Stubenrauch, J.; Garske, B. Peatland governance: The problem of depicting in sustainability governance, regulatory law, and economic instruments. Land 2020, 9, 83. [Google Scholar] [CrossRef] [Green Version]
- Royse, D.J.; Beelman, R.B. Six Steps to Mushroom Farming; The Pennsylvania State University, College of Agricultural Sciences: University Park, PA, USA, 2007; Available online: https://www.mushroomcompany.com/resources/agaricus/SixSteps.pdf (accessed on 13 October 2021).
- Fletcher, J.T.; Gaze, R.H. Mushroom Pest and Disease Control; Manson Publishing: London, UK, 2008; 192p. [Google Scholar]
- Noble, R.; Dobrovin-Pennington, A.; Evered, C.E.; Mead, A. Properties of peat-based casing soils and their influence on the water relations and growth of the mushroom (Agaricus bisporus). Plant Soil 1999, 207, 1–13. [Google Scholar] [CrossRef]
- Barry, J.; Doyle, O.; Grant, J.; Grogan, H. Influence of irrigation management on the quantity and quality of Agaricus bisporus produced on spent mushroom substrate (SMS) based casings. Acta Hortic. 2016, 1112, 299–306. [Google Scholar] [CrossRef]
- Navarro, M.J.; Gea, F.J.; Pardo-Giménez, A.; Martínez, A.; Raz, D.; Levanon, D.; Dannay, O. Agronomical valuation of a drip irrigation system in a comercial mushroom farm. Sci. Hortic. 2020, 265, 109234. [Google Scholar] [CrossRef]
- Beecher, T.M.; Burton, K.S.; Magan, N. Osmotic/matric potential affects mycelial growth and endogenous reserves in Agaricus bisporus. Mushroom Sci. 2000, 15, 455–462. [Google Scholar]
- Vernooij, E. Casing, ‘The skin of the substrate’: Preparation and management of the casing layer. Mushroom Sci. 2008, 17, 875–913. [Google Scholar]
- Saxon, E.B.; Jackson, R.W.; Bhumbra, S.; Smith, T.; Sockett, R.E. Bdellovibriobacteriovorus HD100 guards against Pseudomonas tolaasii brown-blotch lesions on the surface of post-harvest Agaricus bisporus supermarket mushrooms. BMC Microbiol. 2014, 14, 163. [Google Scholar] [CrossRef] [Green Version]
- Navarro, M.J.; Gea, F.J.; González, A.J. Identification, incidence and control of bacterial blotch disease in mushroom crops by management of environmental conditions. Sci. Hortic. 2018, 229, 10–18. [Google Scholar] [CrossRef]
- Reyes, J.E.; Venturini, M.E.; Oria, R.; Blanco, D. Prevalence of Ewingella americana in retail fresh cultivated mushrooms (Agaricus bisporus, Lentinula edodes and Pleurotus ostreatus) in Zaragoza (Spain). FEMS Microbiol. Ecol. 2004, 47, 291–296. [Google Scholar] [CrossRef] [Green Version]
- González, A.J.; Gea, F.J.; Navarro, M.J.; Fernández, A.M. Identification and RAPD-typing of Ewingella americana on cultivated mushrooms in Castilla-La Mancha, Spain. Eur. J. Plant Pathol. 2012, 133, 517–522. [Google Scholar] [CrossRef]
- Fletcher, J.T.; Allan, J.; Seymour, G.K. Managing Cobweb Disease in Australia. Mushroom Sci. 2004, 15, 711–715. [Google Scholar]
- Happ, A.C.; Wuest, P.J. Mushroom yield and incidence of Verticillium disease as influenced by the choice of casing and its treatment with steam. Mushroom Sci. 1978, 10, 303–310. [Google Scholar]
- Pasha, A.Y.; Khoshghalb, A.; Khalili, N. Pitfalls in interpretation of gravimetric water content–based soil-water characteristic curve for deformable porous media. Int. J. Geomech. 2016, 16, D4015004. [Google Scholar] [CrossRef]
- Gea, F.J.; Tello, J.C.; Navarro, M.J. Efficacy and effects on yield of different fungicides for control of wet bubble disease of mushroom caused by the mycoparasite Mycogone perniciosa. Crop Prot. 2010, 29, 1021–1025. [Google Scholar] [CrossRef]
- Pyck, N.; Grogan, H. Fungal Diseases of Mushrooms and Their Control; Factsheet 04/15; Grogan, H., Ed.; MushTV Project: Teagasc, Dublin, 2015; 6p, Available online: https://www.teagasc.ie/media/website/publications/2015/Fungal-diseases-of-mushrooms-and-their-control.pdf (accessed on 13 October 2021).
- Herman, K.C.; Wösten., H.A.; Fricker, M.D.; Bleichrodt, R.J. Growth induced translocation effectively directs an amino acid analogue to developing zones in Agaricus bisporus. Fungal. Biol. 2020, 124, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.; Doyle, O.; Grant, J.; Grogan, H. Influence of rate and grading of sugar beet lime (SBL) waste on the properties and yield potential of a peatbased mushroom casing. Acta Hortic. 2016, 1112, 307–314. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Baars, J.J.P.; Kalkhove, S.I.C.; Lugones, L.G.; Wösten, H.A.B.; Bakker, P.A.H.M. Lecanicillium fungicola: Causal agent of dry bubble disease in white-button mushroom. Mol. Plant Pathol. 2010, 11, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced materials from fungal mycelium: Fabrication and tuning of physical properties. Sci. Rep. 2017, 7, 41292. [Google Scholar] [CrossRef] [PubMed]
- Feofilova, E.P. The fungal cell wall: Modern concepts of its composition and biological function. Microbiology 2010, 79, 711–720. [Google Scholar] [CrossRef]
- Yoshida, S.; Koitabashi, M.; Nakamura, J.; Fukuoka, T.; Sakai, H.; Abe, M.; Kitamoto, H. Effects of biosurfactants, mannosylerythritol lipids, on the hydrophobicity of solid surfaces and infection behaviours of plant pathogenic fungi. J. Appl. Microbiol. 2015, 119, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Appels, F.V.; van den Brandhof, J.G.; Dijksterhuis, J.; de Kort, G.W.; Wösten, H.A. Fungal mycelium classified in different material families based on glycerol treatment. Commun. Biol. 2020, 3, 334. [Google Scholar] [CrossRef] [PubMed]
- Calonje, M.; Mendoza, C.G.; Cabo, A.P.; Bernardo, D.; Novaes- Ledieu, M. Interaction between the mycoparasite Verticillium fungicola and the vegetative mycelial phase of Agaricus bisporus. Mycol. Res. 2000, 104, 988–992. [Google Scholar] [CrossRef]
- Shamshad, A.; Clift, A.D.; Mansfield, S. Host–parasite interaction between cultivated mushroom Agaricus bisporus, hybrid strain Sylvan A15, and the mycoparasite Verticillium fungicola, a causal agent of dry bubble disease. Australas. Plant Pathol. 2009, 38, 74–78. [Google Scholar] [CrossRef]
- Calonje, M.; Garcia Mendoza, C.; Galan, B.; Novaes-Ledieu, M. Enzymic activity of the mycoparasite Verticillium fungicola on Agaricus bisporus fruit body cell walls. Microbiology 1997, 143, 2999–3006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Largeteau, M.L.; Rodier, A.; Rousseau, T.; Juarez del Carmen, S.; Védie, R.; Savoie, J.M. Agaricus susceptibility to Verticillium fungicola. Mushroom Sci. 2004, 16, 504–522. [Google Scholar]
- Carrasco, J.; Preston, G. Growing edible mushrooms: A conversation between bacteria and fungi. Environ. Microbiol. 2020, 22, 858–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noble, R.; Goodchild, M.; Jenkins, D.; Burton, K.; Walker, D.; Moorhouse, E. Precision irrigation of mushroom using a novel dielectric tensiometer. Mushroom News 2017, 65, 8–17. [Google Scholar]
| Trial | Initial Water Content (m3m−3) | Irrigation F1 * (L m−2) | Irrigation F2 ¥ (L m−2) | ||
|---|---|---|---|---|---|
| H1 | H2 | R1 | R2 | R1 and R2 | |
| A | 0.66 | 0.71 | 3.3 | 3.3 + 1.4 | 2.8 |
| L-1 | 0.62 | 0.71 | 3.3 + 0.7 | 3.3 + 2.0 | - |
| L-2 | 0.69 | 0.74 | 3.3 + 0.7 | 3.3 + 2.0 | - |
| C-1 | 0.60 | 0.71 | 3.3 + 0.7 | 3.3 + 2.0 | 2.7 |
| C-2 | 0.55 | 0.71 | 3.3 + 0.7 | 3.3 + 2.0 | 3.7 |
| Treatment | Yield (kg m−2) | Basidiomes | Dry Matter (%) | ||||
|---|---|---|---|---|---|---|---|
| Total | Ø > 40mm | Ø < 40mm | (nº m−2) | 1st Flush | 2nd Flush | 3rd Flush | |
| H1R1 | 19.4 ± 1.6 a | 12.70 ± 2.3 a | 6.7 ± 1.7 b | 136 ± 13.9 b | 9.8 ± 0.5 a | 9.0 ± 0.3 ab | 9.7 ± 1.0 a |
| H2R1 | 21.4 ± 1.0 b | 17.5 ± 2.2 b | 3.8 ± 2.1 a | 113.6 ± 25.0 a | 10.3 ± 0.7 a | 9.5 ± 0.9 b | 9.1 ± 0.9 a |
| H1R2 | 20.3 ±1.5 ab | 15.3 ± 1.3 ab | 5.0 ±1.3 ab | 126.5 ±15.3 ab | 10.3 ± 0.2 a | 8.5 ± 0.4 a | 9.7 ± 0.9 a |
| H2R2 | 23.5 ± 1.6 c | 20.7 ± 2.3 c | 2.9 ± 1.9 a | 114.1 ± 14.2 a | 10.4 ± 0.8 a | 8.9 ± 0.4 ab | 8.8 ± 0.5 a |
| F3.31 | 12.04 | 20.92 | 7.08 | 2.96 | 0.71 | 2.14 | 1.20 |
| P | 0.0000 | 0.0000 | 0.0011 | 0.0496 | 0.5662 | 0.1480 | 0.3515 |
| SED | 0.510517 | 0.738002 | 0.623188 | 6.26 | 0.3 | 0.3 | 0.4 |
| LSD | 1.47891 | 2.85018 | 2.40676 | 18.13 | 0.9 | 0.9 | 1.3 |
| Treatment | 1st Flush | 2nd Flush | 3rd Flush | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L * | b * | AE * | L * | b * | AE * | L * | b * | AE * | |
| H1R1 | 94.4 a ‡ | 9.0 a | 9.5 a | 94.4 a | 9.9 a | 10.3 b | 92.1 a | 10.7 a | 12.1 a |
| H2R1 | 94.7 a | 9.0 a | 9.4 a | 94.7 ab | 9.0 a | 9.4 ab | 93.5 a | 9.3 a | 10.2 a |
| H1R2 | 94.5 a | 8.9 a | 9.4 a | 95.12 b | 9.8 a | 10.0 ab | 92.9 a | 10.4 a | 11.3 a |
| H2R2 | 94.6 a | 8.8 a | 9.2 a | 94.8 ab | 8.9 a | 9.3 a | 92.0 a | 10.2 a | 12.1 a |
| F3.15 | 0.39 | 0.33 | 0.43 | 2.60 | 2.6 | 2.58 | 0.40 | 1.62 | 0.96 |
| p | 0.7594 | 0.8033 | 0.7384 | 0.1002 | 0.1005 | 0.1018 | 0.7527 | 0.2402 | 0.4455 |
| SED | 0.21 | 0.17 | 0.19 | 0.19 | 0.30 | 0.30 | 1.17 | 0.45 | 0.88 |
| LSD | 0.66 | 0.53 | 0.57 | 0.59 | 0.94 | 0.94 | 3.61 | 1.40 | 2.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, M.J.; Carrasco, J.; Gea, F.J. The Role of Water Content in the Casing Layer for Mushroom Crop Production and the Occurrence of Fungal Diseases. Agronomy 2021, 11, 2063. https://doi.org/10.3390/agronomy11102063
Navarro MJ, Carrasco J, Gea FJ. The Role of Water Content in the Casing Layer for Mushroom Crop Production and the Occurrence of Fungal Diseases. Agronomy. 2021; 11(10):2063. https://doi.org/10.3390/agronomy11102063
Chicago/Turabian StyleNavarro, María J., Jaime Carrasco, and Francisco J. Gea. 2021. "The Role of Water Content in the Casing Layer for Mushroom Crop Production and the Occurrence of Fungal Diseases" Agronomy 11, no. 10: 2063. https://doi.org/10.3390/agronomy11102063
APA StyleNavarro, M. J., Carrasco, J., & Gea, F. J. (2021). The Role of Water Content in the Casing Layer for Mushroom Crop Production and the Occurrence of Fungal Diseases. Agronomy, 11(10), 2063. https://doi.org/10.3390/agronomy11102063







