Testing Combined Effect of Amendments and Inoculation with Bacteria for Improving Phytostabilisation of Smelter Waste Extremely Contaminated with Trace Elements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Smelter Waste Material
2.2. Bacterials Strains
2.3. Experimental Setup
2.4. Soil Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Microbiological and Biochemical Indices
3.2. Soil Chemical Parameters and Metal Extractability
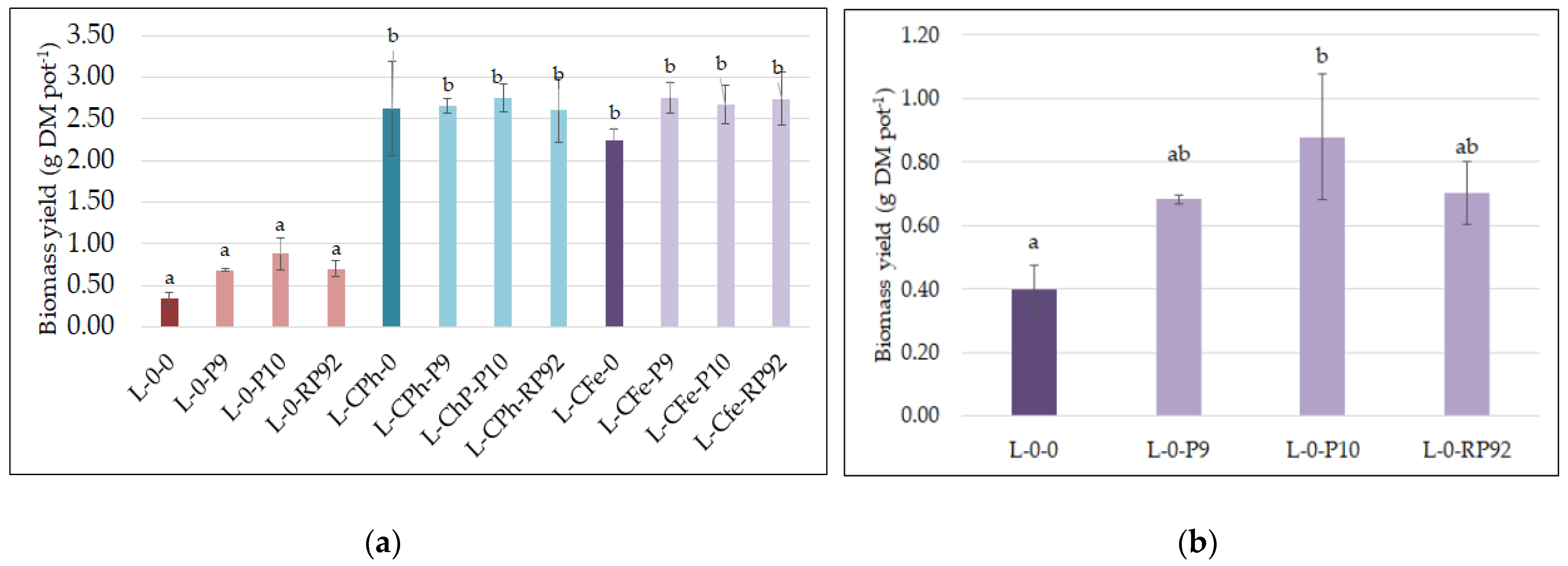
3.3. Plant Growth
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Williamson, A.; Johnson, M.S. Reclamation of metalliferous mine wastes. In Effect of Heavy Metal Pollution on Plants; Applied Sciences Publishing: London, UK, 1981; Volume 2, pp. 185–212. [Google Scholar]
- Patrzalek, A.; Strzyszcz, Z. Field studies on revegetation of tailings. Arch. Environ. Prot. 1980, 3–4, 165–180. (In Polish) [Google Scholar]
- Stuczyński, T.; Siebielec, G.; Daniels, W.; McCarty, G.; Chaney, R. Biological aspects of metal waste reclamation with biosolids. J. Environ. Qual. 2007, 36, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Alvarenga, P.; Gonçalves, A.P.; Fernandes, R.M.; de Varennes, A.; Duarte, E.; Cunha-Queda, A.C.; Vallini, G. Reclamation of a mine contaminated soil using biologically reactive organic matrices. Waste Manag. Res. 2009, 27, 101–111. [Google Scholar] [CrossRef]
- Siebielec, S.; Siebielec, G.; Stuczynski, T.; Sugier, P.; Grzeda, E.; Grzadziel, J. Long term insight into biodiversity of a smelter wasteland reclaimed with biosolids and by-product lime. Sci. Total Environ. 2018, 636, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Siebielec, S.; Siebielec, G.; Sugier, P.; Wożniak, M.; Grzadziel, J.; Gałązka, A.; Stuczyński, T. Activity and diversity of microorganisms in root zone of plant species spontaneously inhabiting smelter waste piles. Molecules 2020, 25, 5638. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Kang, H.B.; Zhang, X.Y.; Shao, H.B.; Chu, L.Y.; Ruan, C.J. A critical review on the bio-removal of hazardous heavy metals from contaminated soils: Issues, progress, eco-environmental concerns and opportunities. J. Hazard. Mater. 2010, 174, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Castro, C.; Prieto-Fernández, Á.; Kidd, P.S.; Weyens, N.; Rodríguez-Garrido, B.; Touceda-González, M.; Acea, M.J.; Vangronsveld, J. Improving performance of Cytisus striatus on substrates contaminated with hexachlorocyclohexane (HCH) isomers using bacterial inoculants: Developing a phytoremediation strategy. Plant Soil 2013, 362, 247–260. [Google Scholar] [CrossRef]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef]
- Haslmayr, H.P.; Meißner, S.; Langella, F.; Baumgarten, A.; Geletneky, J. Establishing best practice for microbially aided phytoremediation. Environ. Sci. Pollut. Res. 2014, 21, 6765–6774. [Google Scholar] [CrossRef] [PubMed]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Newman, L.; Vangronsveld, J. Exploiting plant–microbe partnerships to improve biomass production and remediation. Trends Biotechnol. 2009, 27, 591–598. [Google Scholar] [CrossRef]
- Siebielec, S.; Siebielec, G.; Urbaniak, M.; Smreczak, B.; Grzęda, E.; Wyrwicka, A.; Kidd, P. Impact of rhizobacterial inoculants on plant growth and enzyme activities in soil treated with contaminated bottom sediments. Int. J. Phytoremediat. 2019, 21, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Sessitsch, A.; Kuffner, M.; Kidd, P.S.; Vangronsveld, J.; Wenzel, W.W.; Fallmann, K.; Puschenreiter, M. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol. Biochem. 2013, 60, 182–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidd, P.S.; Álvarez-López, V.; Becerra-Castro, C.; Cabello-Conejo, M.; Prieto-Fernández, Á. Potential role of plant-associated bacteria in plant metal uptake and implications in phytotechnologies. In Phytoremediation; Elsevier Ltd.: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2017; pp. 87–126. ISBN 9780128028537. [Google Scholar]
- Vurukonda, S.S.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Balseiro-Romero, M.; Gkorezis, P.; Kidd, P.S.; Vangronsveld, J.; Monterroso, C. Enhanced degradation of diesel in the rhizosphere of Lupinus luteus after inoculation with diesel-degrading and PGP bacterial strains. J. Environ. Qual. 2016, 45, 924–932. [Google Scholar] [CrossRef]
- Mergeay, M.; Nies, D.; Schlegel, H.G.; Gerits, J.; Charles, P.; Van Gijsegem, F. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J. Bacteriol. 1985, 162, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Hettiarachchi, G.M.; Pierzynski, G.M.; Ransom, M.D. In situ stabilization of soil lead using phosphorus and manganese oxide. Environ. Sci. Technol. 2000, 34, 4614–4619. [Google Scholar] [CrossRef]
- Bruemmer, G.W.; Gerth, J.; Tiller, K.G. Reaction kinetics of the adsorption and desorption of nickel, zinc, and cadmium by goethite. I. Adsorption and diffusion of metals. J. Soil Sci. 1988, 39, 37–52. [Google Scholar] [CrossRef]
- Li, Y.-M.; Chaney, R.L.; Siebielec, G.; Kershner, B.A. Response of four turfgrass cultivars to limestone and biosolids compost amendment of a zinc and cadmium contaminated soil at Palmerton, PA. J. Environ. Qual. 2000, 29, 1440–1447. [Google Scholar] [CrossRef]
- Siebielec, G.; Chaney, R.L. Testing amendments for remediation of military range contaminated soil. J. Environ. Manag. 2012, 108, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Yandigeri, M.S.; Meena, K.K.; Singh, D.; Malviya, N.; Singh, D.P.; Solanki, M.K.; Yadav, A.K.; Arora, D.K. Drought-tolerant endophytic actinobacteria promote growth of wheat (Triticum aestivum) under water stress conditions. Plant Growth Regul. 2012, 68, 411–420. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil Enzymes. In Methods of Soil Analysis. Part 2. Microbiological and Biochemical Properties; SSSA Book Series 5; SSSA: Madison, WI, USA, 1994; pp. 775–826. [Google Scholar]
- Casida, L.E.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenylphosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Wallace, R.H.; Lochhead, A.G. Qualitative studies of soil microorganisms: IX. Amino acid requirements of rhizosphere bacteria. Can. J. Res. 1950, 28, 1–6. [Google Scholar] [CrossRef]
- Fenglerowa, W. Simple method for counting Azotobacter in soil samples. Acta Microbiol. Pol. 1965, 14, 203–206. [Google Scholar]
- Martin, J.P. Use of acid, rose bengal, and streptomycin in the plate method for estimating soil fungi. Soil Sci. 1950, 69, 215–232. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Tiemann, L.K.; Billings, S.A. Indirect effects of nitrogen amendments on organic substrate quality increase enzymatic activity driving decomposition in a mesic grassland. Ecosystems 2011, 14, 234–247. [Google Scholar] [CrossRef]
- Moeskops, B.; Buchan, D.; Sleutel, S.; Herawaty, L.; Husen, E.; Saraswati, R.; Setyorini, D.; De Neve, S. Soil microbial communities and activities under intensive organic and conventional vegetable farming in West Java, Indonesia. Appl. Soil Ecol. 2010, 45, 112–120. [Google Scholar] [CrossRef]
- Kaczyńska, G.; Borowik, A.; Wyszkowska, J. Soil dehydrogenases as an indicator of contamination of the environment with petroleum products. Water Air Soil Pollut. 2015, 226, 372. [Google Scholar] [CrossRef] [Green Version]
- Nannipieri, P.; Grego, S.; Ceccanti, B. Ecological significance of the biological activity in soil. In Soil Biochemistry; Marcel Dekker: New York, NY, USA, 1990; Volume 6, pp. 293–355. [Google Scholar]
- Khan, A.G. Role of soil microbes in the rhizospheres of plants growing on trace metal contaminated soils in phytoremediation. J. Trace Elem. Med. Biol. 2005, 18, 355–364. [Google Scholar] [CrossRef]
- Kumari, S.; Chourasia, S.K.; Singh, U.; Kant, R. Azotobacter: Its role in sustainable agriculture. New Agric. 2017, 28, 485–492. [Google Scholar]
- Lenart, A. Occurrence, characteristics, and genetic diversity of Azotobacter chroococcum in various soils of southern Poland. Pol. J. Environ. Stud. 2012, 21, 415–424. [Google Scholar]
- Billah, M.; Khan, M.; Bano, A.; Hassan, T.U.; Munir, A.; Gurmani, A.R. Phosphorus and phosphate solubilizing bacteria: Keys for sustainable agriculture. Geomicrobiol. J. 2019, 36, 904–916. [Google Scholar] [CrossRef]
- Maharajan, T.; Ceasar, S.A.; Ajeesh Krishna, T.P.; Ramakrishnan, M.; Duraipandiyan, V.; Naif Abdulla, A.D.; Ignacimuthu, S. Utilization of molecular markers for improving the phosphorus efficiency in crop plants. Plant Breed. 2018, 137, 10–26. [Google Scholar] [CrossRef] [Green Version]
- Walpola, B.C.; Yoon, M. Prospectus of phosphate solubilizing microorganisms and phosphorus availability in agricultural soils: A review. Afr. J. Microbiol. Res. 2012, 6, 6600–6605. [Google Scholar]
- Kucey, R.M.N. Phosphate solubilizing bacteria and fungi in various cultivated and fungi in various cultivated and virgin Alberta soils. Can. J. Soil Sci. 1983, 63, 671–678. [Google Scholar] [CrossRef]
- Acosta-Martinez, V.; Down, S.; Sun, Y.; Allen, V. Tag-encoded pyrosequencing analysis of bacterial diversity in a single soil type as affected by management and land use. Soil Biol. Biochem. 2008, 40, 2762–2770. [Google Scholar] [CrossRef]
- Susilowati, L.; Syekhfani, E. Characterization of phosphate solubilizing bacteria isolated from Pb contaminated soils and their potential for dissolving tricalcium phosphate. J. Degrade. Min. Land Manag. 2014, 1, 57–62. [Google Scholar]
- Zinati, G.M.; Li, Y.C.; Bryan, H.H. Utilization of compost increases organic carbon and its humin, humic and fulvic acid fractions in calcareous soil. Compost Sci. Util. 2001, 9, 156–162. [Google Scholar] [CrossRef]
- Siebielec, G.; Smreczak, B.; Klimkowicz-Pawlas, A.; Maliszewska-Kordybach, B.; Terelak, H.; Koza, P.; Łysiak, M.; Gałazka, R.; Pecio, M.; Suszek, B.; et al. Monitoring of Soil Chemical Quality of Agricultural Land in Poland in 2010–2012; Biblioteka Monitoringu Środowiska: Warszawa, Poland, 2012. (In Polish) [Google Scholar]
- Metson, G.S.; Bennett, E.M. Phosphorus cycling in Montreal’s food and urban agriculture systems. PLoS ONE 2015, 10, e0120726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackay, J.E.; Cavagnaro, T.R.; Jakobsen, I.; Macdonald, L.M.; Grønlund, M.; Thomsen, T.P.; Müller-Stover, D.S. Evaluation of phosphorus in thermally converted sewage sludge: P pools and availability to wheat. Plant Soil 2017, 418, 307–317. [Google Scholar] [CrossRef]
- Kumpiene, J.; Bert, V.; Dimitriou, I.; Eriksson, J.; Friesl-Hanl, W.; Galazka, R.; Herzig, R.; Janssen, J.; Kidd, P.; Menchj, M.; et al. Selecting chemical and ecotoxicological test batteries for risk assessment of trace element-contaminated soils (phyto)managed by gentle remediation options (GRO). Sci. Total. Environ. 2014, 496, 510–522. [Google Scholar] [CrossRef]
- Prueß, A. Action values for mobile (NH4NO3-extractable) trace elements in soils based on the German national standard DIN 19730. In Proceedings of the Third International Conference on Biogeochemistry of Trace Elements, Paris, France, 15–19 May 1995. [Google Scholar]
- Rodríguez-Llorente, I.D.; Gamane, D.; Lafuente, A.; Dary, M.; El Hamdaoui, A.; Delgadillo, J.; Doukkali, B.; Caviedes, M.A.; Pajuelo, E. Cadmium biosorption properties of the metal-resistant Ochrobactrum cytisi Azn6.2. Eng. Life Sci. 2010, 10, 49–56. [Google Scholar] [CrossRef]
- Clemente, R.; Walker, D.J.; Pilar Bernal, M. Uptake of heavy metals and As by Brassica juncea grown in a contaminated soil in Aznalcollar (Spain): The effect of soil amendments. Environ. Pollut. 2005, 138, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Dary, M.; Chamber-Pérez, M.A.; Palomares, A.J.; Pajuelo, E. “In situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J. Hazard. Mater. 2010, 177, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, X.; He, X.; Lü, Q.; Qian, X.; Xiao, Q.; Lin, R. Effects of Pseudomonas TCd-1 on rice (Oryza sativa) cadmium uptake, rhizosphere soils enzyme activities and cadmium bioavailability under cadmium contamination. Ecotoxicol. Environ. Saf. 2021, 218, 112249. [Google Scholar] [CrossRef]
- Praburaman, L.; Park, S.H.; Cho, M.; Lee, K.J.; Ko, J.A.; Han, S.S.; Lee, S.H.; Kamala-Kannan, S.; Oh, B.T. Significance of diazotrophic plant growth-promoting Herbaspirillum sp. GW103 on phytoextraction of Pb and Zn by Zea mays L. Environ. Sci. Pollut. Res. Int. 2017, 24, 3172–3180. [Google Scholar] [CrossRef]
- Jeyasundar, P.G.S.A.; Ali, A.; Azeem, M.; Li, Y.; Guo, D.; Sikdar, A.; Abdelrahman, H.; Kwon, E.; Antoniadis, V.; Mani, V.M.; et al. Green remediation of toxic metals contaminated mining soil using bacterial consortium and Brassica juncea. Environ. Pollut. 2021, 277, 116789. [Google Scholar] [CrossRef]
- Zappelini, C.; Alvarez-Lopez, V.; Capelli, N.; Guyeux, C.; Chalot, M. Streptomyces dominate the soil under Betula trees that have naturally colonized a red gypsum landfill. Front. Microbiol. 2018, 9, 1772. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, S. Identification and characterization of the phosphate-solubilizing bacterium Pantoea sp. S32 in reclamation soil in Shanxi, China. Front. Microbiol. 2019, 10, 2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| ID | Plant | Amendment | Bacteria Strain |
|---|---|---|---|
| L-0-0 | L. multiflorum | no amendment | no inoculation |
| L-0-P9 | L. multiflorum | no amendment | P9 |
| L-0-P10 | L. multiflorum | no amendment | P10 |
| L-0-RP92 | L. multiflorum | no amendment | RP92 |
| L-CPh-0 | L. multiflorum | compost plus Ca-phosphate | no inoculation |
| L-CPh-P9 | L. multiflorum | compost plus Ca-phosphate | P9 |
| L-CPh-P10 | L. multiflorum | compost plus Ca-phosphate | P10 |
| L-CPh-RP92 | L. multiflorum | compost plus Ca-phosphate | RP92 |
| L-CFe-0 | L. multiflorum | compost plus Fe oxide | no inoculation |
| L-CFe-P9 | L. multiflorum | compost plus Fe oxide | P9 |
| L-CFe-P10 | L. multiflorum | compost plus Fe oxide | P10 |
| L-CFe-RP92 | L. multiflorum | compost plus Fe oxide | RP92 |
| Ctrl | no plant | no amendment | no inoculation |
| ID | Dehydrogenases μg TPF g Soil DM−1 h−1 | Alkaline Phosphatase μg P-Nitrophenyl g Soil DM−1 h−1 | Acidic Phosphatase μg P-Nitrophenyl g Soil DM−1 h−1 |
|---|---|---|---|
| L-0-0 | 1.03 a1 | 12.89 bc | 20.86 bc |
| L-0-P9 | 0.91 a | 13.22 bc | 18.48 ab |
| L-0-P10 | 0.96 a | 13.53 bc | 17.01 a |
| L-0-RP92 | 1.08 a | 12.65 b | 16.73 a |
| L-CPh-0 | 4.76 d | 12.88 bc | 21.81 cde |
| L-CPh-P9 | 3.60 cd | 14.09 bcd | 22.61 bcd |
| L-CPh-P10 | 3.40 cd | 16.57 e | 24.27 cde |
| L-CPh-RP92 | 3.87 cd | 15.89 de | 24.91 de |
| L-CFe-0 | 3.23 bc | 14.90 cde | 24.91 e |
| L-CFe-P9 | 3.31 bcd | 13.92 bcd | 24.49 de |
| L-CFe-P10 | 3.07 bc | 14.61 bcde | 22.28 cde |
| L-CFe-RP92 | 1.80 ab | 13.29 bc | 24.38 de |
| Ctrl | 0.49 a | 10.18 a | 16.11 a |
| ID | Total Count of Bacteria 107 CFU g Soil DM−1 | Count of Azotobacter 101 CFU g Soil DM−1 | Count of PSB 107 CFU g Soil DM−1 | Total Count of Fungi 104 CFU g Soil DM−1 |
|---|---|---|---|---|
| L-0-0 | 120 ab1 | 0 c | 5.3 bc | 6.0 d |
| L-0-P9 | 138 ab | 0 c | 1.7 cd | 69.7 bc |
| L-0-P10 | 260 a | 0 c | 1.3 cd | 84.0 bc |
| L-0-RP92 | 115 ab | 0 c | 0.3 cd | 15.0 cd |
| L-CPh-0 | 125 ab | 9.7 ab | 4.7 bc | 13.3 cd |
| L-CPh-P9 | 116 ab | 10.0 ab | 22.0 a | 56.0 bcd |
| L-CPh-P10 | 74 bc | 5.0 ab | 10.0 b | 16.0 cd |
| L-CPh-RP92 | 61 bc | 5.0 ab | 0.3 cd | 19.3 cd |
| L-CFe-0 | 39 c | 15.7 a | 3.7 bcd | 156.0 a |
| L-CFe-P9 | 193 a | 13.0 ab | 9.3 b | 108.0 ab |
| L-CFe-P10 | 97 ab | 8.7 ab | 8.3 b | 34.3 cd |
| L-CFe-RP92 | 77 bc | 8.0 ab | 9.3 b | 49.7 bcd |
| Ctrl | 11 d | 0 c | 0 d | 82.7 bc |
| ID | Soil pH pH in H2O | Available Phosphorus mg P2O5 kg−1 | Available Potassium mg K2O kg−1 | Extractable Nitrates mg N-NO3 kg−1 |
|---|---|---|---|---|
| L-0-0 | 7.97 a1 | 12.0 c | 155.9 b | 1.4 d |
| L-0-P9 | 7.90 abc | 12.0 c | 143.7 bc | 1.0 d |
| L-0-P10 | 7.93 ab | 14.2 c | 125.8 bcd | 0.8 d |
| L-0-RP92 | 7.93 ab | 9.6 c | 152.2 b | 0.6 d |
| L-CPh-0 | 7.77 c | 470.1 ab | 108.1 cde | 8.9 bcd |
| L-CPh-P9 | 7.80 bc | 590.8 a | 108.2 cde | 18.34 abc |
| L-CPh-P10 | 7.80 bc | 864.0 a | 109.8 cde | 27.21 a |
| L-CPh-RP92 | 7.80 bc | 762.1 a | 117.7 bcde | 10.08 bcd |
| L-CFe-0 | 7.77 c | 70.3 c | 110.8 cde | 21.33 ab |
| L-CFe-P9 | 7.80 bc | 70.2 c | 90.2 de | 8.32 bcd |
| L-CFe-P10 | 7.80 bc | 76.1 bc | 83.7 e | 5.30 cd |
| L-CFe-RP92 | 7.80 bc | 77.9 bc | 98.1 de | 12.24 bcd |
| Ctrl | 8.00 a | 18.0 c | 206.1 a | 12.53 bcd |
| ID | Cadmium mg kg−1 | Zinc mg kg−1 | Lead mg kg−1 |
|---|---|---|---|
| L-0-0 | 0.31 abc1 | 3.30 bc | 0.73 abc |
| L-0-P9 | 0.30 abc | 2.93 bc | 0.73 abc |
| L-0-P10 | 0.26 bc | 2.86 bc | 0.71 abc |
| L-0-RP92 | 0.20 abc | 2.64 bc | 0.59 abc |
| L-CPh-0 | 0.42 ab | 3.38 abc | 0.92 a |
| L-CPh-P9 | 0.34 abc | 2.54 c | 0.68 abc |
| L-CPh-P10 | 0.28 bc | 2.79 bc | 0.46 c |
| L-CPh-RP92 | 0.35 abc | 3.09 bc | 0.74 abc |
| L-CFe-0 | 0.44 ab | 3.84 ab | 0.80 ab |
| L-CFe-P9 | 0.37 abc | 3.05 bc | 0.58 bc |
| L-CFe-P10 | 0.38 abc | 3.35 abc | 0.66 abc |
| L-CFe-RP92 | 0.37 abc | 3.51 abc | 0.74 abc |
| Ctrl | 0.49 a | 4.51 a | 0.84 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siebielec, S.; Siebielec, G.; Marzec-Grządziel, A.; Pecio, M.; Stuczyński, T. Testing Combined Effect of Amendments and Inoculation with Bacteria for Improving Phytostabilisation of Smelter Waste Extremely Contaminated with Trace Elements. Agronomy 2021, 11, 2064. https://doi.org/10.3390/agronomy11102064
Siebielec S, Siebielec G, Marzec-Grządziel A, Pecio M, Stuczyński T. Testing Combined Effect of Amendments and Inoculation with Bacteria for Improving Phytostabilisation of Smelter Waste Extremely Contaminated with Trace Elements. Agronomy. 2021; 11(10):2064. https://doi.org/10.3390/agronomy11102064
Chicago/Turabian StyleSiebielec, Sylwia, Grzegorz Siebielec, Anna Marzec-Grządziel, Monika Pecio, and Tomasz Stuczyński. 2021. "Testing Combined Effect of Amendments and Inoculation with Bacteria for Improving Phytostabilisation of Smelter Waste Extremely Contaminated with Trace Elements" Agronomy 11, no. 10: 2064. https://doi.org/10.3390/agronomy11102064
APA StyleSiebielec, S., Siebielec, G., Marzec-Grządziel, A., Pecio, M., & Stuczyński, T. (2021). Testing Combined Effect of Amendments and Inoculation with Bacteria for Improving Phytostabilisation of Smelter Waste Extremely Contaminated with Trace Elements. Agronomy, 11(10), 2064. https://doi.org/10.3390/agronomy11102064








