The Promotion of Festuca sinensis under Heavy Metal Treatment Mediated by Epichloë Endophyte
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
2.3. Experimental Evaluations
2.3.1. Determination of Endogenous Phytohormones
2.3.2. Plant Growth
2.3.3. Measurements of Zn2+ and Cd2+ Ions Concentrations
2.3.4. Measurements of Alkaloid Concentrations
2.4. Statistical Analyses
3. Results
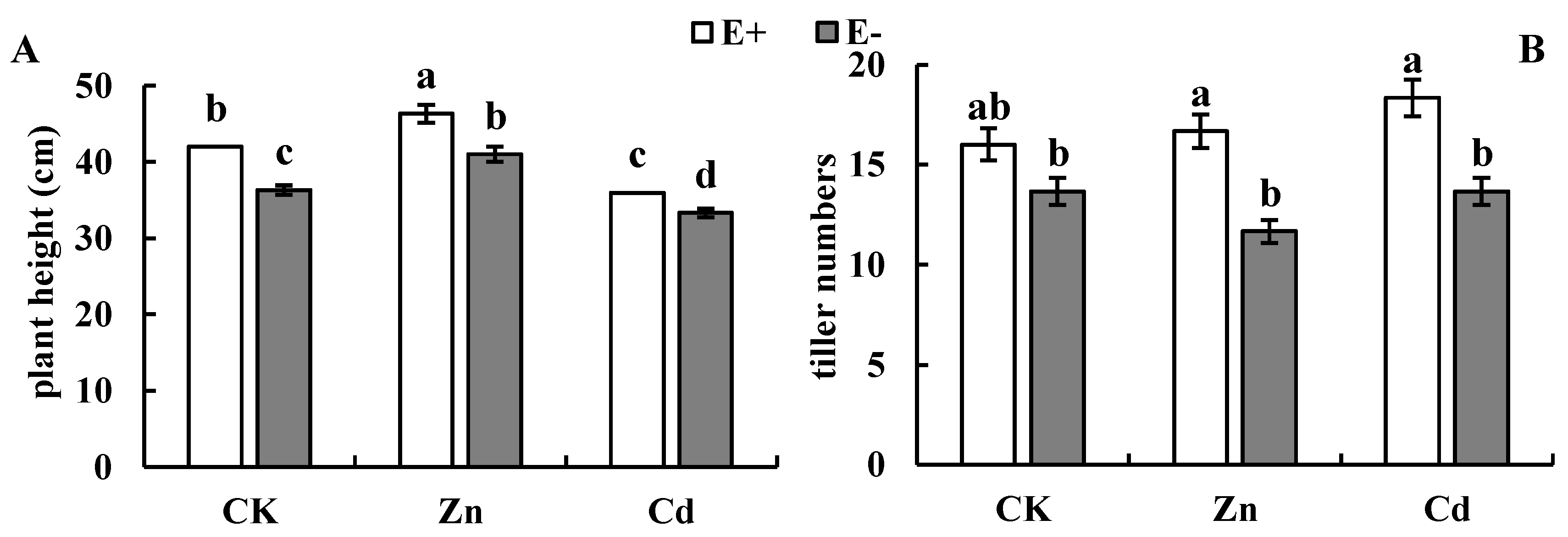
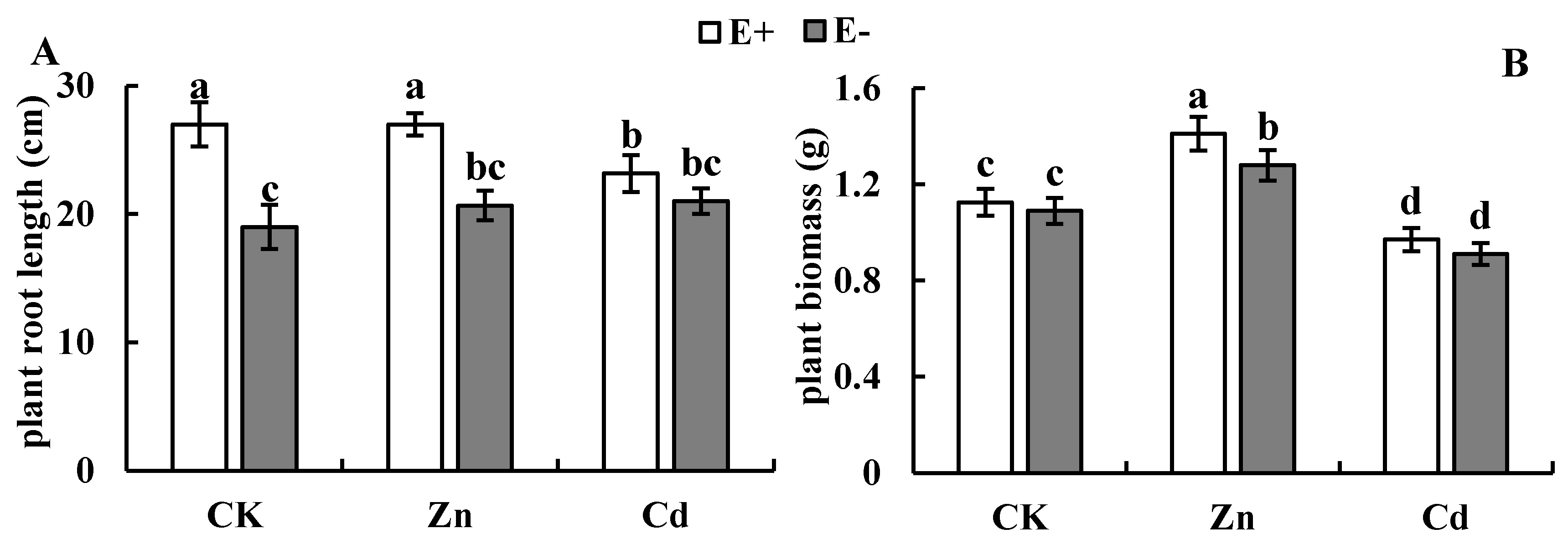
3.1. Plant Growth
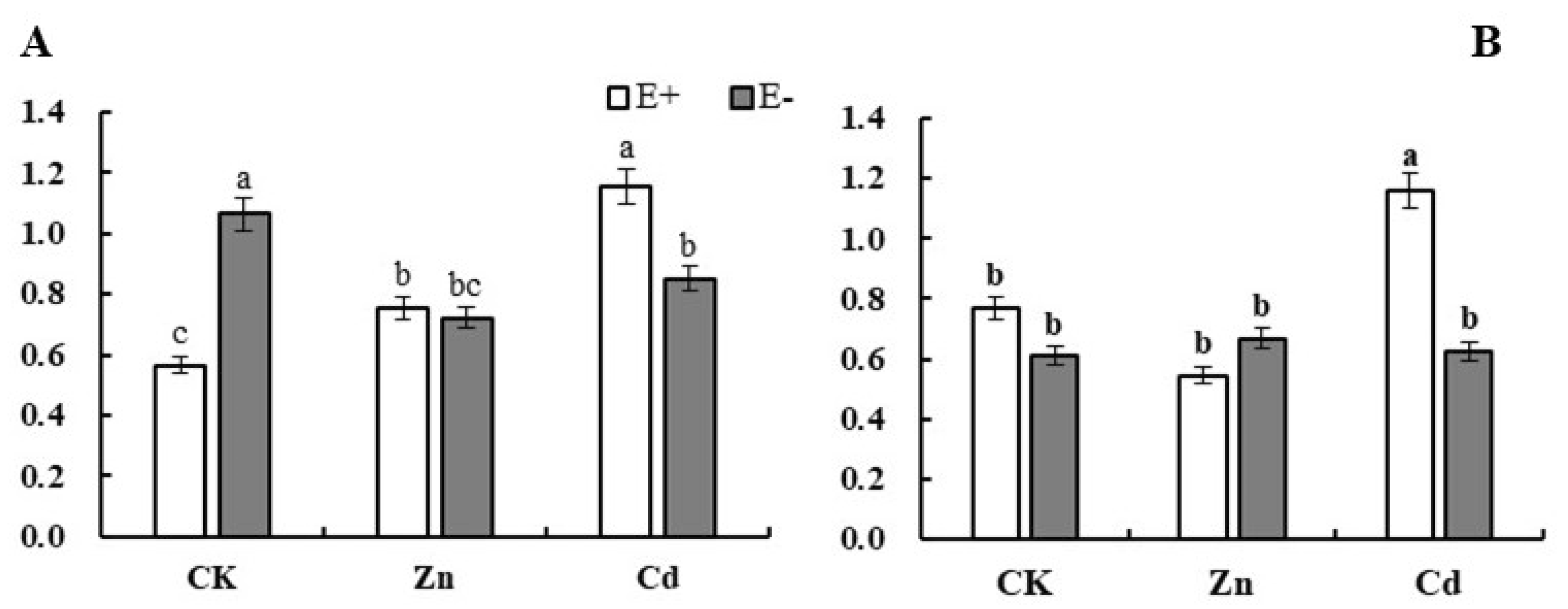
3.2. Cd2+ and Zn2+ Ion Concentration
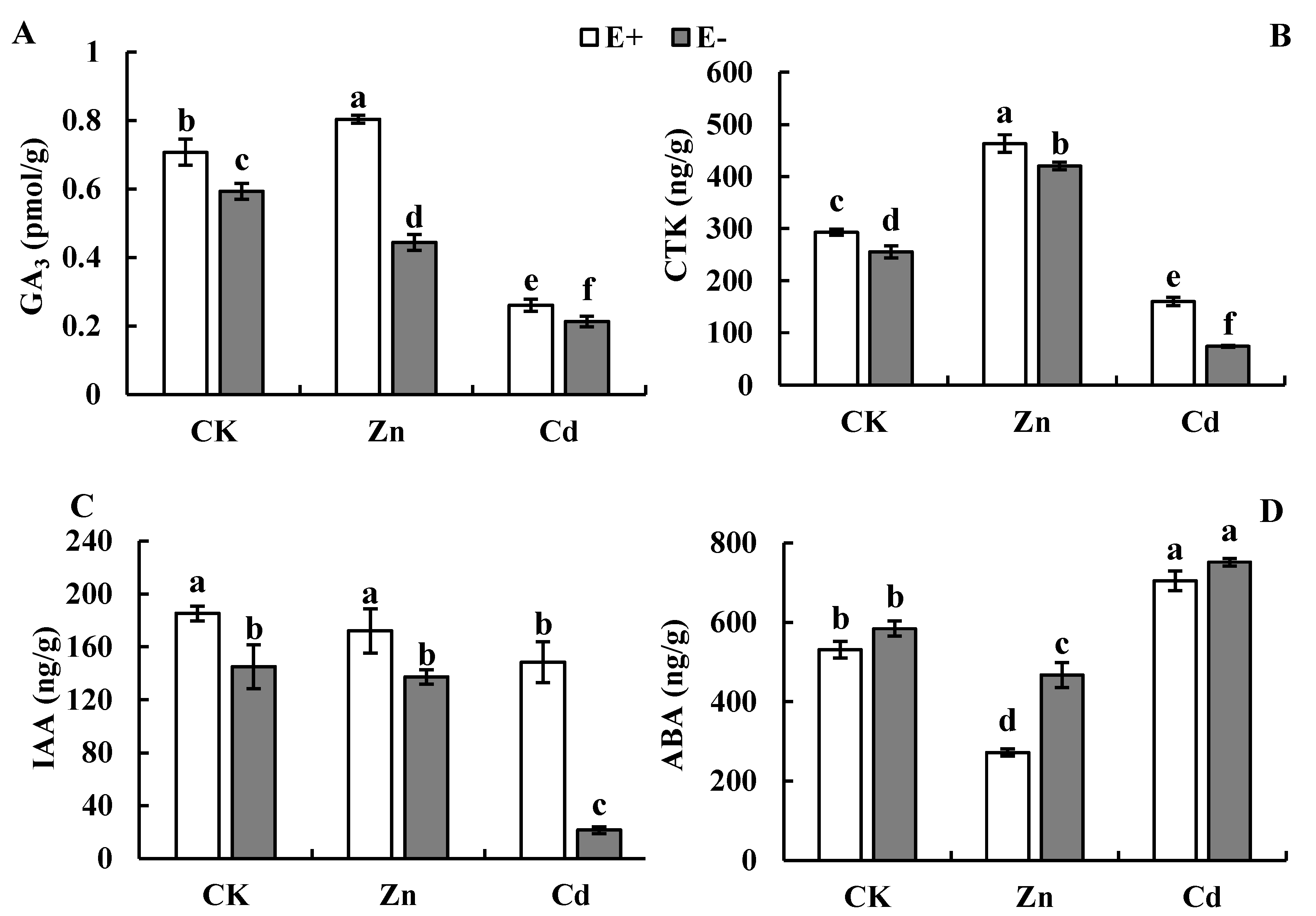
3.3. Plant Hormone Concentrations
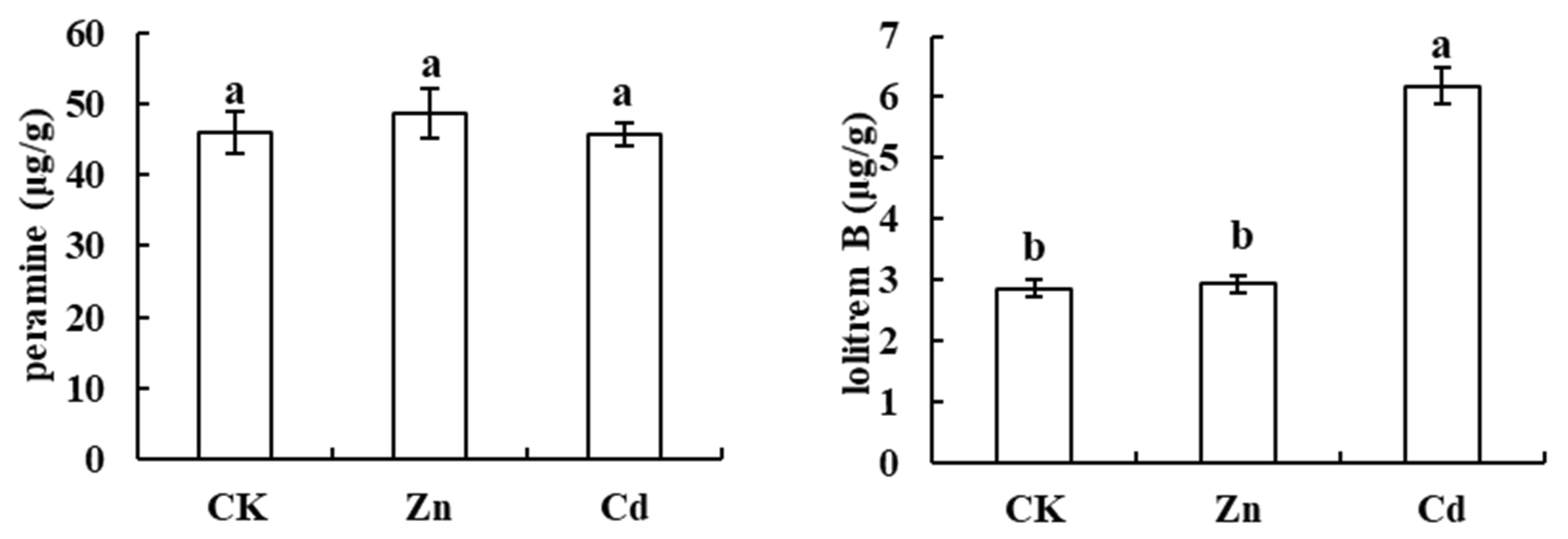
3.4. Alkaloids
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nan, Z.B.; Li, C.J. Neotyphodium in native grasses in China and observations on endophyte/host interactions. In Proceedings of the 4th international Neotyphodium-Grass Interactions Symposium, Soest, Germany, 27–29 September 2000; pp. 41–50. [Google Scholar]
- Li, C.; Ren, A.Z.; Gao, Y.B. Effect of endophyte infection on Zn resistance of tall fescue. Acta. Ecol. Sin. 2010, 30, 1684–1692, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Nan, Z.B. The grassland farming system and sustainable agricultural development in China. Grassl. Sci. 2005, 51, 15–19. [Google Scholar] [CrossRef]
- Nan, Z.B. Incidence and distribution of endophytic fungi in seeds of some native and introduced grasses in China. Acta Prataculturae Sin. 1996, 5, 1–8, (In Chinese with English Abstract). [Google Scholar]
- Nan, Z.B. Incidence and distribution of endophytic fungi in seedlings and plants of some native and introduced grasses in China. Acta Prataculturae Sin. 1996, 5, 13–17, (In Chinese with English Abstract). [Google Scholar]
- Zhou, L.Y.; Li, C.J.; Zhang, X.X.; Johnson, R.; Bao, G.S.; Yao, X.; Chai, Q. Effects of cold shocked Epichloë infected Festuca sinensis on ergot alkaloid accumulation. Fungal Ecol. 2015, 14, 99–104. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Zhang, X.X.; Li, C.J.; Christensen, M.J.; Nan, Z.B. Antifungal activity and phytochemical investigation of the asexual endophyte of Epichloë sp. from Festuca sinensis. Sci. China Life Sci. 2015, 58, 821–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.; Nan, Z.B.; Song, Q.Y.; Xia, C.; Li, X.Z.; Yao, X.; Xu, W.B.; Kuang, Y.; Tian, P.; Zhang, Q.P. Advances in research on Epichloë endophytes in Chinese native grasses. Front. Microbiol. 2016, 7, 1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, P.; Xu, W.B.; Li, C.J.; Song, H.; Wang, M.N.; Schardl, C.L.; Nan, Z.B. Phylogenetic relationship and taxonomy of a hybrid Epichloë species symbiotic with Festuca sinensis. Mycol. Prog. 2020, 19, 1069–1081. [Google Scholar] [CrossRef]
- Saikkonen, K.; Wäli, P.; Helander, M.; Faeth, S.H. Evolution of endophyte-plant symbioses. Trends Plant Sci. 2004, 9, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Li, N.N.; Zhang, Y.W.; Li, C.J.; Zhang, X.X.; Nan, Z.B. Role of Epichloë endophytes in defense responses of cool-season grasses to pathogens: A review. Plant Dis. 2018, 102, 2061–2073. [Google Scholar] [CrossRef] [Green Version]
- Tian, P.; Kuang, Y.; Lin, W.H.; Wang, J.J.; Nan, Z.B. Shoot morphology and alkaloid content of Epichloë endophyte–Festuca sinensis associations. Crop. Pasture Sci. 2018, 69, 430–438. [Google Scholar] [CrossRef]
- Peng, Q.Q.; Li, C.J.; Song, M.L.; Nan, Z.B. Effects of seed hydropriming on growth of Festuca sinensis infected with Neotyphodium endophyte. Fungal Ecol. 2013, 6, 83–91. [Google Scholar] [CrossRef]
- Lin, W.H.; Wang, X.X.; Wang, J.J.; Nzabanita, C.; Xu, W.B.; Yang, L.; Xi, H.F.; Tian, P.; Wang, Y.B.; Li, M.M.; et al. Intra-and interspecific competition of Elymus nutans griseb. and Festuca sinensis keng. ex eb alexeev. infected by Epichloë endophyte. Bangladesh. J. Bot. 2018, 47, 699–709. [Google Scholar]
- Wang, J.J.; Zhou, Y.P.; Lin, W.H.; Li, M.M.; Wang, M.N.; Wang, Z.G.; Kuang, Y.; Tian, P. Effect of an Epichloë endophyte on adaptability to water stress in Festuca sinensis. Fungal Ecol. 2017, 30, 39–47. [Google Scholar] [CrossRef]
- Masindi, V.; Muedi, K.L. Environmental contamination by heavy metals. Heavy Metals. 2018, 10, 115–132. [Google Scholar] [CrossRef] [Green Version]
- Weyens, N.; Lelie, D.V.D.; Taghavi, S.; Vangronsveld, J. Phytoremediation: Lant–endophyte partnerships take the challenge. Curr. Opin. Biotechnol. 2009, 20, 248–254. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, D.; Wang, Q. An overview of field-scale studies on remediation of soil contaminated with heavy metals and metalloids: Technical progress over the last decade. Water Res. 2018, 147, 440–460. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalolam, O.O. A new strategy for heavy metal polluted environments: A review of microbial biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef]
- Malinowski, D.P.; Belesky, D.P. Adaptations of endophyte-infected cool-season grasses to environmental stresses: Mechanisms of drought and mineral stress tolerance. Crop. Sci. 2000, 40, 923–940. [Google Scholar] [CrossRef]
- Bonnet, M.; Camares, O.; Veisseire, P. Effects of zinc and influence of Acremonium lolii on growth parameters, chlorophyll a fluorescence and antioxidant enzyme activities of ryegrass (Lolium perenne L. cv Apollo). J. Exp. Bot. 2000, 51, 945–953. [Google Scholar] [CrossRef]
- Ren, A.Z.; Gao, Y.B.; Zhang, L.; Xie, F. Effects of cadmium on growth parameters of endophyte-infected endophyte-free ryegrass. J. Plant Nutr. Soil Sci. 2006, 169, 857–860. [Google Scholar] [CrossRef]
- Malinowski, D.P.; Belesky, D.P. Epichloë (formerly Neotyphodium) fungal endophytes increase adaptation of cool-season perennial grasses to environmental stresses. Acta Agrobot. 2019, 72, 1–26. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Du, M.W.; Eneji, E.A.; Wang, B.M.; Duan, L.S.; Li, Z.H.; Tian, X.L. Mechanism of phytohormone involvement in feedback regulation of cotton leaf senescence induced by potassium deficiency. J. Exp. Bot. 2012, 63, 5887–5901. [Google Scholar] [CrossRef] [Green Version]
- Fargaová, A. Toxicity comparison of some possible toxic metals (Cd, Cu, Pb, Se, Zn) on young seedlings of Sinapis alba L. Plant Soil Environ. 2004, 50, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Nusrat JalbaniArain, M.B.; Kazi, T.G.; Jamali, M.K.; Jalbani, N.; Afridi, H.I.; Baig, J.A. Speciation of heavy metals in untreated domestic waste water sludge by time saving BCR sequential extraction method. Environ. Sci. Health A 2007, 42, 649–659. [Google Scholar]
- Sidhu, G.P.S.; Bali, A.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Insights into the tolerance and phytoremediation potential of Coronopus didymus L.(Sm) grown under zinc stress. Chemosphere 2020, 244, 125350. [Google Scholar] [CrossRef]
- Gallagher, R.T.; Hawkes, A.D.; Stewart, J.M. Rapid determination of the neurotoxin lolitrem B in perennial ryegrass by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. A 1985, 321, 217–226. [Google Scholar] [CrossRef]
- Ball, O.J.P.; Prestidge, R.A.; Sprosen, J.M. Interrelationships between Acremonium lolii, peramine, and lolitrem B in perennial ryegrass. Appl. Environ. Microbiol. 1995, 61, 1527–1533. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, D.S.; Totawat, K.L. Zinc and phosphorus interaction on growth and nutrient uptake by wheat. Ann. Arid. Zone 1985, 24, 31–38. [Google Scholar]
- Zhao, D.D. Studies on Growth and Resistance of Festuca Arundinacea under the Stress of Cd2+ and Cd2+-Zn2+; Sichuan Normal University: Chengdu, China, 2008; (In Chinese with English Abstract). [Google Scholar]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, C.J.; Nan, Z.B. Effects of cadmium stress on growth and anti-oxidative systems in Achnatherum inebrians, symbiotic with Neotyphodium gansuens. J. Hazard. Mater. 2010, 175, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Li, C.J.; Nan, Z.B. Effects of cadmium stress on seed germination and seedling growth of Elymus dahuricus infected with the Neotyphodium endophyte. Sci. China Life Sci. 2012, 55, 793–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malinowski, D.P.; Alloush, G.A.; Belesky, D.P. Evidence for chemical changes on the root surface of tall fescue in response to infection with the fungal endophyte Neotyphodium coenophialum. Plant Soil 1998, 205, 1–12. [Google Scholar] [CrossRef]
- Malinowski, D.; Elesky, D.P. Infection with leaf fungal endophyte Neotyphodium coenophialum increase aluminium sequestration on root surfaces of tall fescue. J. Plant Nutr. 1999, 22, 1335–1349. [Google Scholar] [CrossRef]
- Malinowski, D.P.; Zuo, H.; Belesky, D.P.; Alloush, G.A. Evidence for copper binding by extracellular root exudates of tall fescue but not perennial ryegrass infected with Neotyphodium spp. Endophytes. Plant Soil 2004, 267, 1–12. [Google Scholar] [CrossRef]
- Creek, R.; Wade, G.L. Excretion of phenolic compounds from the roots of Festuca arundinacea, Eragrostis curvula, and Lespedeza striat. Trans. Ky. Acad. Sci. 1985, 46, 51–55. [Google Scholar]
- Lanaras, T.; Moustakas, M.; Symeonidis, L.; Diamantoglou, S.; Karataglis, S. Plant metal content, growth responses and some photosynthetic measurements on field-cultivated wheat growing on ore bodies enriched in Cu. Physiol. Plant. 1993, 88, 307–314. [Google Scholar] [CrossRef]
- Argueso, C.T.; Raines, T.; Kieber, J.J. Cytokinin signaling and transcriptional networks. Curr. Opin. Plant Biol. 2010, 13, 533–559. [Google Scholar] [CrossRef]
- Qin, H.; Gu, Q.; Zhang, J.L.; Sun, L.; Kuppu, S.; Zhang, Y.Z.; Burow, M.; Payton, P.; Blumwald, E.; Zhang, H. Regulated expression of an Isopentenyltransferase Gene (IPT) in peanut significantly improves drought tolerance and increases yield under field conditions. Plant Cell Physiol. 2011, 52, 1904–1914. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, S.; Kudoyarova, G.R.; Veselov, D.S.; Arkhipova, T.N.; Davies, W.J. Plant hormone interactions: Innovative targets for crop breeding and management. J. Exp. Bot. 2012, 63, 3499–3509. [Google Scholar] [CrossRef]
- Srivastava, S.; Chiappetta, A.; Beatrice, M. Identification and profiling of arsenic stress-induced miRNAs in Brassica juncea. J. Exp. Bot. 2013, 64, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Elobeid, M.; Go¨bel, C.; Feussner, I.; Polle, A. Cadmium interferes with auxin physiology and lignification in Poplar. J. Exp. Bot. 2012, 63, 1413–1421. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.F.; Jiang, T.; Wang, Z.W.; Lei, G.J.; Shi, Y.Z.; Li, G.X.; Zheng, S.J. Gibberellic acid alleviates cadmium toxicity by reducing nitric oxide accumulation and expression of IRT1 in Arabidopsis thaliana. J. Hazard. Mater. 2012, 239, 302–307. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Rathinasabapathi, B. Auxin and its transport play a role in plant tolerance to arsenite-induced oxidative stress in Arabidopsis thaliana. Plant Cell Environ. 2013, 36, 1838–1849. [Google Scholar] [CrossRef]
- Mei, Y.X. Effect of Endophyte Gao Y1-1 Infection on Endogenous Hormones and Organic Acids of Rice Seedlings under Cd and/or Al Stress; Shenyang Normal University: Shenyang, China, 2017; (In Chinese with English Abstract). [Google Scholar]
- Battista, J.P.D.; Bouton, J.H.; Bacon, C.W.; Siegel, M.R. Rhizome and herbage production of endophyte-removed tall fescue clones and populations. Crop Sci. 1990, 82, 651–654. [Google Scholar] [CrossRef]
- Bunyard, B.; Mclnnis, T.M. Evidence for elevated phytohormone levels in endophyte-infected tall fescue. In Proceedings of the International Symposium on Acremonium/Grass Interactions; Quisenberry, S.S., Joost, R.E., Eds.; Louisiana Agricultural Experiment Station: Baton Rouge, LA, USA, 1990; pp. 185–188. [Google Scholar]
- Joost, R.E. Acremonium in fescue and ryegrass: Boon or bane? A review. J. Anim. Sci. 1995, 73, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Qin, J.H.; Chen, W.; Zhou, Y.; Ren, A.Z.; Gao, Y.B. Pathogen resistant advantage of endophyte-infected over endophyte-free Leymus chinensis is strengthened by pre-drought treatment. Eur. J. Plant Pathol. 2016, 144, 477–486. [Google Scholar] [CrossRef]
- Battista, J.P.D.; Bacon, C.W.; Severson, R.F.; Plattner, R.D.; Bouton, J.H. Indole acetic acid production by the fungal endophyte of tall fescue. Agron. J. 1990, 82, 878–880. [Google Scholar] [CrossRef]
- Yue, Q.; Miller, C.J.; White, J.F.; Richardson, M.D. Isolation and characterization of fungal inhibitors from Epichloë festucae. J. Agric. Food Chem. 2000, 48, 4687–4692. [Google Scholar] [CrossRef]
- Bush, L.P.; Wilkinson, H.H.; Schardl, C.L. Bioprotective alkaloids of grass-fungal endophyte symbioses. Plant Physiol. 1997, 114, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Easton, H.S.; Latch, G.C.M.; Tapper, B.A.; Ball, O.J.P. Ryegrass host genetic control of contentrations of endophyte-derived alkaloids. Crop Sci. 2002, 42, 51–57. [Google Scholar] [PubMed]
- Porter, J.K. Analysis of endophyte toxins: Fescue and other grasses toxic to livestock. J. Anim. Sci. 1995, 73, 871–880. [Google Scholar] [CrossRef] [Green Version]
- Rowan, D.D.; Dymock, J.J.; Brimble, M.A. Effect of fungal metabolite peramine and analogs on feeding and development of Argentine stem weevil (Listronotus bonariensis). J. Chem. Ecol. 1990, 16, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Sabzalian, M.R.; Mirlohi, A.; Sharifnabi, B. Reaction to powdery mildew fungus, Blumeria graminis in endophyte-infected and endophyte-free tall and meadow fescues. Australas. Plant Pathol. 2012, 41, 565–572. [Google Scholar] [CrossRef]
- Ma, M.Z.; Christensen, M.J.; Nan, Z.B. Effects of the endophyte Epichloë festucae var. lolii of perennial ryegrass (Lolium perenne) on indicators of oxidative stress from pathogenic fungi during seed germination and seedling growth. Eur. J. Plant Pathol. 2015, 141, 571–583. [Google Scholar] [CrossRef]
- Popay, A.J.; Cotching, B.; Moorhead, A.; Ferguson, C.M. AR37 endophyte effects on porina and root aphid populations and ryegrass damage in the field. Proc. N. Zeal. Grassl Assoc. 2012, 74, 165–170. [Google Scholar] [CrossRef]
- Matsukura, K.; Shiba, T.; Sasaki, T.; Yoshida, K.; Matsumura, M. Dynamics of Neotyphodium uncinatum and N-formylloline in Italian ryegrass, and their relation to insect resistance in the field. J. Appl. Microbiol. 2014, 116, 400–407. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, S.; Parsons, A.J.; Bassett, S.; Christensen, M.J.; Hume, D.E.; Johnson, L.J.; Johnson, R.D.; Simpson, W.R.; Stacke, C.; Voisey, C.R. High nitrogen supply and carbohydrate content reduce fungal endophyte and alkaloid concentration in Lolium perenne. New Phytol. 2007, 173, 787–797. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Tian, P.; Gao, M.; Li, M. The Promotion of Festuca sinensis under Heavy Metal Treatment Mediated by Epichloë Endophyte. Agronomy 2021, 11, 2049. https://doi.org/10.3390/agronomy11102049
Wang M, Tian P, Gao M, Li M. The Promotion of Festuca sinensis under Heavy Metal Treatment Mediated by Epichloë Endophyte. Agronomy. 2021; 11(10):2049. https://doi.org/10.3390/agronomy11102049
Chicago/Turabian StyleWang, Meining, Pei Tian, Min Gao, and Miaomiao Li. 2021. "The Promotion of Festuca sinensis under Heavy Metal Treatment Mediated by Epichloë Endophyte" Agronomy 11, no. 10: 2049. https://doi.org/10.3390/agronomy11102049
APA StyleWang, M., Tian, P., Gao, M., & Li, M. (2021). The Promotion of Festuca sinensis under Heavy Metal Treatment Mediated by Epichloë Endophyte. Agronomy, 11(10), 2049. https://doi.org/10.3390/agronomy11102049




