Effects of Climate Change on the Distribution of Key Native Dung Beetles in South American Grasslands
Abstract
1. Introduction
2. Materials and Methods
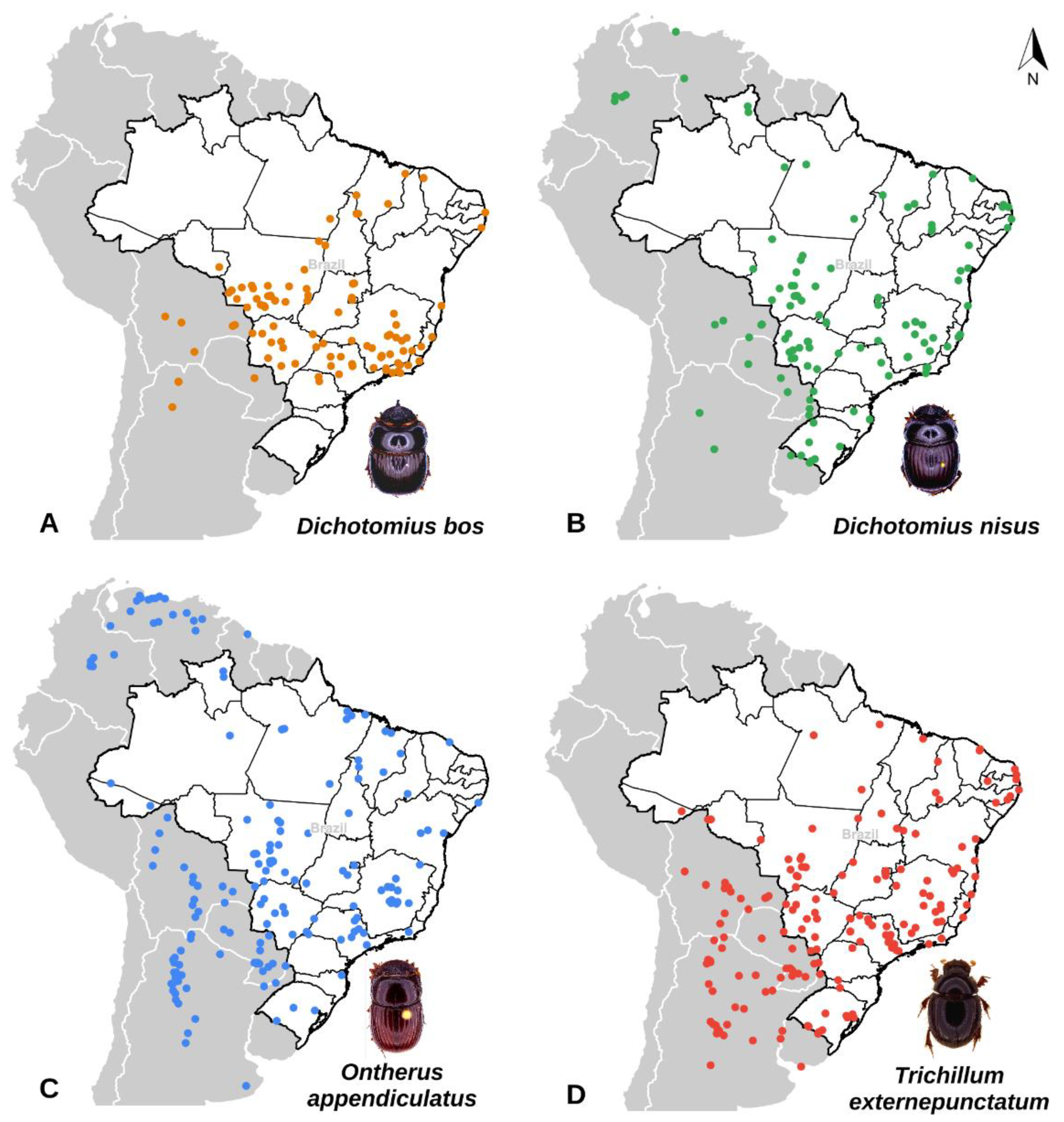
2.1. Dung Beetles Data Source
2.2. Climatic Variables and Future Climate Simulations
2.3. Niche-Based Model Building
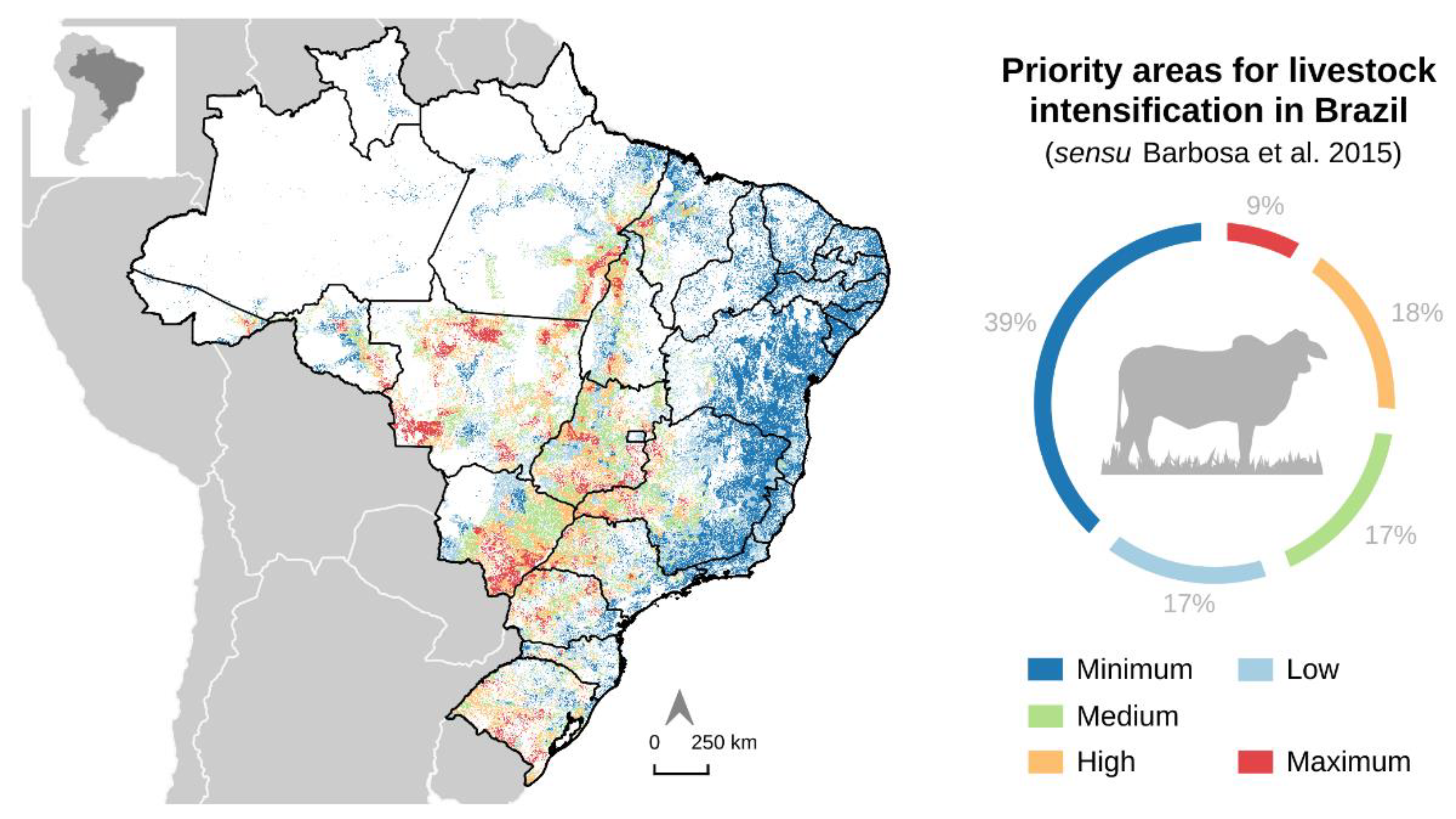
2.4. Priority Areas for Livestock Intensification in Brazil
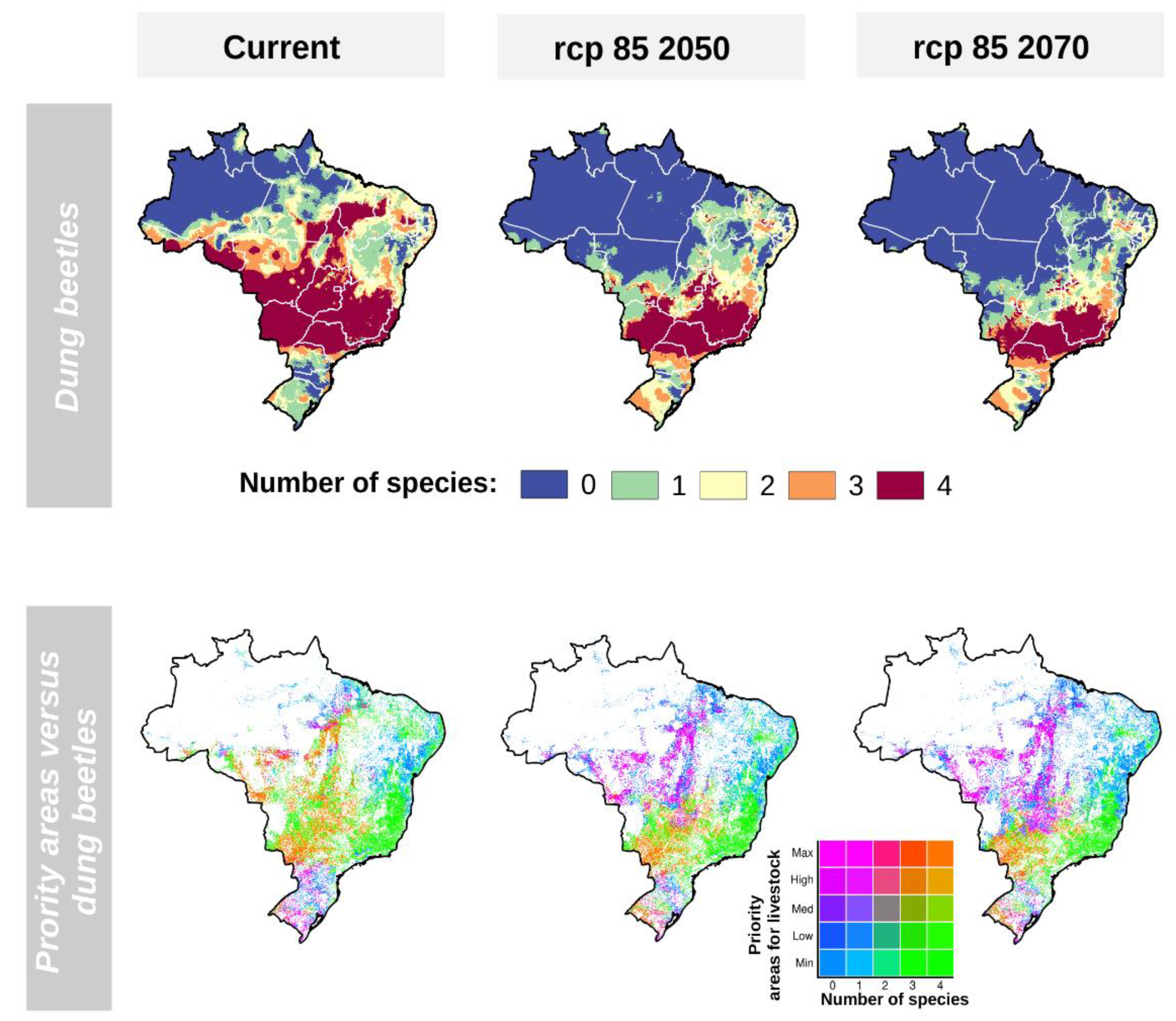
2.5. Effects of Climate Change on Key Dung Beetle Species Spatial Distributions and Livestock Priority Areas
2.6. Effects of Climate Change on Extent of Occurrence and Area of Species Occupancy
3. Results
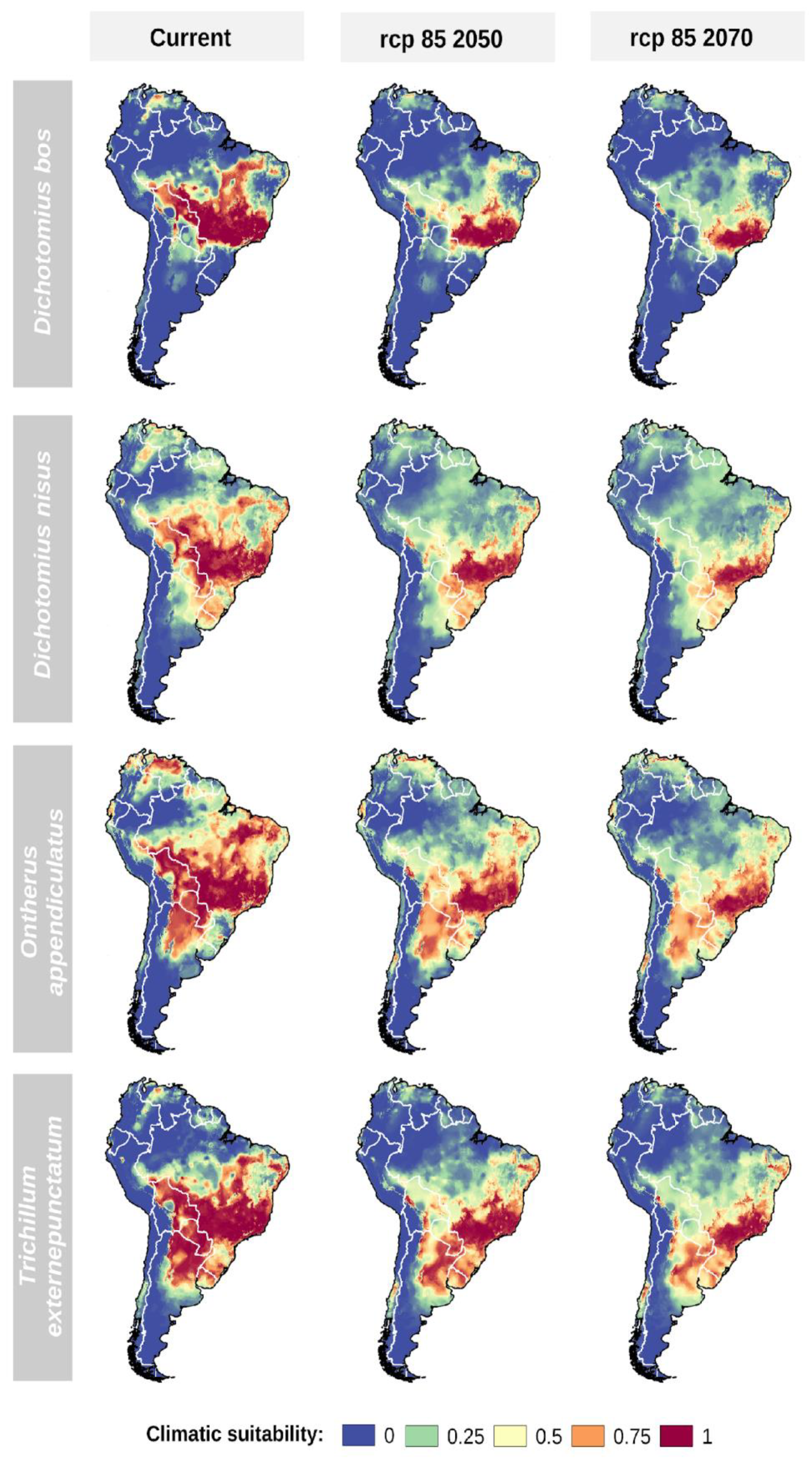
3.1. Key Dung Beetles Spatial Distribution in the Present
3.2. Effects of Climate Change on Key Dung Beetles Range
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilman, D.; Knops, J.; Wedin, D.; Reich, P.; Ritchie, M.; Siemann, E. The influence of functional diversity and composition on ecosystem processes. Science 1997, 277, 1300–1302. [Google Scholar] [CrossRef]
- Flynn, D.F.; Mirotchnick, N.; Jain, M.; Palmer, M.I.; Naeem, S. Functional and phylogenetic diversity as predictors of biodiversity–ecosystem-function relationships. Ecology 2011, 92, 1573–1581. [Google Scholar] [CrossRef]
- Srivastava, D.S.; Cadotte, M.W.; MacDonald, A.A.M.; Marushia, R.G.; Mirotchnick, N. Phylogenetic diversity and the functioning of ecosystems. Ecol. Lett. 2012, 15, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Dirzo, R.; Young, H.S.; Galetti, M.; Ceballos, G.; Isaac, N.J.; Collen, B. Defaunation in the Anthropocene. Science 2014, 345, 401–406. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.C.; Williams, S.E. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355. [Google Scholar] [CrossRef]
- Dirzo, R.; Raven, P.H. Global state of biodiversity and loss. Annu. Rev. Environ. Resour. 2003, 28, 137–167. [Google Scholar] [CrossRef]
- Araújo, M.; Cabeza, M.; Thuiller, W.; Hannah, L.; Williams, P. Would climate change drive species out of reserves? Testing the robustness of existing reserve-selection methods in Europe. Glob. Chang. Biol. 2004, 10. [Google Scholar] [CrossRef]
- Da Silveira, N.S.; Vancine, M.H.; Jahn, A.E.; Pizo, M.A.; Sobral-Souza, T. Future climate change will impact the size and location of breeding and wintering areas of migratory thrushes in South America. Condor 2021, 123, duab006. [Google Scholar] [CrossRef]
- Van der Putten, W.H.; Macel, M.; Visser, M.E. Predicting species distribution and abundance responses to climate change: Why it is essential to include biotic interactions across trophic levels. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2025–2034. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Arico, S.; Aronson, J.; Baron, J.S.; Bridgewater, P.; Cramer, V.A.; Zobel, M. Novel ecosystems: Theoretical and management aspects of the new ecological world order. Glob. Ecol. Biogeogr. 2006, 15, 1–7. [Google Scholar] [CrossRef]
- USDA Foreign Agricultural Service—United States Department of Agriculture. Livestock and Poultry: World Markets and Trade. 10 October 2019. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/73666448x/g445ct12h/ff365k146/Livestock_poultry.pdf (accessed on 14 July 2021).
- Grisi, L.; Leite, R.C.; Martins, J.R.D.S.; Barros, A.T.M.D.; Andreotti, R.; Cançado, P.H.D.; León, A.A.; Pereira, J.B.; Villela, H.S. Reassessment of the potential economic impact of cattle parasites in Brazil. Rev. Bras. Parasitol. Veterinária 2014, 23, 150–156. [Google Scholar] [CrossRef]
- Bornemissza, G.F. Could dung eating insects improve our pastures? J. Aust. Inst. Agric. Sci. 1960, 26, 54–56. [Google Scholar]
- Bornemissza, G.F. Insectary studies on the control of dung breeding flies by the activity of the dung beetle, Onthophagus gazella F. (Coleoptera: Scarabaeinae). Aust. J. Entomol. 1970, 9, 31–41. [Google Scholar] [CrossRef]
- Bornemissza, G.F. The Australian dung beetle project 1965–75. AMRC Review. Aust. Meat Res. Comm. 1976, 30, 1–32. [Google Scholar]
- Halffter, G.; Matthews, E.G. The natural history of dung beetles of the subfamily Scarabaeinae (Coleoptera: Scarabaeidae). Folia Entomol. Mex. 1966, 12, 1–312. [Google Scholar]
- Bang, H.S.; Lee, J.H.; Kwon, O.S.; Na, Y.E.; Jang, Y.S.; Kim, W.H. Effects of paracoprid dung beetles (Coleoptera: Scarabaeidae) on the growth of pasture herbage and on the underlying soil. Appl. Soil Ecol. 2005, 29, 165–171. [Google Scholar] [CrossRef]
- Nichols, E.; Spector, S.; Jouzada, J.; Larsen, T.; Amezquita, S.; Favila, M.E. The Scarabaeinae Research Network. Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol. Conserv. 2008, 141, 1461–1474. [Google Scholar] [CrossRef]
- Losey, J.E.; Vaughan, M. The economic value of ecological services provided by insects. Bioscience 2006, 56, 311–323. [Google Scholar] [CrossRef]
- Beynon, S.A.; Wainwright, W.A.; Christie, M. The application of an ecosystem services framework to estimate the economic value of dung beetles to the UK cattle industry. Ecol. Entomol. 2015, 40, 124–135. [Google Scholar] [CrossRef]
- Slade, E.; Riutta, T.; Roslin, T.; Tuomisto, H.L. The role of dung beetles in reducing greenhouse gas emissions from cattle farming. Sci. Rep. 2016, 6, 18140. [Google Scholar] [CrossRef]
- Verdú, J.R.; Sánchez-Piñero, F.; Lobo, J.M.; Cortez, V. Evaluating long-term ivermectin use and the role of dung beetles in reducing short-term CH4 and CO2 emissions from livestock faeces: A mesocosm design under Mediterranean conditions. Ecol. Entomol. 2020, 45, 109–120. [Google Scholar] [CrossRef]
- De Oliveira Tissiani, A.S.; Vaz-de-Mello, F.Z.; Campelo-Júnior, J.H. Dung beetles of Brazilian pastures and key to genera identication (Coleoptera: Scarabaeidae). Pesqui. Agropecuária Bras. 2017, 52, 401–418. [Google Scholar] [CrossRef]
- Noriega, J.A.; Floate, K.D.; Génier, F.; Reid, C.A.; Kohlmann, B.; Horgan, F.G.; Davis, A.L.; Forgie, S.A.; Aguilar, C.; Ibarra, M.G.; et al. Global distribution patterns provide evidence of niche shift by the introduced African dung beetle Digitonthophagus gazella. Entomol. Exp. Et Appl. 2020, 168, 766–782. [Google Scholar] [CrossRef]
- Sales, L.P.; Galetti, M.; Pires, M.M. Climate and land-use change will lead to a faunal “savannization” on tropical rainforests. Glob. Chang. Biol. 2020, 26, 7036–7044. [Google Scholar] [CrossRef] [PubMed]
- Génier, F. A revision of the Neotropical genus Ontherus Erichson (Coleoptera: Scarabaeidae, Scarabaeinae). Mem. Entomol. Soc. Can. 1996, 170, 1–169. [Google Scholar] [CrossRef]
- Vaz-de-Mello, F.Z. Synopsis of the new subtribe Scatimina (Coleoptera: Scarabaeidae: Ateuchini), with descriptions of twelve new genera and review of Genieridium, new genus. Zootaxa 2008, 1955, 1–75. [Google Scholar] [CrossRef]
- Luederwaldt, H. As espécies brasileiras do gênero Pinotus. Rev. Do Mus. Paul. 1929, 16, 603–775. [Google Scholar]
- Cassenote, S.; Valois, M.C.; Maldaner, M.E.; Vaz-de-Mello, F.Z. Taxonomic revision of Dichotomius (Selenocopris) nisus (Olivier, 1719) and Dichotomius (Selenocopris) superbus (Felsche, 1901). Rev. Bras. Entomol. 2020, 64. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological niches and geographic distributions (MPB-49); Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Sobral-Souza, T.; Francini, R.B.; Lima-Ribeiro, M.S. Species extinction risk might increase out of reserves: Allowances for conservation of threatened butterfly Actinote quadra (Lepidoptera: Nymphalidae) under global warming. Nat. Conserv. 2015, 13, 159–165. [Google Scholar] [CrossRef][Green Version]
- IPCC. 2014: Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team; Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Package ‘Dismo’. 2020. Available online: http://cran.r-project.org/web/packages/dismo/index.html (accessed on 14 July 2021).
- Hijmans, R.J. Package ‘Raster’. 2021. Available online: https://cran.r-project.org/web/packages/raster/index.html (accessed on 14 July 2021).
- Karatzoglou, A.; Smola, A.; Hornik, K.; Zeileis, A. Kernlab—An S4 Package for Kernel Methods in R. J. Stat. Softw. 2004, 11, 1–20. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 14 July 2021).
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Diniz-Filho, J.A.F.; Bini, L.M.; Rangel, T.F.; Loyola, R.D.; Hof, C.; Nogués-Bravo, D.; Araújo, M.B. Partitioning and mapping uncertainties in ensembles of forecasts of species turnover under climate change. Ecography 2009, 32, 897–906. [Google Scholar] [CrossRef]
- Nix, H.A. A biogeographic analysis of Australian elapid snakes. Atlas Elapid Snakes Aust. 1986, 7, 4–15. [Google Scholar]
- Carpenter, G.; Gillison, A.N.; Winter, J. DOMAIN: A flexible modelling procedure for mapping potential distributions of plants and animals. Biodivers. Conserv. 1993, 2, 667–680. [Google Scholar] [CrossRef]
- Farber, O.; Kadmon, R. Assessment of alternative approaches for bioclimatic modeling with special emphasis on the Mahalanobis distance. Ecol. Model. 2003, 160, 115–130. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudik, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Tax, D.M.J.; Duin, R.P.W. Support vector data description. Mach. Learn. 2004, 54, 45–66. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef]
- Araújo, M.B.; Anderson, R.P.; Barbosa, A.M.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B.; et al. Standards for distribution models in biodiversity assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef]
- Barbosa, F.A.; Soares-Filho, B.S.; Merry, F.D.; Azevedo, H.O.; Costa, W.L.S.; Coe, M.T.; Batista, E.L.S.; Maciel, T.G.; Sheepers, L.C.; Oliveira, A.R.; et al. Cenários Para a Pecuária de Corte Amazônica; IGC/UFMG: Belo Horizonte, Brasil, 2015; p. 146. [Google Scholar]
- Tomczak, M.; Tomczak, E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trends Sports Sci. 2014, 1, 1–25. [Google Scholar]
- Ogle, D.H.; Doll, J.C.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis. 2021. R Package Version 0.9. Available online: https://cran.r-project.org/web/packages/FSA/ (accessed on 14 July 2021).
- Wickham, H.; Chang, W.; Henry, L. ggplot2. Comput. Softw. J. 2012. Available online: http://ggplot2.org (accessed on 14 July 2021).
- QGIS Development Team. QGIS Geographic Information System. 2021. Open Source Geospatial Foundation Project. Available online: http://qgis.osgeo.org (accessed on 14 July 2021).
- Griffith, G.E.; Omernik, J.M.; Azevedo, S.H. Ecological Classification of the Western Hemisphere; Unpublished Report; U.S. Environmental Protection Agency, Western Ecology Division: Corvallis, OR, USA, 1998; p. 49.
- Alves, S.B.; Nakano, O. Estudo da biologia do Dichotomius anaglypticus (Mannerheim, 1829) (Coleoptera, Scarabaeidae) [Coleoptera]. Ecossistema 1978, 3, 1–20. [Google Scholar]
- López, D.A.; Halffter, G.; Vaz-de-Mello, F.Z. Nesting Behavior in Trichillum Harold, 1868 and Related Genera (Coleoptera: Scarabaeidae: Scarabaeinae: Ateuchini: Scatimina): A Primitive Process or a Loss of Nidification? Coleopt. Bull. 2009, 63, 289–297. [Google Scholar]
- Vaz-de-Mello, F.Z.; (Federal University of Mato Grosso, Cuiabá, Mato Grosso, Brazil). Personal observation including 30 years of field observations, collection, and specimens received for identification from the whole continent, 1991–2021.
- Brazilian Federal Law. Law No 11.428, from 22 December 2006. D.O.U. 26/12/2006, P.1. Available online: http://www.planalto.gov.br/ccivil_03/_ato2004-2006/2006/lei/l11428.htm (accessed on 6 August 2021).
- ABIEC—Associação Brasileira das Indústrias Exportadoras de Carne. Beef Report: Perfil da Pecuária no Brasil; ABIEC: São Paulo, Brazil, 2019; p. 47. [Google Scholar]
- Dias-Filho, M.B. Diagnóstico das pastagens no Brasil; Embrapa Amazonia Oriental: Belém, Brazil, 2014. [Google Scholar]
- Vaz-de-Mello, F.Z. Scarabaeidae in Catálogo Taxonômico da Fauna do Brasil. PNUD. 2021. Available online: http://fauna.jbrj.gov.br/fauna/faunadobrasil/127498 (accessed on 25 August 2021).
- Mesquita Filho, W.; Flechtmann, C.A.; Godoy, W.A.; Bjornstad, O.N. The impact of the introduced Digitonthophagus gazella on a native dung beetle community in Brazil during 26 years. Biol. Invasions 2018, 20, 963–979. [Google Scholar] [CrossRef]
- Zunino, M.; Barbero, E. Escarabajos, ganado, pastizales: Algunas consideraciones deontológicas. Folia Entomológica Mex. 1993, 87, 95–101. [Google Scholar]
- Tissiani, A.S.O. Variabilidade climática e meteorológica na distribuição de Scarabaeinae Coprófagos. Ph.D. Thesis, Universidade Federal de Mato Grosso, Cuiabá, Brazil, 2014. [Google Scholar]
- Halffter, G.; Edmonds, W.D. The Nesting Behavior of Dung Beetles (Scarabaeinae). An Ecological and Evolutive Approach; Instituto de Ecología: México City, México, 1982; p. 176. [Google Scholar]
- Vaz-de-Mello, F.Z.; (Federal University of Mato Grosso, Cuiabá, Mato Grosso, Brazil). Personal observation including 20 years of direct field observations, 2001–2021.
- Lumaret, J.P.; Galante, E.; Lumbreras, C.; Mena, J.; Bertrand, M.; Bernal, J.L.; Cooper, J.F.; Kadiri, N.; Crowe, D. Field effects of ivermectin residues on dung beetles. J. Appl. Ecol. 1993, 30, 428. [Google Scholar] [CrossRef]
- Wardhaugh, K.G.; Longstaff, B.C.; Lacey, M.J. Effects of residues of deltamethrin in cattle faeces on the development and survival of three species of dungbreeding insect. Aust. Vet. J. 1998, 76, 273–280. [Google Scholar] [CrossRef]
- Wardhaugh, K.G.; Longstaff, B.C.; Morton, R. A comparison of the development and survival of the dung beetle, Onthophagus taurus (Schreb.) when fed on the faeces of cattle treated with pour-on formulations of eprinomectin or moxidectin. Vet. Parasitol. 2001, 99, 155–168. [Google Scholar] [CrossRef]
- Errouissi, F.; Alvinerie, M.; Galtier, P.; Kerboeuf, D.; Lumaret, J.P. The negative effects of the residues of ivermectin in cattle dung using a sustained-release bolus on Aphodius constans (Duft.) (Coleoptera: Aphodiidae). Vet. Res. 2001, 32, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Mougin, C.; Kollmann, A.; Dubroca, J.; Ducrot, P.H.; Alvinerie, M.; Galtier, P. Fate of the veterinary medicine ivermectin in soil. Environ. Chem. Lett. 2003, 1, 131–134. [Google Scholar]
- Floate, K.D.; Wardhaugh, K.G.; Boxall, A.B.; Sherratt, T.N. Fecal residues of veterinary parasiticides: Nontarget effects in the pasture environment. Annu. Rev. Entomol. 2005, 50, 153–179. [Google Scholar] [CrossRef] [PubMed]
- Adler, N.; Bachmann, J.; Blanckenhorn, W.U.; Floate, K.D.; Jensen, J.; Römbke, J. Effects of ivermectin application on the diversity and function of dung and soil fauna: Regulatory and scientific background information. Environ. Toxicol. Chem. 2016, 35, 1914–1923. [Google Scholar] [CrossRef]
- Nieman, C.C.; Floate, K.D.; Düring, R.A.; Heinrich, A.P.; Young, D.K.; Schaefer, D.M. Eprinomectin from a sustained release formulation adversely affected dung breeding insects. PLoS ONE 2018, 13, e0201074. [Google Scholar] [CrossRef] [PubMed]
- Verdú, J.R.; Lobo, J.M.; Sánchez-Piñero, F.; Gallego, B.; Numa, C.; Lumaret, J.P.; Cortez, V.; Ortiz, A.J.; Tonelli, M.; García-Teba, J.P.; et al. Ivermectin residues disrupt dung beetle diversity, soil properties and ecosystem functioning: An interdisciplinary field study. Sci. Total Environ. 2018, 618, 219–228. [Google Scholar] [CrossRef]
- Tonelli, M. Some considerations on the terminology applied to dung beetle functional groups. Ecol. Entomol. 2021. [Google Scholar] [CrossRef]
- Strong, L.; Wall, R.; Woolford, A.; Djeddour, D. The effect of faecally excreted ivermectin and fenbendazole on the insect colonisation of cattle dung following the oral administration of sustained-release boluses. Vet. Parasitol. 1996, 62, 253–266. [Google Scholar] [CrossRef]
- Sands, B.; Noll, M. Toxicity of ivermectin residues in aged farmyard manure to terrestrial and freshwater invertebrates. Insect Conserv. Divers. 2021. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldaner, M.E.; Sobral-Souza, T.; Prasniewski, V.M.; Vaz-de-Mello, F.Z. Effects of Climate Change on the Distribution of Key Native Dung Beetles in South American Grasslands. Agronomy 2021, 11, 2033. https://doi.org/10.3390/agronomy11102033
Maldaner ME, Sobral-Souza T, Prasniewski VM, Vaz-de-Mello FZ. Effects of Climate Change on the Distribution of Key Native Dung Beetles in South American Grasslands. Agronomy. 2021; 11(10):2033. https://doi.org/10.3390/agronomy11102033
Chicago/Turabian StyleMaldaner, Maria Eduarda, Thadeu Sobral-Souza, Victor Mateus Prasniewski, and Fernando Z. Vaz-de-Mello. 2021. "Effects of Climate Change on the Distribution of Key Native Dung Beetles in South American Grasslands" Agronomy 11, no. 10: 2033. https://doi.org/10.3390/agronomy11102033
APA StyleMaldaner, M. E., Sobral-Souza, T., Prasniewski, V. M., & Vaz-de-Mello, F. Z. (2021). Effects of Climate Change on the Distribution of Key Native Dung Beetles in South American Grasslands. Agronomy, 11(10), 2033. https://doi.org/10.3390/agronomy11102033