Soilless System with Supplementary LED Light to Obtain a High-Quality Out-of-Season Production of Green Beans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Plant Material and Growing Condition
2.3. Supplementary Light Treatments
2.4. Growth Analysis, Yield, Fruit Dry Weight, and Physiological Parameters
2.5. Statistical Analysis
3. Results
3.1. Yield, Morphological Parameters, and Colour of Leaves and Pods
3.2. Physiological Parameters: Photosynthesis, Chlorophyll Content, and Chlorophyll Fluorescence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sánchez-Reinoso, A.D.; Ligarreto-Moreno, G.A.; Restrepo-Díaz, H. Drought-tolerant Common Bush Bean Physiological Parameters as Indicators to Identify Susceptibility. HortScience 2019, 54, 2091–2098. [Google Scholar] [CrossRef] [Green Version]
- Folta, K.M.; Childers, K.S. Light as a Growth Regulator: Controlling Plant Biology with Narrow-bandwidth Solid-state Lighting Systems. HortScience 2008, 43, 1957–1964. [Google Scholar] [CrossRef] [Green Version]
- Ouzounis, T.; Fretté, X.; Rosenqvist, E.; Ottosen, C.-O. Spectral effects of supplementary lighting on the secondary metabolites in roses, chrysanthemums, and campanulas. J. Plant Physiol. 2014, 171, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.W.; Park, S.; Jin, J.S.; Seo, O.N.; Kim, G.-S.; Kim, Y.-H.; Bae, H.; Lee, G.; Kim, S.T.; Lee, W.S.; et al. Influences of Four Different Light-Emitting Diode Lights on Flowering and Polyphenol Variations in the Leaves of Chrysanthemum (Chrysanthemum morifolium). J. Agric. Food Chem. 2012, 60, 9793–9800. [Google Scholar] [CrossRef]
- Heuvelink, E.; Bakker, M.; Hogendonk, L.; Janse, J.; Kaarsemaker, R.; Maaswinkel, R. Horticultural lighting in the netherlands: New developments. Acta Hortic. 2006, 25–34. [Google Scholar] [CrossRef]
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130243. [Google Scholar] [CrossRef]
- Polzella, A.; Terzaghi, M.; Trupiano, D.; Baronti, S.; Scippa, G.S.; Chiatante, D.; Montagnoli, A. Morpho-Physiological Responses of Pisum sativum L. to Different Light-Emitting Diode (LED) Light Spectra in Combination with Biochar Amendment. Agronomy 2020, 10, 398. [Google Scholar] [CrossRef] [Green Version]
- Goto, E. Plant production in a closed plant factory with artificial lighting. Acta Hortic. 2012, 37–49. [Google Scholar] [CrossRef]
- Piovene, C.; Orsini, F.; Bosi, S.; Sanoubar, R.; Bregola, V.; Dinelli, G.; Gianquinto, G.P. Optimal red:blue ratio in led lighting for nutraceutical indoor horticulture. Sci. Hortic. 2015, 193, 202–208. [Google Scholar] [CrossRef]
- Paucek, I.; Pennisi, G.; Pistillo, A.; Appolloni, E.; Crepaldi, A.; Calegari, B.; Spinelli, F.; Cellini, A.; Gabarrell, X.; Orsini, F.; et al. Supplementary LED Interlighting Improves Yield and Precocity of Greenhouse Tomatoes in the Mediterranean. Agronomy 2020, 10, 1002. [Google Scholar] [CrossRef]
- Palmitessa, O.D.; Leoni, B.; Montesano, F.F.; Serio, F.; Signore, A.; Santamaria, P. Supplementary Far-Red Light Did Not Affect Tomato Plant Growth or Yield under Mediterranean Greenhouse Conditions. Agronomy 2020, 10, 1849. [Google Scholar] [CrossRef]
- Chen, X.-L.; Guo, W.-Z.; Xue, X.-Z.; Wang, L.-C.; Qiao, X.-J. Growth and quality responses of ‘Green Oak Leaf’ lettuce as affected by monochromic or mixed radiation provided by fluorescent lamp (FL) and light-emitting diode (LED). Sci. Hortic. 2014, 172, 168–175. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2014, 95, 869–877. [Google Scholar] [CrossRef]
- Kopsell, D.; Sams, C.E.; Morrow, R.C. Blue Wavelengths from LED Lighting Increase Nutritionally Important Metabolites in Specialty Crops. HortScience 2015, 50, 1285–1288. [Google Scholar] [CrossRef] [Green Version]
- Qian, H.; Liu, T.; Deng, M.; Miao, H.; Cai, C.; Shen, W.; Wang, Q. Effects of light quality on main health-promoting compounds and antioxidant capacity of Chinese kale sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Samuolienė, G.; Brazaitytė, A.; Jankauskienė, J.; Viršilė, A.; Sirtautas, R.; Novičkovas, A.; Sakalauskienė, S.; Sakalauskaitė, J.; Duchovskis, P. LED irradiance level affects growth and nutritional quality of Brassica microgreens. Open Life Sci. 2013, 8, 1241–1249. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Sakalauskienė, S.; Samuolienė, G.; Jankauskienė, J.; Viršilė, A.; Novičkovas, A.; Sirtautas, R.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Dabašinskas, L.; et al. The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef]
- Meas, S.; Luengwilai, K.; Thongket, T. Enhancing growth and phytochemicals of two amaranth microgreens by LEDs light irradiation. Sci. Hortic. 2020, 265, 109204. [Google Scholar] [CrossRef]
- Wojciechowska, R.; Kołton, A.; Długosz-Grochowska, O.; Knop, E. Nitrate content in Valerianella locusta L. plants is affected by supplemental LED lighting. Sci. Hortic. 2016, 211, 179–186. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Sirtautas, R.; Novičkovas, A.; Duchovskis, P. The effect of supplementary led lighting on the antioxidant and nutritional properties of lettuce. Acta Hortic. 2012, 835–841. [Google Scholar] [CrossRef]
- Signore, A.; Bell, L.; Santamaria, P.; Wagstaff, C.; Van Labeke, M.-C. Red Light Is Effective in Reducing Nitrate Concentration in Rocket by Increasing Nitrate Reductase Activity, and Contributes to Increased Total Glucosinolates Content. Front. Plant Sci. 2020, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; van Iersel, M.; Bugbee, B. Why Far-Red Photons Should Be Included in the Definition of Photosynthetic Photons and the Measurement of Horticultural Fixture Efficacy. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.G.; Mele, B.H.; Vitale, L.; Vitale, E.; Arena, C. The role of monochromatic red and blue light in tomato early photomorphogenesis and photosynthetic traits. Environ. Exp. Bot. 2020, 179, 104195. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, O.K. Blue Light added with Red LEDs Enhance Growth Characteristics, Pigments Content, and Antioxidant Capacity in Lettuce, Spinach, Kale, Basil, and Sweet Pepper in a Controlled Environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, E.; Ouzounis, T.; Giday, H.; Schipper, R.; Heuvelink, E.; Marcelis, L.F.M. Adding Blue to Red Supplemental Light Increases Biomass and Yield of Greenhouse-Grown Tomatoes, but Only to an Optimum. Front. Plant Sci. 2019, 9, 2002. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Y.; Xu, X.M.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 2014, 53, 213–222. [Google Scholar] [CrossRef]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Mekbib, F. Yield stability in common bean (Phaseolus vulgaris L.) genotypes. Euphytica 2003, 130, 147–153. [Google Scholar] [CrossRef]
- Graham, P.; Ranalli, P. Common bean (Phaseolus vulgaris L.). Field Crop. Res. 1997, 53, 131–146. [Google Scholar] [CrossRef]
- El Youssfi, L.; Choukr-Allah, R.; Santamaria, P.; Montesano, F.F. Soilless Closed Cycle Production Of Green Bean (Phaseolus vulgaris L.) Using Subirrigation: Effects On Yield, Fruit Quality, Substrate And Nutrient Solution Parameters. Acta Hortic. 2012, 383–390. [Google Scholar] [CrossRef]
- Palmitessa, O.; Pantaleo, M.; Santamaria, P. Applications and Development of LEDs as Supplementary Lighting for Tomato at Different Latitudes. Agronomy 2021, 11, 835. [Google Scholar] [CrossRef]
- Leser, S. The 2013 FAO report on dietary protein quality evaluation in human nutrition: Recommendations and implications. Nutr. Bull. 2013, 38, 421–428. [Google Scholar] [CrossRef]
- Kusumah, S.H.; Andoyo, R.; Rialita, T. Isolation and Characterization of Red Bean and Green Bean Protein using the Extraction Method and Isoelectric pH. SciMedicine J. 2020, 2, 77–85. [Google Scholar] [CrossRef]
- Sancarlos, A.; Cameron, M.; Abel, A.; Cueto, E.; Duval, J.L.; Chinesta, F. From ROM of Electrochemistry to AI-Based Battery Digital and Hybrid Twin; Springer: Berlin/Heidelberg, Germany, 2021; Volume 28, ISBN 0123456789. [Google Scholar]
- Johnson, R.E.; Kong, Y.; Zheng, Y. Elongation growth mediated by blue light varies with light intensities and plant species: A comparison with red light in arugula and mustard seedlings. Environ. Exp. Bot. 2019, 169, 103898. [Google Scholar] [CrossRef]
- Huché-Thélier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations—Perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Renna, M.; D’Imperio, M.; Gonnella, M.; Parente, A.; Santamaria, P.; Serio, F. Barattiere: An Italian Local Variety of Cucumis melo L. with Quality Traits between Melon and Cucumber. Plants 2020, 9, 578. [Google Scholar] [CrossRef] [PubMed]
- Pathare, P.; Opara, U.L.; Al-Said, F.A.-J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2012, 6, 36–60. [Google Scholar] [CrossRef]
- Paciulli, M.; Palermo, M.; Chiavaro, E.; Pellegrini, N. Chlorophylls and Colour Changes in Cooked Vegetables. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health; Yahia, E.M., Ed.; John Wiley & Sons Ltd: Hoboken, NJ, USA, 2018; pp. 703–719. ISBN 9781119158042. [Google Scholar]
- Pola, W.; Sugaya, S.; Photchanachai, S. Color Development and Phytochemical Changes in Mature Green Chili (Capsicum annuum L.) Exposed to Red and Blue Light-Emitting Diodes. J. Agric. Food Chem. 2019, 68, 59–66. [Google Scholar] [CrossRef]
- Hogewoning, S.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Caemmerer, S.; Evans, J. Temperature responses of mesophyll conductance differ greatly between species. Plant Cell Environ. 2014, 38, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lysenko, V.; Guo, Y.; Kosolapov, A.; Usova, E.; Varduny, T.; Krasnov, V. Polychromatic Fourier-PAM fluorometry and hyperspectral analysis of chlorophyll fluorescence from Phaseolus vulgaris leaves: Effects of green light. Inf. Process. Agric. 2019, 7, 204–211. [Google Scholar] [CrossRef]

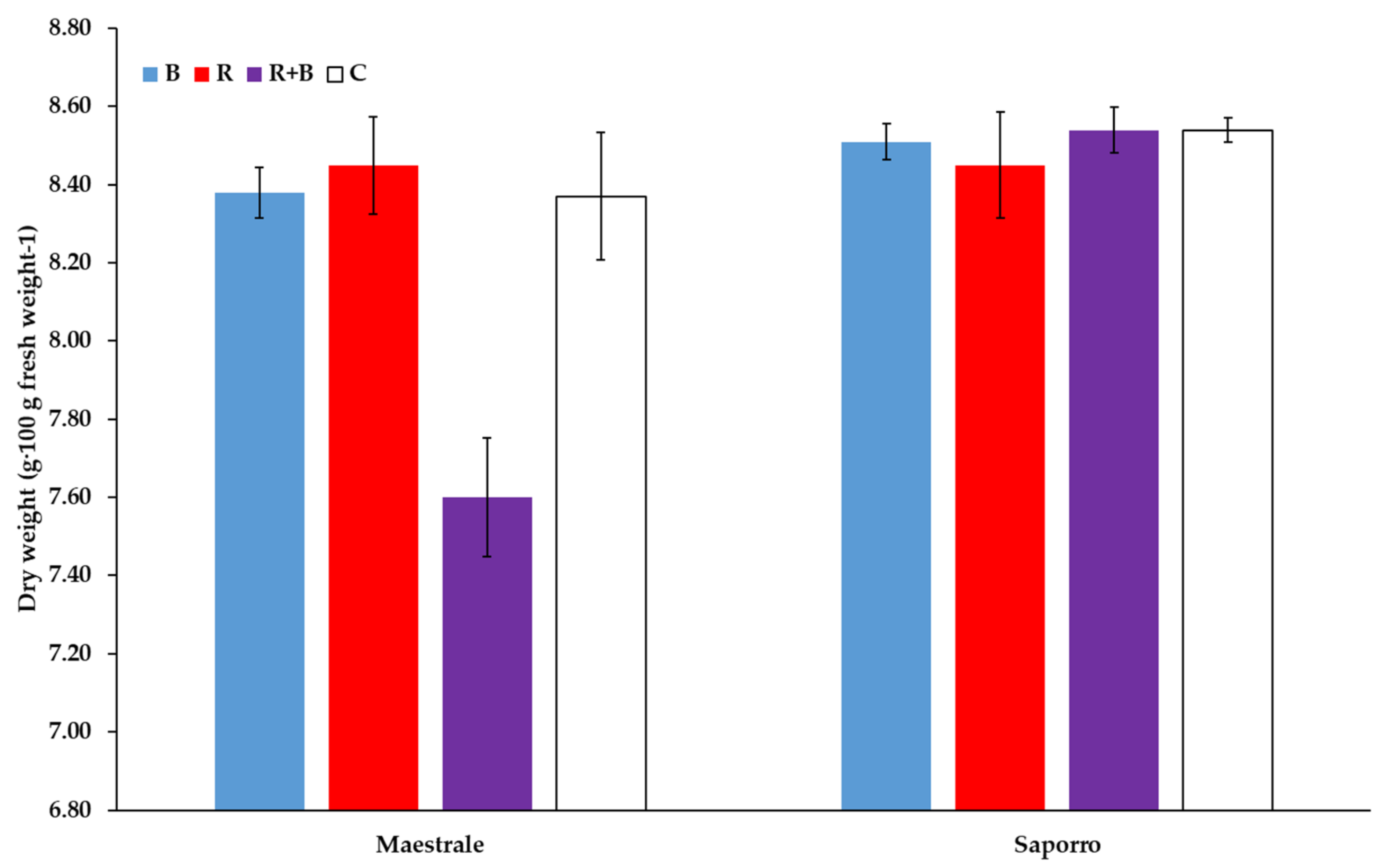
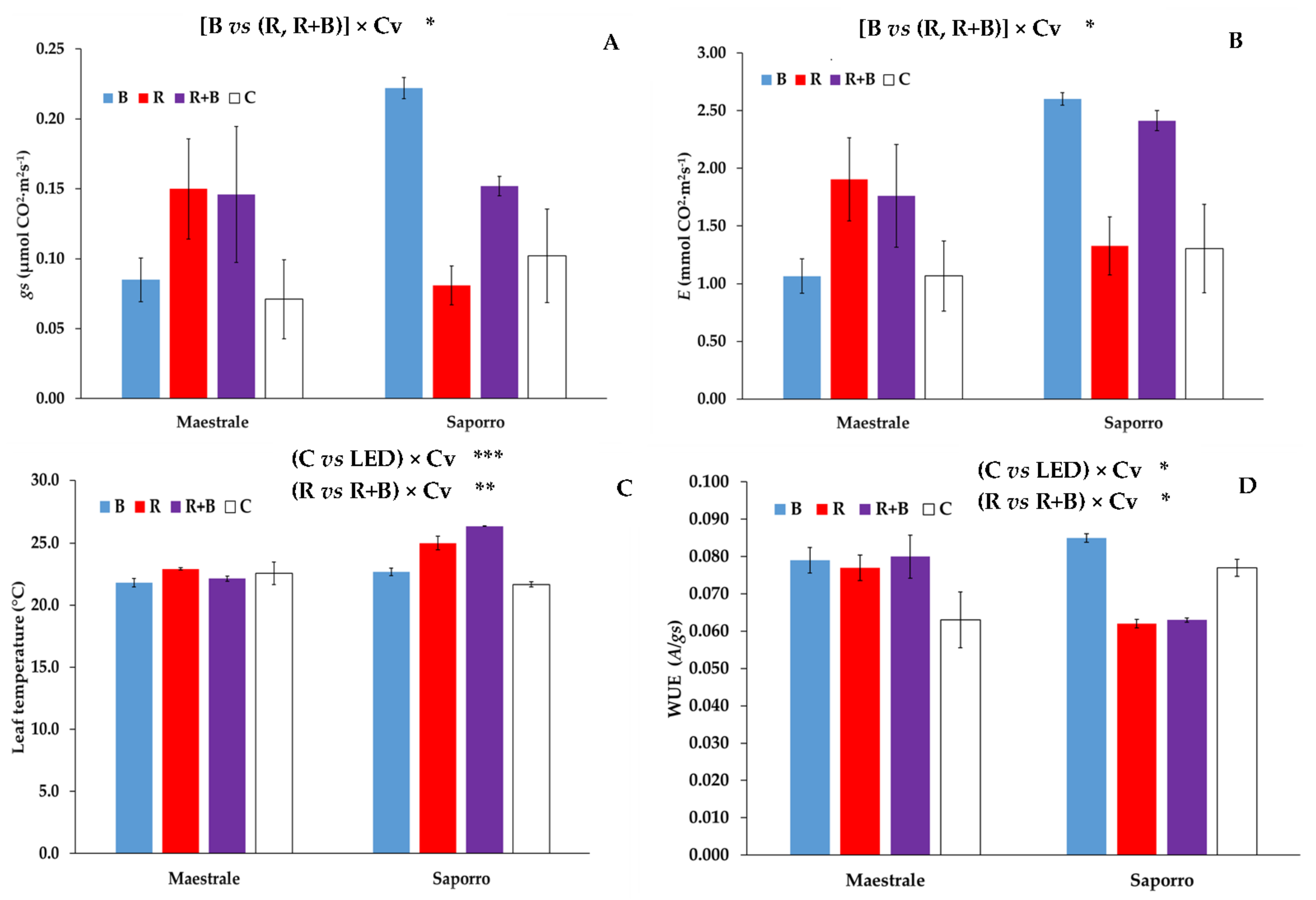
| Fresh Weight | Number | Height | Leaves | Leaf Area | Dry Matter | |
|---|---|---|---|---|---|---|
| g∙Plant−1 | n.∙Plant−1 | cm | n. | cm2 | g∙100 g−1 Fresh Weight | |
| Light (L) | ||||||
| Blue (B) | 154.5 | 76.4 | 53.35 | 27.5 | 4141 | 8.45 |
| Red + Blue (R + B) | 140.0 | 70.7 | 54.27 | 26.7 | 4368 | 8.07 |
| Red (R) | 141.9 | 70.3 | 54.33 | 26.5 | 4372 | 8.45 |
| Control (C) | 122.5 | 66.5 | 51.02 | 25.3 | 3982 | 8.46 |
| Cultivar (Cv) | ||||||
| Maestrale | 149.2 | 73.6 | 54.63 | 27.58 | 4546 | 8.20 |
| Saporro | 130.3 | 68.4 | 51.85 | 25.42 | 3885 | 8.51 |
| Significance 1 | ||||||
| C vs. LED | * | ns | * | ns | ns | ns |
| B vs. (R, R + B) | ns | ns | ns | ns | ns | * |
| R vs. R + B | ns | ns | ns | ns | ns | ** |
| Cv | ** | * | ns | ns | * | * |
| (C vs. LED) × Cv | ns | ns | ns | ns | ns | ns |
| [B vs. (R, R + B)] × Cv | ns | ns | ns | ns | ns | ns |
| (R vs. R + B) × Cv | ns | ns | ns | ns | ns | ** |
| L* | a* | b* | h° | C | |
|---|---|---|---|---|---|
| (0–100) | (−60/+60) | (−60/+60) | √(a2 + b2) | (0–360)° | |
| Light (L) | |||||
| Blue (B) | 42.70 | −15.35 | 25.92 | 120.66 | 31.28 |
| Red + Blue (R + B) | 40.37 | −15.29 | 26.02 | 120.53 | 30.12 |
| Red (R) | 39.18 | −14.69 | 25.28 | 120.20 | 29.24 |
| Control (C) | 41.34 | −15.81 | 26.39 | 120.93 | 30.76 |
| Cultivar (Cv) | |||||
| Maestrale | 39.10 | −14.77 | 25.02 | 120.61 | 26.60 |
| Saporro | 42.69 | −15.80 | 26.78 | 120.54 | 31.10 |
| Significance 1 | |||||
| C vs. LED | ns | ns | ns | ns | ns |
| B vs. (R, R + B) | ** | ns | ns | ns | ns |
| R vs. R + B | ns | ns | ns | ns | * |
| Cv | * | ** | * | ns | * |
| (C vs. LED) × Cv | ns | ns | ns | ns | ns |
| [B vs. (R, R + B)] × Cv | ns | ns | ns | ns | ns |
| (R vs. R + B) × Cv | ns | ns | ns | ns | ns |
| L* | a* | b* | h° | C | Chlorophyll Content | |
|---|---|---|---|---|---|---|
| (0–100) | (−60/+60) | (−60/+60) | √(a2 + b2) | (0–360)° | µmol∙m−2 | |
| Light (L) | ||||||
| Blue (B) | 35.79 | 363 | −12.57 | 14.16 | 131.69 | 18.94 |
| Red + Blue (R + B) | 36.12 | 318 | −12.70 | 14.72 | 130.80 | 19.44 |
| Red (R) | 37.13 | 305 | −14.02 | 16.41 | 130.53 | 21.59 |
| Control (C) | 35.92 | 289 | −13.29 | 15.08 | 131.39 | 20.10 |
| Cultivar (Cv) | ||||||
| Maestrale | 36.98 | 325 | −12.91 | 14.80 | 131.15 | 16.64 |
| Saporro | 36.49 | 313 | −13.38 | 15.39 | 131.06 | 20.39 |
| Significance 1 | ||||||
| C vs. LED | ns | * | ns | ns | ns | ns |
| B vs. (R, R + B) | ns | * | ns | * | * | * |
| R vs. R + B | ns | ns | * | * | ns | * |
| Cv | ns | ns | ns | ns | ns | ns |
| (C vs. LED) × Cv | ns | ns | ns | ns | ns | ns |
| [B vs. (R, R + B)] × Cv | ns | ns | ns | ns | ns | ns |
| (R vs. R + B) × Cv | ns | ns | ns | ns | ns | ns |
| A | gs | E | Leaf T | WUE | |
|---|---|---|---|---|---|
| µmol CO2·m2s−1 | µmol H2O·m2s−1 | mmol H2O·m2s−1 | °C | A/gs | |
| Light (L) | |||||
| Blue (B) | 13.4 | 0.153 | 1.83 | 22.26 | 0.082 |
| Red + Blue (R + B) | 13.5 | 0.149 | 2.09 | 24.26 | 0.071 |
| Red (R) | 13.3 | 0.116 | 1.62 | 23.97 | 0.070 |
| Control (C) | 11.1 | 0.087 | 1.19 | 22.13 | 0.070 |
| Cultivar (Cv) | |||||
| Maestrale | 12.3 | 0.113 | 1.45 | 22.37 | 0.075 |
| Saporro | 13.4 | 0.140 | 1.91 | 23.93 | 0.072 |
| Significance 1 | |||||
| C vs. LED | * | * | ** | ** | ns |
| B vs. (R, R + B) | ns | ns | ns | ns | * |
| R vs. R + B | ns | ns | ns | ** | ns |
| Cv | ns | ns | ns | *** | ns |
| (C vs. LED) × Cv | ns | ns | ns | *** | * |
| [B vs. (R, R × B)] x Cv | ns | * | * | ns | * |
| (R vs. R + B) × Cv | ns | ns | ns | ** | ns |
| F | F0′ | Fm′ | ΦPSII | NPQ | qP | Fv/Fm | |
|---|---|---|---|---|---|---|---|
| Light (L) | |||||||
| Blue (B) | 0.544 | 0.371 | 1.154 | 0.528 | 0.356 | 0.779 | 0.771 |
| Red + Blue (R + B) | 0.570 | 0.382 | 1.132 | 0.495 | 0.483 | 0.752 | 0.769 |
| Red (R) | 0.542 | 0.366 | 1.010 | 0.453 | 0.637 | 0.733 | 0.775 |
| Control (C) | 0.582 | 0.380 | 1.135 | 0.488 | 0.476 | 0.709 | 0.786 |
| Cultivar (Cv) | |||||||
| Maestral | 0.559 | 0.378 | 1.076 | 0.476 | 0.537 | 0.733 | 0.768 |
| Saporro | 0.560 | 0.372 | 1.114 | 0.507 | 0.439 | 0.753 | 0.783 |
| Significance 1 | |||||||
| C vs. LED | ns | ns | ns | ns | ns | ns | ns |
| B vs. (R, R + B) | ns | ns | * | ns | ns | ns | ns |
| R vs. R + B | ns | ns | ns | ns | ns | ns | ns |
| Cv | ns | ns | ns | * | ns | ns | ns |
| (C vs. LED) × Cv | ns | ns | ns | ns | ns | ns | ns |
| [B vs. (R, R + B)] × Cv | ns | ns | ns | ns | ns | ns | ns |
| (R vs. R + B) × Cv | ns | ns | ns | ns | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signore, A.; Leoni, B.; Palmitessa, O.D.; Santamaria, P. Soilless System with Supplementary LED Light to Obtain a High-Quality Out-of-Season Production of Green Beans. Agronomy 2021, 11, 1999. https://doi.org/10.3390/agronomy11101999
Signore A, Leoni B, Palmitessa OD, Santamaria P. Soilless System with Supplementary LED Light to Obtain a High-Quality Out-of-Season Production of Green Beans. Agronomy. 2021; 11(10):1999. https://doi.org/10.3390/agronomy11101999
Chicago/Turabian StyleSignore, Angelo, Beniamino Leoni, Onofrio Davide Palmitessa, and Pietro Santamaria. 2021. "Soilless System with Supplementary LED Light to Obtain a High-Quality Out-of-Season Production of Green Beans" Agronomy 11, no. 10: 1999. https://doi.org/10.3390/agronomy11101999
APA StyleSignore, A., Leoni, B., Palmitessa, O. D., & Santamaria, P. (2021). Soilless System with Supplementary LED Light to Obtain a High-Quality Out-of-Season Production of Green Beans. Agronomy, 11(10), 1999. https://doi.org/10.3390/agronomy11101999









