Phosphorus Behaviour and Its Basic Indices under Organic Matter Transformation in Variable Moisture Conditions: A Case Study of Fen Organic Soils in the Odra River Valley, Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. Methods
2.3. Statistics
3. Results and Discussion
3.1. Morphology of Soils
3.2. Basic Soil Characteristics
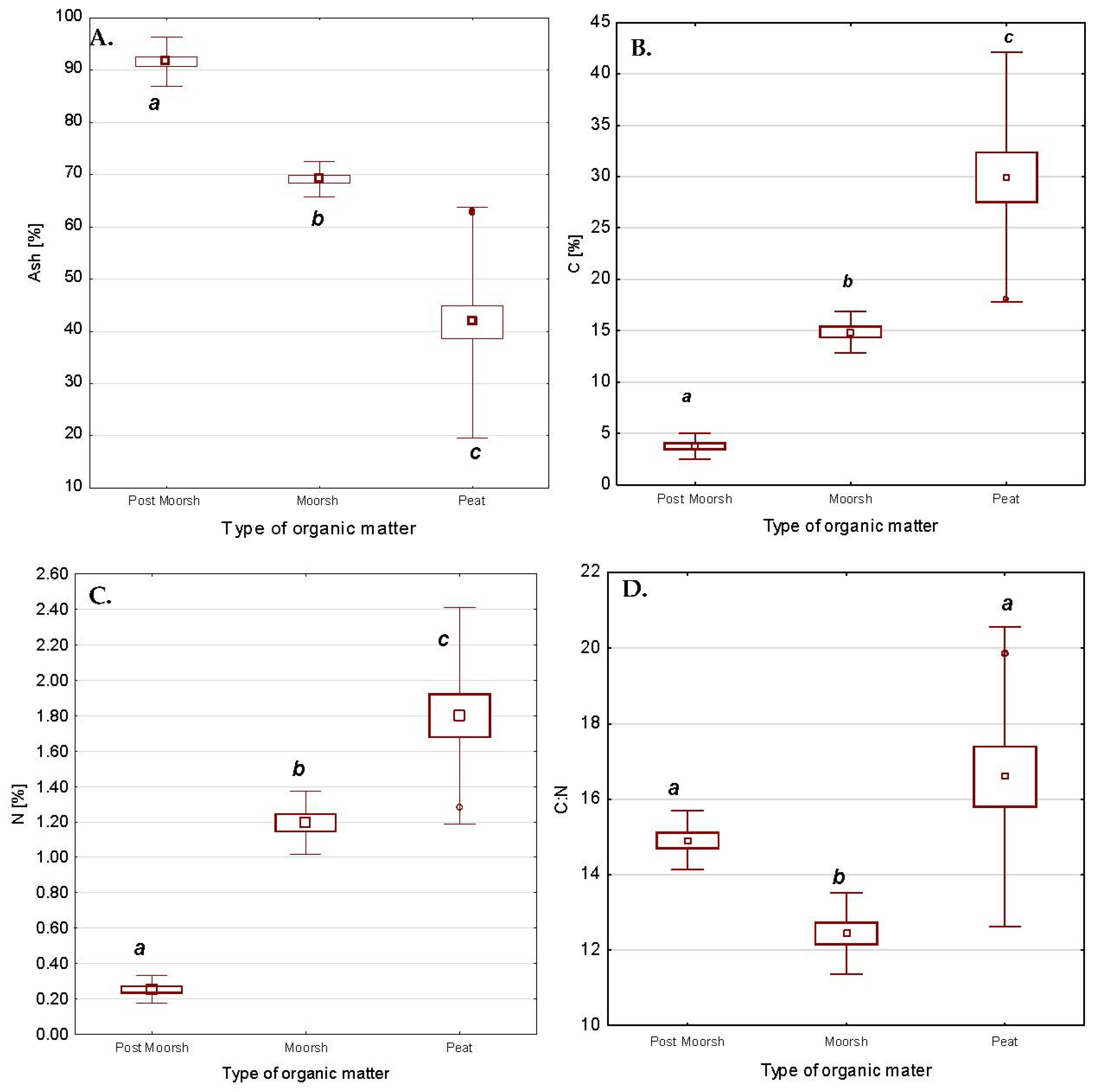
3.2.1. Degree of Peat Decomposition
3.2.2. Ash Content
3.2.3. pH Value and CaCO3 Content
3.2.4. Content of C and N
3.2.5. C:N Ratio
3.2.6. Sorption Properties
3.2.7. Alox and Feox Content
3.2.8. The Phosphorus Sorption Capacity
3.3. Phosphorus Behaviour in Soil
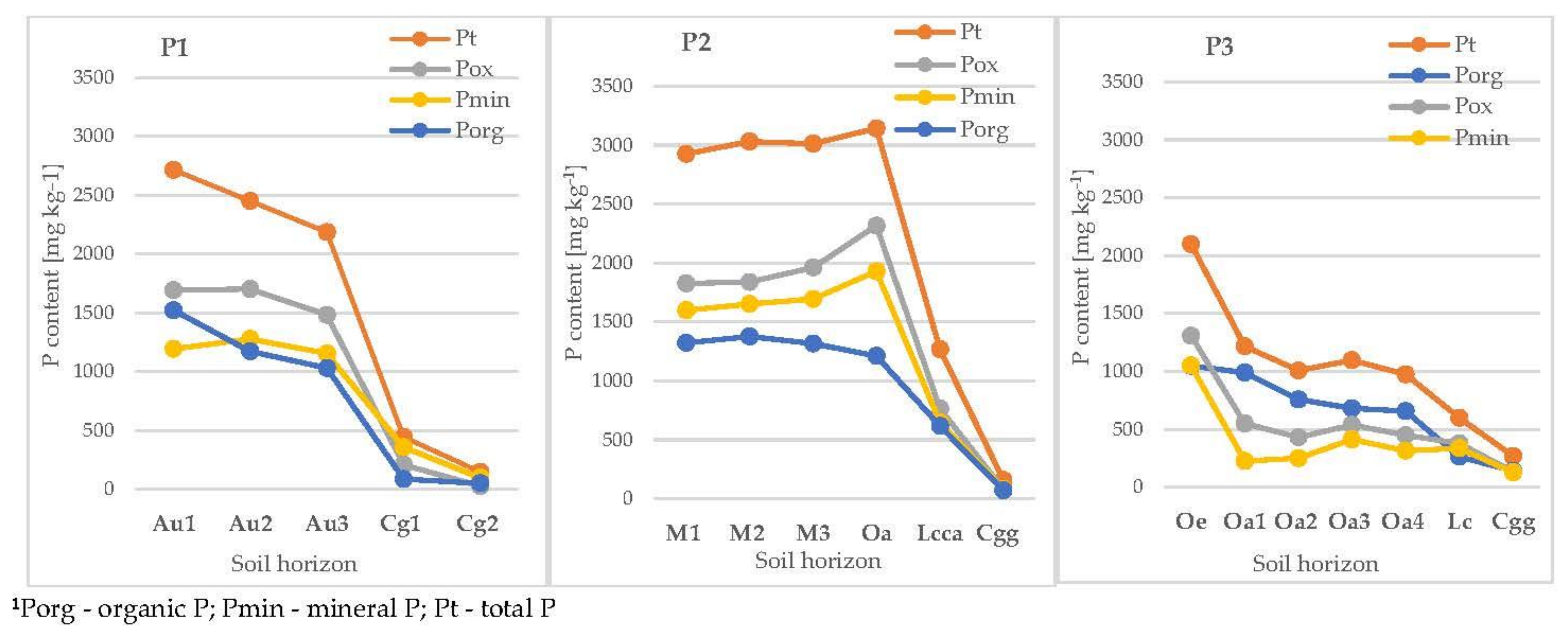
3.3.1. Total and Mineral Phosphorus
3.3.2. Organic Phosphorus
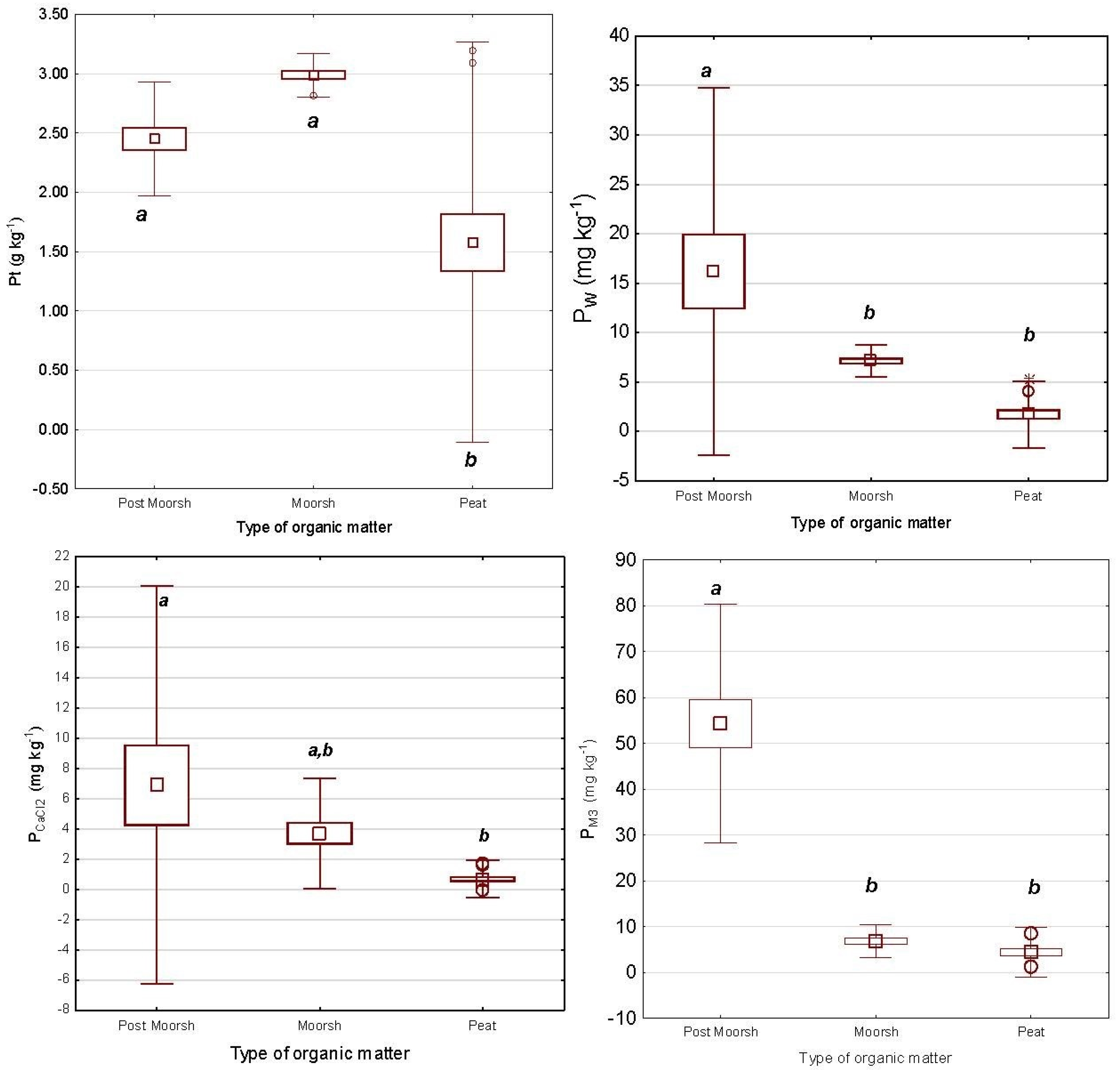
3.3.3. Soluble Forms of Phosphorus
3.4. Indicators of Phosphorus Accumulation and/or Release in the Soil
3.4.1. Phosphorus Saturation Degree (PSD)
3.4.2. Ratios of C:P and N:P
3.4.3. Solubility Index (Ip)
3.5. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports; FAO: Rome, Italy, 2015. [Google Scholar]
- Xu, J.; Morris, P.J.; Liu, J.; Holden, J. Peatmap: Refining estimates of global peatland distribution based on a meta-analysis. Catena 2018, 160, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Joosten, H.; Tapio-Biström, M.L.; Tol, S. Peatlands: Guidance for Climate Change Mitigation through Conservation, Rehabilitation and Sustainable Use, 2nd ed.; Food and Agriculture Organization of the United Nations and Wetlands International: Rome, Italy, 2012; p. 100. [Google Scholar]
- Tubiello, F.N.; Biancalani, R.; Salvatore, M.; Rossi, S.; Conchedda, G. A worldwide assessment of greenhouse gas emissions from drained organic soils. Sustainability 2016, 8, 371. [Google Scholar] [CrossRef] [Green Version]
- Szuflicki, M.; Malon, A.; Tymiński, M. Balance Sheet of Mineral Resources in Poland as at 31.XII.2020 (In Polish); Państwowy Instytut Geologiczny-Państwowy Instytut Badawczy: Warsaw, Poland, 2021. [Google Scholar]
- Delarue, F.; Laggoun-Défarge, F.; Buttler, A.; Gogo, S.; Jassey, V.E.J.; Disnar, J.R. Effects of short-term ecosystem experimental warming on water-extractable organic matter in an ombrotrophic Sphagnum peatland (Le Forbonnet, France). Organic. Geochem. 2011, 42, 1016–1024. [Google Scholar] [CrossRef] [Green Version]
- Keller, J.K.; Medvedeff, C.A. Soil Organic Matter. In Wetland Soils: Genesis, Hydrology, Landscapes, and Classification, 2nd ed.; Vepraskas, M.J., Craft, C.B., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 165–188. [Google Scholar]
- Grenon, G.; Singh, B.; de Sena, A.; Madramootoo, C.A.; von Sperber, C.; Goyal, M.K.; Zhang, T. Phosphorus fate, transport and management on subsurface drained agricultural organic soils: A review. Environ. Res. Lett. 2021, 16, 013004. [Google Scholar] [CrossRef]
- Piaścik, H.; Gotkiewicz, T. Transformation of drained peat soils as a cause of their degradation (In Polish). Soil Sci. Ann. 2004, 40, 331–338. [Google Scholar]
- Szajdak, L.; Brandyk, T.; Szatyłowicz, J. Chemical properties of different peat-moorsh soils from the Biebrza River Valley. Agron. Res. 2007, 5, 165–174. [Google Scholar]
- Kalisz, B.; Lachacz, A.; Glazewski, R. Effects of peat drainage on labile organic carbon and water repellency in NE Poland. Turkish J. Agric. For. 2015, 39, 20–27. [Google Scholar] [CrossRef]
- Zając, E.; Zarzycki, J.; Ryczek, M. Degradation of peat surface on an abandoned post extracted bog and implications for re-vegetation. Appl Ecol Environ. Res. 2018, 16, 3363–3380. [Google Scholar] [CrossRef]
- Ilnicki, P. Peatlands and Peat (In Polish); Wydawnictwo Akademii Rolniczej: Poznań, Poland, 2002. [Google Scholar]
- Ilnicki, P.; Zeitz, J. Irreversible Loss of Organic Soil Functions after Reclamation. In Organic Soils and Peat. Materials for Sustainable Agriculture; Parent, L.E., Ilnicki, P., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 15–32. [Google Scholar]
- Okruszko, H.; Ilnicki, P. The Moorsh Horizons as Quality Indicators of Reclaimed Organic Soils. In Organic Soils and Peat. Materials for Sustainable Agriculture, Parent, L.E., Ilnicki, P., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 1–14. [Google Scholar]
- Roj-Rojewski, S.; Korol, A.; Zienkiewicz, A. Water conditions influence on physical properties and plant cover of muck soils located on drained mud habitats (In Polish). Inż. Ekol. 2012, 29, 141–152. [Google Scholar]
- Becher, M.; Pakuła, K.; Jaremko, D. Phosphorus accumulation in the dehydrated peat soils of the Liwiec river valley. J. Ecol. Eng. 2020, 21, 213–220. [Google Scholar] [CrossRef]
- Łachacz, A.; Nitkiewicz, M.; Kalisz, B. Water repellency of post boggy soils with a various content of organic matter (In Polish). Biologia 2009, 64, 634–638. [Google Scholar] [CrossRef]
- Zheng, Z.M.; Zhang, T.Q.; Wen, G.; Kessel, C.; Tan, C.S.; O’Halloran, I.P.; Reid, D.K.; Nemeth, D.; Speranzini, D. Soil testing to predict dissolved reactive phosphorus loss in surface runoff from organic soils. Soil Sci. Soc. Am. J. 2014, 78, 1786–1796. [Google Scholar] [CrossRef]
- Zheng, Z.M.; Zhang, T.Q.; Kessel, C.; Tan, C.S.; O’Halloran, I.P.; Wang, Y.T.; Speranzini, D.; Van Eerd, L.L. Approximating phosphorus leaching from agricultural organic soils by soil testing. J. Environ. Qual. 2015, 44, 1871–1882. [Google Scholar] [CrossRef]
- Sapek, B. Soil phosphorus accumulation and release—Sources, processes, causes (In Polish). Water-Environ.-Rural. Areas 2014, 14, 77–100. [Google Scholar]
- Stępniewska, Z.; Borkowska, A.; Kotowska, U. Phosphorus from peat soils under flooded conditions of the Łęczyńsko-Włodawskie Lake District. Int. Agrophysics 2006, 20, 237–243. [Google Scholar]
- Kang, J.; Hesterberg, D.; Osmond, D.L. Soil organic matter effects on phosphorus sorption: A Path Analysis. Soil Sci. Soc. Am. J. 2009, 73, 360–366. [Google Scholar] [CrossRef]
- Sapek, B. Phosphorus sorption by muck and peat formations in the Biebrza River valley (In Polish). Water-Environ.-Rural. Areas 2011, 11, 219–235. [Google Scholar]
- Becher, M.; Pakuła, K.; Pielech, J.; Trzcińska, E. Phosphorus resources and fractions in peat-muck soils. Environ. Prot. Nat. Resour. 2018, 29, 1–6. [Google Scholar] [CrossRef]
- Riddle, M.; Bergström, L.; Schmieder, F.; Kirchmann, H.; Condron, L.; Aronsson, H. Phosphorus Leaching from an Organic and a Mineral Arable Soil in a Rainfall Simulation Study. J. Environ. Qual. 2018, 47, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, J.; Stroetenga, M.; Bos, L.; van Bodegom, P.M.; Verhoef, H.A.; Aerts, R. Restoring natural seepage conditions on former agricultural grasslands does not lead to reduction of organic matter decomposition and soil nutrient dynamics. Biogeochem. 2004, 71, 317–337. [Google Scholar] [CrossRef]
- Richardson, C.J. Mechanisms controlling phosphorus retention capacity in fresh-water wetlands. Science 1985, 228, 1424–1427. [Google Scholar] [CrossRef]
- Rockwell, D.C.; Warren, G.J.; Bertram, P.E.; Salisbury, D.K.; Burns, N.M. The US EPA lake Erie indicators monitoring program 1983–2002: Trends in phosphorus, silica, and chlorophyll α in the central basin. J. Great Lakes Res. 2005, 31, 23–34. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.J.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, K.W.; Williams, M.R.; Macrae, M.L.; Fausey, N.R.; Frankenberger, J.; Smith, D.R.; Kleinman, P.J.A.; Brown, L.C. Phosphorus transport in agricultural subsurface drainage: A review. J. Environ. Qual. 2015, 44, 467–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joosten, H.; Clarke, D. Wise Use of Mires and Peatlands—Background and Principles Including a Framework for Decision-Making; International Mire Conservation Group/International Peat Society: Saarijärvi, Finland, 2002; p. 304. [Google Scholar]
- Heller, C.; Zeitz, J. Stability of soil organic matter in two northeastern German fen soils: The influence of site and soil development. J. Soils Sediments 2012, 12, 1231–1240. [Google Scholar] [CrossRef]
- Gnatowski, T.; Szatyłowicz, J.; Brandyk, T.; Kechavarzi, C. Hydraulic properties of fen peat soils in Poland. Geoderma 2010, 154, 188–195. [Google Scholar] [CrossRef]
- Kalembasa, D.; Pakuła, K.; Becher, M.; Jaremko, D. Trace elements in the peat-muck soils in the upper Liwiec river valley. Fresen Environ. Bull. 2009, 18, 1201–1204. [Google Scholar]
- Lipka, K. Peatlands in the Vistula basin as an element of the natural environment (In Polish). Zesz. Nauk. AR Krakowie Ser. Rozpr. 2000, 255, 1–148. [Google Scholar]
- Lipka, K.; Zając, E.; Wdowik, W. The impact of land use on the disappearance of peat-moorsh soils in the Mrowla River valley near Rzeszów (In Polish). Zesz. Probl. Post. Nauk Roln. 2005, 507, 349–355. [Google Scholar]
- Glina, B.; Gajewski, P.; Kaczmarek, Z.; Owczarzak, W.; Rybczyński, P. Current state of peatland soils as an effect of long-term drainage—Preliminary results of peatland ecosystems investigation in the Grójecka Valley (central Poland). Soil Sci. Ann. 2016, 67, 3–9. [Google Scholar] [CrossRef]
- Litaor, M.I.; Reichmann, O.; Auerswald, K.; Haimand, A.; Shenker, M. The geochemistry of phosphorus in peat soils of a semiarid altered wetland. Soil Sci. Soc. Am. J. 2004, 68, 2078–2085. [Google Scholar] [CrossRef]
- Giedrojc, B. Physical and chemical properties of some organic soils in the Średzianka Valley in Lower Silesia (In Polish). Soil Sci. Ann. 1985, 36, 51–65. [Google Scholar]
- Stankiewicz, A. Chemical properties of peat soils of the meadow part of the spring fens near Przedmość (In Polish). Master’s Thesis, University of Agriculture in Wroclaw, Wrocław, Poland, 1983. [Google Scholar]
- Szulc, A.; Bogacz, A. Evolution of physical properties of mucky peat soils in differently dried forest and meadows areas (In Polish). Zesz. Nauk. Uniw. Zielonogórskiego Inżynieria Sr. 2002, 12, 347–352. [Google Scholar]
- Bogacz, A.; Szulc, A.; Bober, A.; Pląskowska, E.; Matkowski, K. Influence of the degree of peat mucking on the composition and abundance of soil fungi of the Przedmoście site (In Polish). Soil Sci. Ann. 2004, 55, 39–51. [Google Scholar]
- Bogacz, A.; Grodzińska, D.; Chachuła, K.; Kasowska, D. Characteristics of meadow hydrogenic soil in Przedmoście against the background of forming plant communities. Acta Agrophysica 2001, 50, 17–28. [Google Scholar]
- Kiryluk, A. Influence of perennial meadow use of a lowland peatland on the quality of biomass and formation of plant communities in the Supraśl river valley (In Polish). Zesz. Probl. Post. Nauk Roln. 1996, 38, 154–199. [Google Scholar]
- Verhoeven, J.T.A. (Ed.) Fens and Bogs in The Netherlands: Vegetation, History, Nutrient Dynamics and Conservation; Kluwer Acad. Publ: Dordrecht, The Netherlands, 1992; p. 472. [Google Scholar]
- Szulc, A.; Bogacz, A. Formation of properties of muck soils of meadow and forest sites under different degrees of drying (In Polish). Zesz. Nauk. Uniw. Zielonogórskiego Inżynieria Sr. 2004, 12, 347–352. [Google Scholar]
- Lynn, W.C.; McKinzie, W.E.; Grossman, R.B. Field Laboratory Tests for Characterization of Histosols. In Histosols: Their Characteristics, Classification and Use; Stelly, M., Ed.; SSSA Spec.: Madison, WI, USA, 1974; pp. 11–20. [Google Scholar]
- Şenlikci, A.; Doğu, M.; Eren, E.; Çetinkaya, E.; Karadağ, S. Pressure calcimeter as a simple method for measuring the CaCO3 content of soil and comparison with Scheibler calcimeter. Soil-Water J. 2015, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer: Berlin, Heidelberg, 2006; pp. 596–599. [Google Scholar]
- McKeague, J.A.; Day, J.H. Dithionite and oxalate Fe and Al as aids in differentiating various classes of soils. Can. J. Soil Sci. 1966, 46, 13–22. [Google Scholar] [CrossRef]
- van der Zee, S.E.A.T.M.; Fokkink, L.G.J.; van Riemsdijk, W.H. A new technique for assessment of reversibly adsorbed phosphate. Soil Sci. Soc. Am. J. 1987, 51, 599–604. [Google Scholar] [CrossRef]
- van der Zee, S.E.A.T.M.; van Riemsdijk, W.H. Model for long-term phosphate reaction kinetics in soil. J. Environ. Qual. 1988, 17, 35–41. [Google Scholar]
- Freese, D.; van der Zee, S.E.A.T.M.; van Riemsdijk, W.H. Comparison of different models for phosphate sorption as a function of the iron and aluminium oxides of soils. J. Soil Sci. 1992, 43, 729–738. [Google Scholar] [CrossRef]
- Zhang, H.; Shroder, J.L.; Fuhrman, J.K.; Basta, N.T.; Storm, D.E.; Payton, M.E. Path and multiple regression analyses of phosphorus sorption capacity. Soil Sci. Soc. Am. J. 2005, 69, 96–106. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Houba, V.J.G.; Temminghoff, E.J.M. Behaviour of phosphate in soil extracts using weak unbuffered extracting solutions. Commun. Soil Sci. Plant Anal. 1999, 30, 1367–1370. [Google Scholar] [CrossRef]
- Saunders, W.M.H.; Williaus, E.G. Observations on the determination of total organic phosphorus in soils. J. Soil Sci. 1955, 6, 254–267. [Google Scholar] [CrossRef]
- Walker, T.W.; Adams, A.F.R. Studies on soil organic matter. J. Soil Sci. 1958, 85, 307–318. [Google Scholar] [CrossRef]
- Shah, R.; Syers, J.K.; Williams, J.D.H. The forms of inorganic phosphorus extracted from soils by N Sulfuric acid, N.Z.J. Agric. Res. 1968, 11, 184–192. [Google Scholar]
- TIBCO Software Inc. Statistica (Data Analysis Software System), 2017, Version 13. Available online: http://statistica.io (accessed on 29 September 2021).
- Polish Soil Classification (Systematyka Gleb Polski). Soil Science Society of Poland, Commission on Soil Genesis, Classification and Cartography; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu, Polskie Towarzystwo Gleboznawcze, Wrocław: Warszawa, Poland, 2019; p. 235. [Google Scholar]
- Biester, H.; Knorr, K.H.; Schellekens, J.; Basler, A.; Hermanns, Y.M. Comparison of different methods to determine the degree of peat decomposition in peat bogs. Biogeosciences 2014, 11, 2691–2707. [Google Scholar] [CrossRef] [Green Version]
- Leifeld, J.; Klein, K.; Wüst-Galley, C. Soil organic matter stoichiometry as indicator for peatland degradation. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Bengtsson, G.; Bengtson, P.; Månsson, K.F. Gross nitrogen mineralization-, immobilization-, and nitrification-rates as a function of soil C/N ratio and microbial activity. Soil Biol. Biochem. 2003, 35, 143–154. [Google Scholar] [CrossRef]
- Dettmann, U.; Kraft, N.N.; Rech, R.; Heidkamp, A.; Tiemeyer, B. Analysis of peat soil organic carbon, total nitrogen, soil water content and basal respiration: Is there a ‘best’ drying temperature? Geoderma 2021, 403, 115231. [Google Scholar] [CrossRef]
- Preston, M.D.; Smemo, K.A.; McLaughlin, J.W.; Basiliko, N. Peatland microbial communities and decomposition processes in the James Bay Lowlands, Canada. Front. Microbiol. 2012, 3, 70. [Google Scholar] [CrossRef] [Green Version]
- van de Riet, B.P.; Hefting, M.M.; Verhoeven, J.T.A. Rewetting drained peat meadows: Risks and benefits in terms of nutrient release and greenhouse gas exchange. Water Air Soil Pollut. 2013, 224, 1440. [Google Scholar] [CrossRef]
- Glina, B.; Bogacz, A.; Gulyás, M.; Zawieja, B.; Gajeski, P.; Kaczmarek, Z. The effect of long-term forestry drainage on the current state of peatland soils: A case study from the Central Sudetes, SW Poland. Mires Peat 2016, 18, 1–11. [Google Scholar]
- Daly, K.; Jeffrey, D.; Tunney, H. The effect of soil type on phosphorus sorption capacity and desorption dynamics in Irish grassland soils. Soil Use Manag. 2001, 17, 12–20. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.; Strong, J.; Wang, H. Are the biogeochemical cycles of carbon, nitrogen, sulfur, and phosphorus driven by the ‘FeIII-FeII redox wheel’ in dynamic redox environments? J. Soils Sediments 2012, 12, 683–693. [Google Scholar] [CrossRef]
- Pote, D.H.; Daniel, T.C.; Sharpley, A.N.; Moore, P.A.; Edwards, D.R.; Nichols, D.J. Relating extractable soil phosphorus to phosphorus losses in runoff. Soil Sci. Soc. Am. J. 1996, 60, 855–859. [Google Scholar] [CrossRef]
- McDowell, R.W.; Sharpley, A.N. Approximating Phosphorus Release from Soils to Surface Runoff and Subsurface Drainage. J. Environ. Qual. 2001, 30, 508–520. [Google Scholar] [CrossRef] [Green Version]
- Blombäck, K.; Bolster, C.H.; Lindsjö, A.; Hesse, K.; Linefur, H.; Parvage, M.M. Comparing measures for determination of phosphorus saturation as a method to estimate dissolved P in soil solution. Geoderma 2021, 383, 114708. [Google Scholar] [CrossRef]
- Eriksson, A.K.; Ulén, B.; Berzina, L.; Iital, A.; Jansons, V.; Sileika, A.S.; Toomsoo, A. Phosphorus in agricultural soils around the Baltic Sea—Comparison of laboratory methods as indices for phosphorus leaching to waters. Soil Use Manag. 2013, 29, 5–14. [Google Scholar] [CrossRef]
- Nair, V.D.; Portier, K.M.; Graetz, D.A.; Walker, M.L. An environmental threshold for degree of phosphorus saturation in sandy soils. J. Environ. Qual. 2004, 33, 107–113. [Google Scholar] [CrossRef]
- Sánchez-Alcalá, I.; del Campillo, M.C.; Torrent, J. Extraction with 0.01 m CaCl2 underestimates the concentration of phosphorus in the soil solution. Soil Use Manag. 2014, 30, 297–302. [Google Scholar] [CrossRef]
- Barrow, N.J.; Shaw, T.C. Effects of solution:soil ratio and vigour of shaking on the rate of phosphate adsorption by soil. J. Soil Sci. 1979, 30, 67–76. [Google Scholar] [CrossRef]
- Evans, R.L.; Sorensen, R.C. The influence of ionic strength and monovalent cation source in a leaching solution of phosphorus extractability in a coarse-textured soil. Soil Sci. 1986, 141, 38–42. [Google Scholar] [CrossRef]
- Sinaj, S.; Mächler, F.; Frossard, E.; Faïsse, C.; Oberson, A.; Morel, C. Interference of colloidal particles in the determination of orthophosphate concentrations in soil water extracts. Commun. Soil Sci. Plant Anal. 1998, 29, 1091–1105. [Google Scholar] [CrossRef]
- Koopmans, G.F.; Chardon, W.J.; van der Salm, C. Disturbance of water-extractable phosphorus determination by colloidal particles in a heavy clay soil from the Netherlands. J. Environ. Qual. 2005, 34, 1446–1450. [Google Scholar] [CrossRef]
- Breeuwsma, A.; Schoumans, O.F. Forecasting Phosphate Leaching on a Regional Scale. In Proceedings of the International Conference on Vulnerability of Soil and Groundwater to Pollutants, Noordwijkaan Zee, The Netherlands, 30 March–3 April 1987; Proceedings and Information No. 38. van Duijvenboode, W., van Waegeningh, H.G., Eds.; TNO Committee on Hydrological Research: The Hague, The Netherlands, 1987. [Google Scholar]
- Ige, D.V.; Akinremi, O.O.; Flaten, D.N. Environmental index for estimating the risk of phosphorus loss in calcareous soils of Manitoba. J. Environ. Qual. 2005, 34, 1944–1951. [Google Scholar] [CrossRef]
- Ohno, T.; Hoskins, B.R.; Erich, M.S. Soil organic matter effects on plant available and water soluble phosphorus. Biol Fertil Soil. 2007, 43, 683–690. [Google Scholar] [CrossRef]
- Abdala, D.B.; da Silva, I.R.; Vergütz, L.; Sparks, D.L. Long-term manure application effects on phosphorus speciation, kinetics and distribution in highly weathered agricultural soils. Chemosphere 2015, 119, 504–514. [Google Scholar] [CrossRef]
- Jamroz, E.; Bekier, J.; Medynska-Juraszek, A.; Kaluza-Haladyn, A.; Cwielag-Piasecka, I.; Bednik, M. The contribution of water extractable forms of plant nutrients to evaluate MSW compost maturity: A case study. Sci. Rep. 2020, 10, 12842. [Google Scholar] [CrossRef] [PubMed]
- Conwenberg, J. Grenhause gass emission for managed peat soil: In the IPCC guidance realistics? Mires and Peat 2011, 8, 1–10. [Google Scholar]
- Grosshans, R.E.; Venema, H.D.; Cicek, N.; Goldsborough, G. Cattail Farming for Water Quality: Harvesting Cattails for Nutrient Removal and Phosphorus Recovery in the Watershed. In Proceedings of the WEA-IWA Nutrient Recovery and Management, Miami, FL, USA, 9–12 January 2011. [Google Scholar]
- Negassa, W.; Michalik, D.; Klysubem, W.; Leinweber, P. Phosphorus speciation in long term drained and rewetted peatlands of Northern Germany. Soil Syst. 2020, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Dommain, R.; Couwenberg, J.; Joosten, H. Hydrological self-regulation of drained peat swamps in South-East Asia and consequences for conservation and restoration. Mires Peat 2010, 6, 1–17. [Google Scholar]
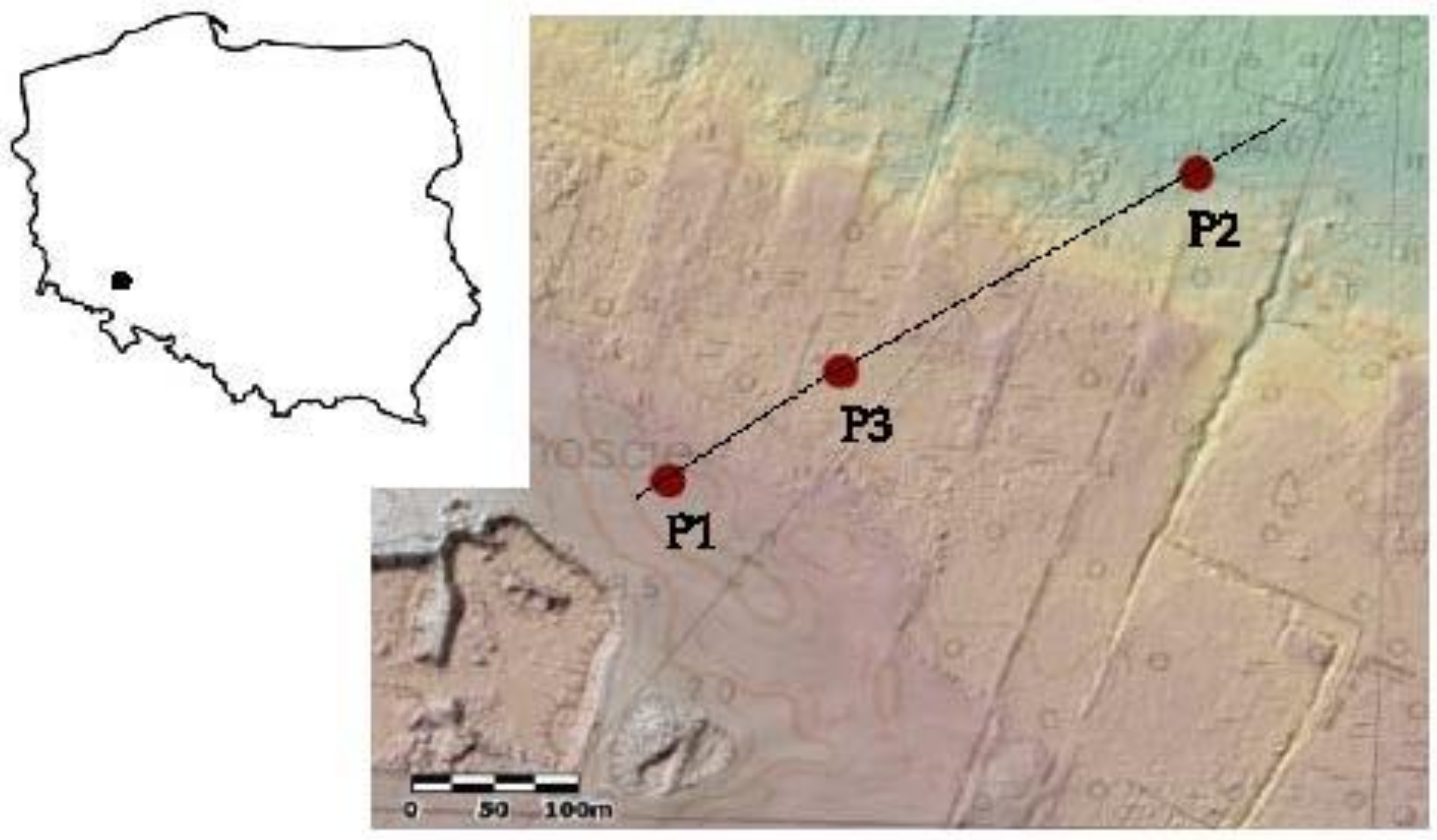



| Soil Profile | Soil Horizons | Depth (cm) | Munsell Colour Scale | Soil Moisture | Specific Features |
|---|---|---|---|---|---|
| P1 | Gleyic Umbrisol (Arenic) [1] Postmurshic soil [62] GPS coordinates: 51°11′45.5″ N; 16°40′42.7″ E | ||||
| Au1 | 0–8 | 10YR 2/2 | Slightly moist | Humic topsoil horizon with labile humus material combined with mineral fraction (quartz); granular structure; reductomorphic and oxymorphic stains; Fe precipitates (fine, 10% coverage); roots moderately fine; smooth boundary | |
| Au2 | 8–18 | 10YR 3/1 | Humic horizon with labile humus material combined with mineral fraction (quartz); granular structure; roots fine; smooth boundary | ||
| Au3 | 18–30 | 10YR 3/1 | Humic horizon with labile humus material combined with mineral fraction (quartz); granular structure; roots fine; abrupt boundary | ||
| Cg1 | 30–45 | 10YR 6/5 | Mineral horizon; subangular structure, oxymorphic colours 5 YR 4/5; Fe precipitates (medium-fine, 20–30% coverage); smooth boundary | ||
| Cg2 | >45 | 10Y/R 7/3 | Mineral horizon; subangular structure; oxymorphic colours 5 YR 4/5; Fe precipitates (medium-fine, 10% coverage) | ||
| P2 | Calcic Drainic Sapric Murshic Histosols (Limnic) [1] (thin) murshic soil [62] GPS coordinates: 51°11′52.2″ N 16°40′58.8″ E | ||||
| M1 | 0–10 | 10YR 2/1 | Moist | Moorsh horizons; fine and medium granular structure; roots of medium thickness and highly abundant; numerous earthworms; smooth boundaries | |
| M2 | 10–25 | 10YR 2/1 | Moist | ||
| M3 | 25–42 | 10YR 2/1 | Moist | Moorsh horizon; blocky structure; small roots and few in number; earthworms few in number; clear boundary | |
| Oa | 42–55 | 10YR 1/1 | Wet | Sapric sedge peat horizon; fibre amorphous structure; abrupt boundary | |
| Lcca | 55–70 | 7.5 YR 5/6 | Wet | Limnic horizon with Fe and gypsum with wood pieces, Fe precipitates (fine-medium, 60% coverage); calcium carbonate present; single, fine roots; abrupt boundary | |
| Cgg | >70 | 7.5 YR 4/3 | Wet | Mineral horizon with gley reductomorphic colours; Fe precipitates (fine-medium, 60% coverage) | |
| P3 | Calcic Sapric Histosols (Limnic) [1] Sapric peat soil [62] GPS coordinates: 51°11′47.9″ N 16°40′47.9″ E | ||||
| Oe | 0–14 | 10YR 2/2 | Wet | Hemic sedge peat topsoil horizon; fibrous structure; abrupt boundary | |
| Oa1 | 14–34 | 10YR 2/1 | Wet | Sapric reed and sedge peat; silted; amorphous structure; single plant fragments; smooth boundary | |
| Oa2 | 34–62 | 10YR 2/1.5 | Wet | Sapric reed and sedge peat; amorphous structure; single plant fragments, smooth boundary | |
| Oa3 | 62–70 | 10YR 2/2 | Wet | Sapric sedge peat; amorphous structure; single plant fragments; smooth boundary | |
| Oa4 | 70–83 | 10YR 2/1 | Wet | Sapric sedge peat amorphous structure; single plant fragments; abrupt boundary | |
| Lc | 83–106 | 10YR 3/3 | Wet | Limnic horizon—gyttja with bryales peat fragments and sand; sulphur content; abrupt boundary | |
| Cgg | 106–120 | 10YR 4/1 | Wet | Mineral horizon—sand with gyttja, gleization | |
| Number of Profile | Soil Horizons | Depth of Horizon (cm) | pH H2O | pH KCl | pH CaCl2 | CaCO3 | C | N | Ash | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | ||||||||||||
| P1 | Au1 | 0–8 | 5.7 | ±0.09 | 4.6 | ±0.08 | 4.9 | ±0.11 | 0.2 | 4.27 | 0.29 | 88.60 |
| Au2 | 8–18 | 5.7 | ±0.10 | 4.1 | ±0.10 | 4.6 | ±0.07 | - | 3.03 | 0.21 | 93.22 | |
| Au3 | 18–30 | 5.5 | ±0.08 | 4.3 | ±0.08 | 4.6 | ±0.01 | 0.1 | 3.98 | 0.26 | 93.18 | |
| Cg1 | 30–45 | 5.9 | ±0.05 | 4.8 | ±0.07 | 5.5 | ±0.08 | - | 0.15 | 0.01 | 99.57 | |
| Cg2 | >45 | 6.0 | ±0.08 | 4.9 | ±0.05 | 5.6 | ±0.02 | - | 0.10 | 0.00 | 99.72 | |
| P2 | M1 | 0–10 | 7.4 | ±0.08 | 6.9 | ±0.04 | 7.1 | ±0.01 | 20.9 | 15.89 | 1.30 | 67.20 |
| M2 | 10–25 | 7.5 | ±0.08 | 7.1 | ±0.01 | 7.2 | ±0.02 | 26.9 | 13.88 | 1.15 | 69.32 | |
| M3 | 25–42 | 7.5 | ±0.10 | 7.0 | ±0.02 | 7.3 | ±0.03 | 28.6 | 14.82 | 1.14 | 70.97 | |
| Oa | 42–55 | 7.2 | ±0.07 | 6.7 | ±0.02 | 7.2 | ±0.04 | 4.3 | 18.05 | 1.28 | 62.78 | |
| Lcca | 55–70 | 6.8 | ±0.05 | 6.6 | ±0.10 | 6.9 | ±0.03 | 0.9 | 11.69 | 0.70 | 75.86 | |
| Cgg | >70 | 6.4 | ±0.08 | 6.5 | ±0.02 | 6.3 | ±0.03 | - | 0.54 | 0.03 | 98.11 | |
| P3 | Oe | 0–14 | 6.1 | ±0.08 | 4.5 | ±0.27 | 5.3 | ±0.16 | 0.1 | 34.22 | 2.09 | 31.72 |
| Oa1 | 14–34 | 5.4 | ±0.10 | 4.6 | ±0.02 | 4.8 | ±0.15 | 0.3 | 29.70 | 1.96 | 46.13 | |
| Oa2 | 34–62 | 5.8 | ±0.06 | 4.6 | ±0.02 | 5.0 | ±0.22 | 0.2 | 34.12 | 2.03 | 32.54 | |
| Oa3 | 62–70 | 5.9 | ±0.07 | 4.7 | ±0.04 | 5.2 | ±0.28 | 0.3 | 31.56 | 1.83 | 39.83 | |
| Oa4 | 70–83 | 5.8 | ±0.08 | 4.7 | ±0.03 | 5.0 | ±0.10 | 0.1 | 32.03 | 1.61 | 37.43 | |
| Lc | 83–106 | 5.8 | ±0.09 | 3.8 | ±0.31 | 5.0 | ±0.11 | - | 11.66 | 0.43 | 83.19 | |
| Cgg | 106–120 | 5.6 | ±0.13 | 3.9 | ±0,20 | 4.8 | ±0.11 | - | 2.20 | 0.09 | 95.51 | |
| Soil Profile | Genetic Horizon | Depth (cm) | Feox | Alox | PSC 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g kg−1) | ||||||||||||||
| 1 | Au1 | 0–8 | 16.73 | ± | 0.43 * | a | 1.03 | ± | 0.24 | a | 8.88 | ± | 0.34 | a |
| Au2 | 8–18 | 20.60 | ± | 0.62 | a | 1.49 | ± | 0.17 | a | 11.04 | ± | 0.22 | a | |
| Au3 | 18–30 | 19.60 | ± | 0.18 | a | 1.35 | ± | 0.04 | a | 10.48 | ± | 0.07 | a | |
| Cg1 | 30–45 | 5.52 | ± | 0.06 | 0.42 | ± | 0.03 | 2.97 | ± | 0.02 | ||||
| Cg2 | >45 | 0.53 | ± | 0.29 | 0.12 | ± | 0.02 | 0.33 | ± | 0.15 | ||||
| 2 | M1 | 0–10 | 31.75 | ± | 0.10 | a | 1.40 | ± | 0.03 | a | 16.58 | ± | 0.04 | a |
| M2 | 10–25 | 32.89 | ± | 1.04 | a | 1.46 | ± | 0.07 | a | 17.17 | ± | 0.55 | a | |
| M3 | 25–42 | 34.52 | ± | 1.13 | a | 1.21 | ± | 0.05 | a | 17.86 | ± | 0.59 | a | |
| Oa | 42–55 | 45.05 | ± | 0.71 | b | 7.5 | ± | 0.14 | b | 26.15 | ± | 0.43 | b | |
| Lcca | 55–70 | 78.53 | ± | 7.71 | 13.29 | ± | 0.29 | 45.91 | ± | 4.00 | ||||
| Cgg | >70 | 13.73 | ± | 0.45 | 0.66 | ± | 0.10 | 7.19 | ± | 0.27 | ||||
| 3 | Oe | 0–14 | 59.48 | ± | 3.39 | a | 2.50 | ± | 0.00 | a | 30.99 | ± | 1.70 | a |
| Oa1 | 14–34 | 23.78 | ± | 1.14 | b | 3.46 | ± | 0.28 | a | 13.62 | ± | 0.71 | b | |
| Oa2 | 34–62 | 17.57 | ± | 0.14 | c | 2.01 | ± | 0.06 | ab | 9.79 | ± | 0.10 | c | |
| Oa3 | 62–70 | 17.70 | ± | 0.33 | c | 17.63 | ± | 0.81 | c | 17.66 | ± | 0.57 | d | |
| Oa4 | 70–83 | 13.47 | ± | 0.01 | c | 13.44 | ± | 0.19 | d | 13.45 | ± | 0.09 | b | |
| Lc | 83–106 | 15.93 | ± | 0.24 | 7.11 | ± | 0.27 | 11.52 | ± | 0.25 | ||||
| Cgg | 106–120 | 9.12 | ± | 1.56 | 1.82 | ± | 0.39 | 5.47 | ± | 0.98 | ||||
| Soil Profile | Genetic Horizon | Depth (cm) | PSD 1 (%) | IP (%) | C:N | C:Pt | N:Pt | C:N:Pt | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Au1 | 0–8 | 19.1 | ± | 0.4 | a | 1.03 | a | 15:1 | a | 16:1 | a | 1:1 | a | 16:1:1 |
| Au2 | 8–18 | 15.4 | ± | 0.2 | b | 0.48 | b | 14:1 | b | 12:1 | a | 1:1 | a | 14:1:1 | |
| Au3 | 18–30 | 14.2 | ± | 0.2 | b | 0.41 | b | 15:1 | a | 18:1 | a | 1:1 | a | 18:1:1 | |
| Cg1 | 30–45 | 7.0 | ± | 1.7 | 0.35 | 15:1 | 3:1 | 1:1 | 15:1:5 | ||||||
| Cg2 | >45 | 9.6 | ± | 5.2 | 0.58 | - | 6:1 | - | - | ||||||
| 2 | M1 | 0–10 | 11.0 | ± | 0.4 | a | 0.28 | a | 12:1 | a | 54:1 | a | 4:1 | a | 54:4:1 |
| M2 | 10–25 | 10.7 | ± | 0.6 | a | 0.23 | a | 12:1 | a | 46:1 | a | 4:1 | a | 46:4:1 | |
| M3 | 25–42 | 11.0 | ± | 0.5 | a | 0.21 | a | 13:1 | b | 49:1 | a | 4:1 | a | 49:4:1 | |
| Oa | 42–55 | 8.9 | ± | 0.3 | b | 0.15 | a | 14:1 | c | 57:1 | b | 4:1 | b | 57:4:1 | |
| Lcca | 55–70 | 1.7 | ± | 0.2 | 0.08 | 17:1 | 92:1 | 6:1 | 92:6:1 | ||||||
| Cgg | >70 | 1.1 | ± | 0.1 | - | 17:1 | 34:1 | 2:1 | 34:2:1 | ||||||
| 3 | Oe | 0–14 | 4.2 | ± | 0.3 | a | 0.14 | a | 16:1 | a | 163:1 | a | 10:1 | a | 163:10:1 |
| Oa1 | 14–34 | 4.1 | ± | 0.3 | a | 0.09 | a | 15:1 | b | 244:1 | b | 16:1 | b | 244:16:1 | |
| Oa2 | 34–62 | 4.4 | ± | 0.8 | a | 0.10 | a | 17:1 | c | 338:1 | c | 20:1 | c | 338:20:1 | |
| Oa3 | 62–70 | 3.1 | ± | 0.1 | a | 0.06 | a | 17:1 | d | 287:1 | d | 17:1 | d | 288:17:1 | |
| Oa4 | 70–83 | 3.3 | ± | 0.7 | a | - | 20:1 | e | 356:1 | e | 18:1 | e | 356:18:1 | ||
| Lc | 83–106 | 3.3 | ± | 0.7 | - | 27:1 | 194:1 | 7:1 | 194:7:1 | ||||||
| Cgg | 106–120 | 2.4 | ± | 0.1 | - | 24:1 | 81:1 | 3:1 | 81:3:1 | ||||||
| Soil Profile | Genetic Horizon | Depth (cm) | PW 1 | PCaCl2 | PM3 | Porg | Pmin | Pox | Pt | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg kg−1) | ||||||||||||||||||||||||||||||
| 1 | Au1 | 0–8 | 27.9 | ± | 4.0 * | a | 15.3 | ± | 1.8 | a | 70.4 | ± | 14.1 | a | 1522.5 | ± | 91.9 | a | 1193.5 | ± | 106.1 | a | 1692.8 | ± | 95.8 | a | 2716.0 | ± | 14.1 | a |
| Au2 | 8–18 | 11.8 | ± | 0.1 | b | 3.2 | ± | 0.2 | b | 50.1 | ± | 10.0 | b | 1173.0 | ± | 7.1 | ab | 1277.5 | ± | 5.7 | a | 1702.8 | ± | 7.4 | a | 2450.5 | ± | 1.4 | ab | |
| Au3 | 18–30 | 8.9 | ± | 0.6 | b | 2.2 | ± | 0.1 | b | 42.3 | ± | 8.5 | c | 1032.0 | ± | 103.9 | b | 1155.5 | ± | 2.8 | a | 1483.3 | ± | 13.1 | a | 2187.5 | ± | 101.1 | b | |
| Cg1 | 30–45 | 1.6 | ± | 0.2 | <0.32 ** | 6.2 | ± | 1.2 | 86.5 | ± | 21.7 | 357.4 | ± | 7.2 | 206.9 | ± | 47.8 | 443.9 | ± | 14.5 | ||||||||||
| Cg2 | >45 | 0.8 | ± | 0.1 | <0.32 | 3.5 | ± | 0.7 | 49.4 | ± | 13.5 | 97.3 | ± | 1.9 | 27.3 | ± | 1.9 | 146.8 | ± | 15.4 | ||||||||||
| 2 | M1 | 0–10 | 8.1 | ± | 0.5 | a | 5.7 | ± | 1.4 | a | 9.1 | ± | 1.9 | a | 1323.5 | ± | 181.0 | a | 1599.3 | ± | 28.6 | a | 1825.5 | ± | 67.2 | a | 2922.8 | ± | 152.4 | a |
| M2 | 10–25 | 6.9 | ± | 0.2 | a | 2.9 | ± | 1.2 | ab | 5.8 | ± | 1.2 | b | 1376.5 | ± | 105.4 | a | 1651.0 | ± | 32.5 | a | 1838.8 | ± | 51.3 | a | 3027.5 | ± | 72.8 | a | |
| M3 | 25–42 | 6.5 | ± | 0.1 | a | 2.5 | ± | 1.2 | ab | 5.6 | ± | 1.1 | b | 1317.5 | ± | 34.6 | a | 1694.3 | ± | 3.9 | a | 1960.5 | ± | 161.9 | a | 3011.8 | ± | 30.8 | a | |
| Oa | 42–55 | 4.7 | ± | 1.0 | a | 1.0 | ± | 0.1 | b | 5.5 | ± | 1.1 | b | 1211.8 | ± | 90.9 | a | 1929.5 | ± | 19.1 | b | 2315.5 | ± | 34.6 | b | 3141.3 | ± | 71.8 | a | |
| Lcca | 55–70 | 1.0 | ± | 0.0 | <0.32 | 0.8 | ± | 0.2 | 619.0 | ± | 40.3 | 648.5 | ± | 6.4 | 765.0 | ± | 7.1 | 1267.5 | ± | 33.9 | ||||||||||
| Cgg | >70 | <0.48 ** | <0.32 | 1.2 | ± | 0.2 | 73.4 | ± | 24.6 | 85.1 | ± | 1.3 | 80.8 | ± | 1.3 | 158.5 | ± | 23.3 | ||||||||||||
| 3 | Oe | 0–14 | 2.9 | ± | 0.2 | a | 1.7 | ± | 0.1 | a | 8.5 | ± | 0.7 | a | 1048.0 | ± | 184.6 | a | 1051.3 | ± | 27.2 | a | 1308.8 | ± | 30.1 | a | 2099.3 | ± | 157.3 | a |
| Oa1 | 14–34 | 1.1 | ± | 0.0 | a | 0.9 | ± | 0.0 | a | 5.9 | ± | 0.3 | b | 990.3 | ± | 112.5 | a | 226.0 | ± | 6.1 | b | 551.0 | ± | 14.1 | b | 1216.3 | ± | 106.4 | b | |
| Oa2 | 34–62 | 1.0 | ± | 0.0 | a | 0.7 | ± | 0.0 | a | 3.7 | ± | 0.2 | c | 758.3 | ± | 123.4 | a | 251.0 | ± | 1.4 | b | 430.8 | ± | 86.3 | b | 1009.3 | ± | 124.8 | b | |
| Oa3 | 62–70 | 0.6 | ± | 0.2 | a | <0.32 | a | 1.5 | ± | 0.2 | d | 682.2 | ± | 138.5 | a | 414.1 | ± | 16.7 | bc | 539.0 | ± | 7.1 | b | 1096.3 | ± | 155.2 | b | |||
| Oa4 | 70–83 | <0.48 | a | <0.32 | a | 1.2 | ± | 0.3 | d | 658.9 | ± | 17.6 | a | 315.6 | ± | 1.4 | b | 450.4 | ± | 90.7 | b | 974.5 | ± | 19.1 | b | |||||
| Lc | 83–106 | <0.48 | <0.32 | 1.1 | ± | 1.2 | 262.4 | ± | 90.5 | 338.1 | ± | 7.0 | 380.3 | ± | 88.7 | 600.5 | ± | 97.6 | ||||||||||||
| Cgg | 106–120 | <0.48 | <0.32 | 1.4 | ± | 1.7 | 142.4 | ± | 17.4 | 128.0 | ± | 11.0 | 133.5 | ± | 20.9 | 270.4 | ± | 28.3 | ||||||||||||
| Variable | PW 1 | PCaCl2 | PM3 | Porg | Pmin | Pox | Pt |
|---|---|---|---|---|---|---|---|
| pHH2O | −0.10 | 0.03 | −0.42 | 0.55 | 0.76 | 0.63 | 0.73 |
| pHKCl | −0.20 | −0.03 | −0.55 | 0.49 | 0.64 | 0.50 | 0.62 |
| pHCaCl2 | 0.00 | 0.10 | −0.32 | 0.61 | 0.79 | 0.69 | 0.78 |
| CaCO3 | 0.00 | 0.12 | −0.30 | 0.56 | 0.62 | 0.49 | 0.64 |
| Feox | −0.20 | −0.14 | −0.31 | 0.39 | 0.51 | 0.45 | 0.50 |
| Alox | −0.44 | −0.43 | −0.41 | −0.65 | −0.45 | −0.51 | −0.54 |
| Ash | 0.73 | 0.57 | 0.77 | 0.55 | 0.61 | 0.70 | 0.62 |
| C | −0.75 | −0.60 | −0.77 | −0.59 | −0.65 | −0.73 | −0.66 |
| N | −0.76 | −0.57 | −0.85 | −0.42 | −0.50 | −0.61 | −0.51 |
| C:N | −0.32 | −0.36 | −0.13 | −0.83 | −0.79 | −0.77 | −0.85 |
| N:P | −0.68 | −0.57 | −0.64 | −0.75 | −0.84 | −0.90 | −0.86 |
| C:Pt | −0.64 | −0.55 | −0.58 | −0.80 | −0.86 | −0.92 | −0.89 |
| C:Porg | −0.68 | −0.58 | −0.65 | −0.78 | −0.77 | −0.85 | −0.82 |
| Ip | 0.99 | 0.92 | 0.93 | 0.58 | 0.27 | 0.44 | 0.39 |
| PSD | 0.89 | 0.78 | 0.87 | 0.65 | 0.57 | 0.69 | 0.63 |
| Variable | Ash | C | N | N:Pt | C:Pt | C:Porg | Ip | PSD |
|---|---|---|---|---|---|---|---|---|
| Ip 1 | 0.73 | −0.75 | −0.77 | −0.65 | −0.62 | −0.67 | - | 0.89 |
| PSD | 0.94 | −0.95 | −0.94 | −0.87 | −0.84 | −0.88 | 0.89 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debicka, M.; Bogacz, A.; Kowalczyk, K. Phosphorus Behaviour and Its Basic Indices under Organic Matter Transformation in Variable Moisture Conditions: A Case Study of Fen Organic Soils in the Odra River Valley, Poland. Agronomy 2021, 11, 1997. https://doi.org/10.3390/agronomy11101997
Debicka M, Bogacz A, Kowalczyk K. Phosphorus Behaviour and Its Basic Indices under Organic Matter Transformation in Variable Moisture Conditions: A Case Study of Fen Organic Soils in the Odra River Valley, Poland. Agronomy. 2021; 11(10):1997. https://doi.org/10.3390/agronomy11101997
Chicago/Turabian StyleDebicka, Magdalena, Adam Bogacz, and Karolina Kowalczyk. 2021. "Phosphorus Behaviour and Its Basic Indices under Organic Matter Transformation in Variable Moisture Conditions: A Case Study of Fen Organic Soils in the Odra River Valley, Poland" Agronomy 11, no. 10: 1997. https://doi.org/10.3390/agronomy11101997
APA StyleDebicka, M., Bogacz, A., & Kowalczyk, K. (2021). Phosphorus Behaviour and Its Basic Indices under Organic Matter Transformation in Variable Moisture Conditions: A Case Study of Fen Organic Soils in the Odra River Valley, Poland. Agronomy, 11(10), 1997. https://doi.org/10.3390/agronomy11101997







