Modeling of Phosphorus Nutrition to Obtain Maximum Yield, High P Use Efficiency and Low P-Loss Risk for Wheat Grown in Sandy Calcareous Soils
Abstract
:1. Introduction
2. Material and Methods
2.1. Field Experiment
2.2. Collection and Analysis of Soil Plant Samples
2.3. Data Analysis
3. Results
3.1. Effect of P Fertilization Rates on P in Soil and Plant
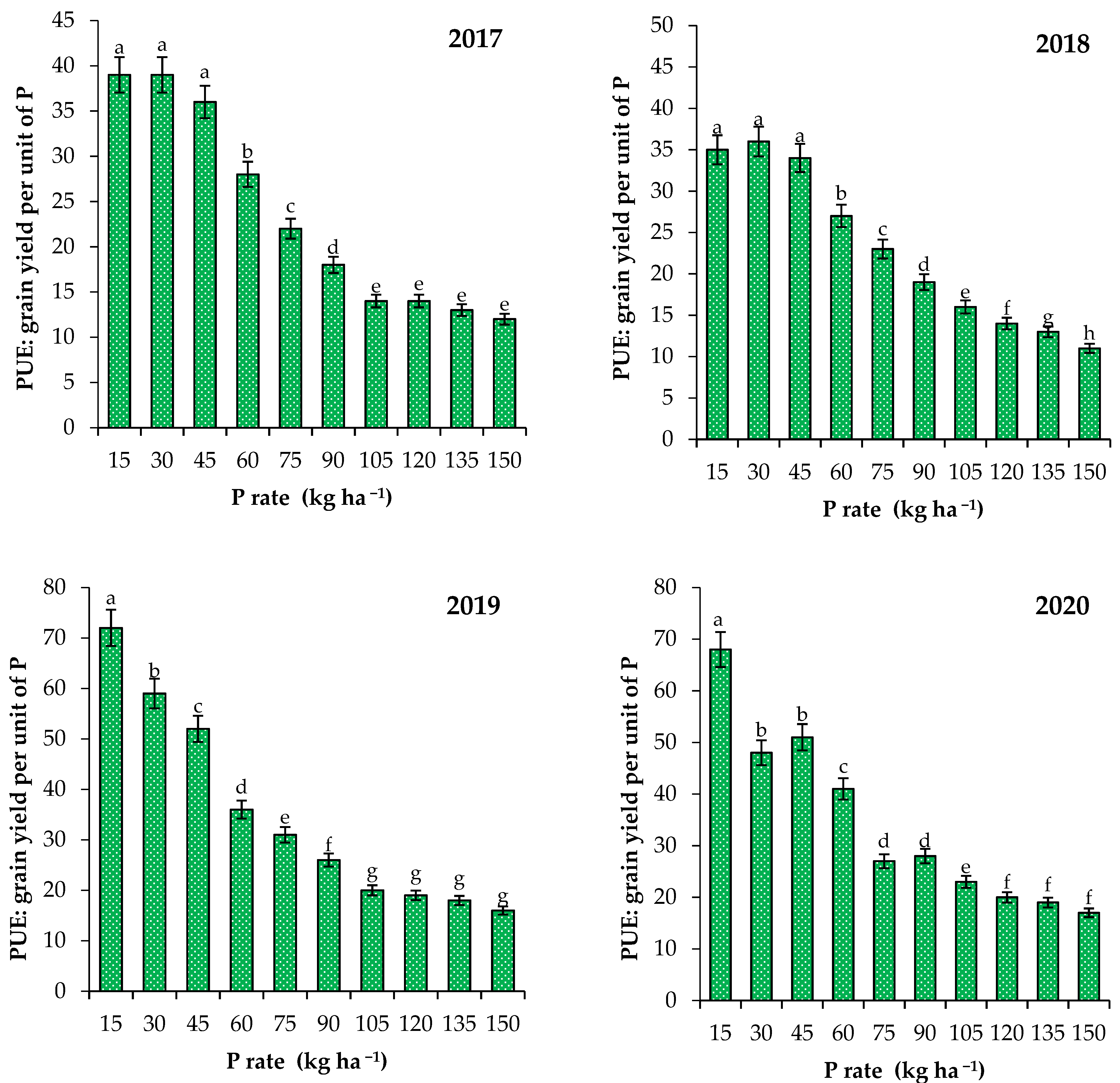
3.2. Effect of P Fertilizer Rates on Yield of Wheat and P Use Efficiency
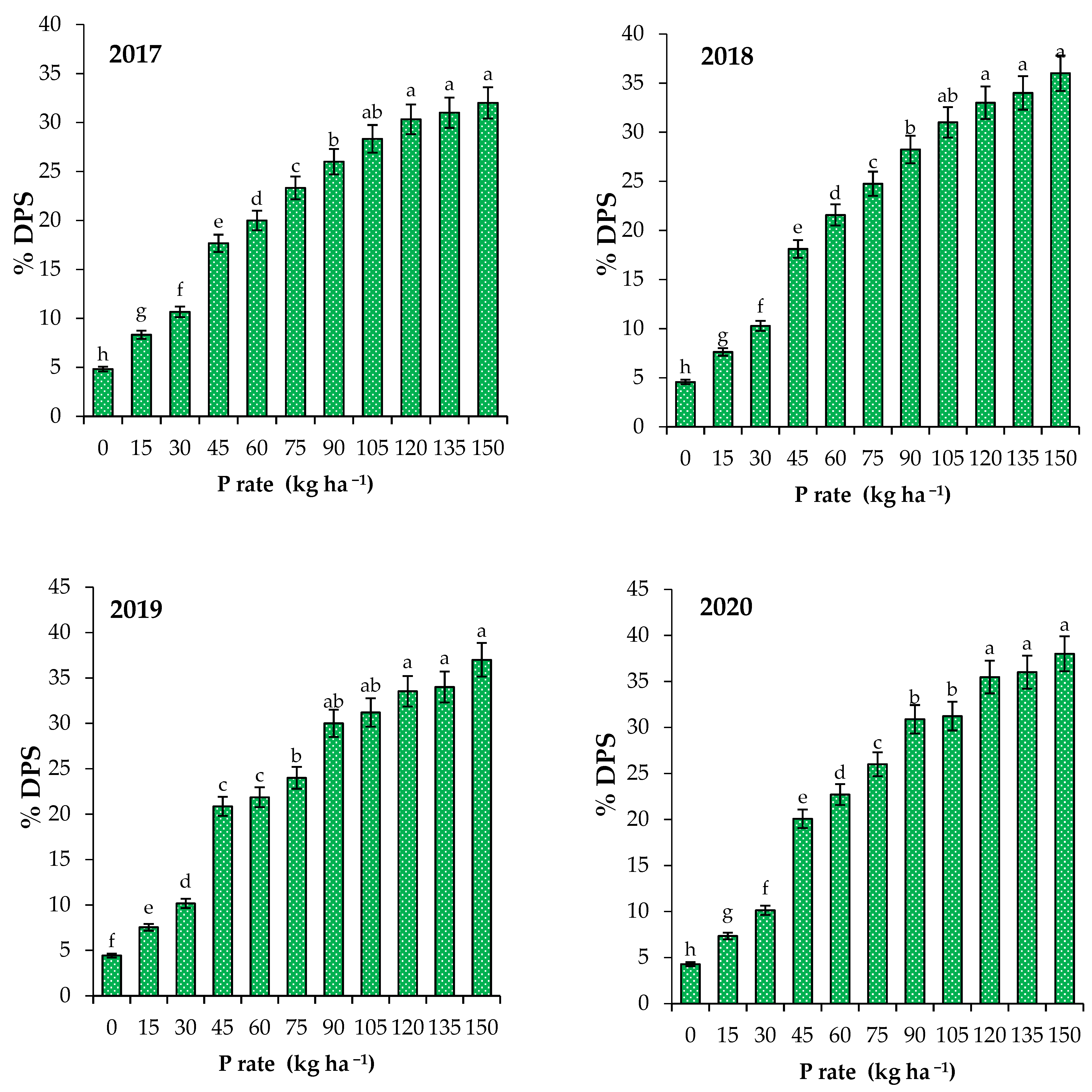
3.3. Critical P in Soil and Plant and P Loss Risk
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide Research Trends on Wheat and Barley: A Bibliometric Comparative Analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef] [Green Version]
- Eissa, M.A. Nutrition of drip irrigated corn by phosphorus under sandy calcareous soils. J. Plant Nutr. 2016, 39, 1620–1626. [Google Scholar] [CrossRef]
- Kolahchi, Z.; Jalali, M. Phosphorus Movement and Retention by Two Calcareous Soils. Soil Sediment Contam. Int. J. 2013, 22, 21–38. [Google Scholar] [CrossRef]
- Gitari, H.I.; Shadrack, N.; Kamau, S.; Karanja, N.N.; Gachene, C.K.K.; Schulte-Geldermann, E. Agronomic assessment of phosphorus efficacy for potato (Solanum tuberosum L) under legume intercrops. J. Plant Nutr. 2020, 43, 864–878. [Google Scholar] [CrossRef]
- Silvertooth, C.J. Fertigation in Arid Regions and Saline Soils. In Proceedings of the IPINATESC-CAUCAAS international Symposium on Fertigation, Beijing, China, 20–24 September 2005. [Google Scholar]
- Badr, M.A.; El-Tohamy, W.A.; Zaghloul, A.M. Yield and water use efficiency of potato grown under different irrigation and nitrogen levels in an arid region. Agric. Water Manag. 2012, 110, 9–15. [Google Scholar] [CrossRef]
- Manghabati, H.; Kohlpaintner, M.; Ettl, R.; Mellert, K.; Blum, U.; Göttlein, A. Correlating phosphorus extracted by simple soil extraction methods with foliar phosphorus concentrations of Picea abies (L.) H. Karst. and Fagus sylvatica (L.). J. Plant Nutr. Soil Sci. 2018, 181, 547–556. [Google Scholar] [CrossRef]
- Gachene, C.K.K.; Nyawade, S.O.; Karanja, N.N. Soil and Water Conservation: An Overview. In Encyclopedia of the UN Sustainable Development Goals; Leal, F.W., Azul, A., Brandli, L., Ozuyar, P., Wall, T., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Lemming, C.; Oberson, A.; Magid, J.; Bruun, S.; Scheutz, C.; Frossard, E.; Jensen, L.S. Residual phosphorus availability after long-term soil application of organic waste. Agric. Ecosyst. Environ. 2019, 270–271, 65–75. [Google Scholar] [CrossRef]
- Al-Rohily, K.M.; Ghoneim, A.M.; Modaihsh, A.S.; Mahjoub, M.O. Phosphorus Availability in Calcareous Soil Amend with Chemical Phosphorus Fertilizer, Cattle Manure Compost and Sludge Manure. Int. J. Soil Sci. 2013, 8, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Naeem, A.; Akhtar, M.; Ahmad, W. Optimizing available phosphorus in calcareous soils fertilized with diammonium phosphate and phosphoric acid using Freundlich adsorption isotherm. Sci. World J. 2013, 2013, 680257. [Google Scholar] [CrossRef]
- Shi, L.L.; Shen, M.X.; Lu, C.Y.; Wang, H.H.; Zhou, X.W.; Jin, M.J.; Wu, T.D. Soil phosphorus dynamic, balance and critical P values in long-term fertilization experiment in Taihu Lake region, China. J. Integr. Agric. 2015, 14, 2446–2455. [Google Scholar] [CrossRef]
- Antoniadis, V.; Koliniati, R.; Efstratiou, E.; Golia, E.; Petropoulos, S. Effect of soils with varying degree of weathering and pH values on phosphorus sorption. CATENA 2016, 139, 214–219. [Google Scholar] [CrossRef]
- Bell, L.C.; Black, C.A. Transformation of Dibasic Calcium Phosphate Dihydrate and Octacalcium Phosphate in Slightly Acid and Alkaline Soils1. Soil Sci. Soc. Am. J. 1970, 34, 583–587. [Google Scholar] [CrossRef]
- Li, J.M.; Gao, J.S.; Liu, J.; Xu, M.G.; Ma, Y.B. Predictive Model for Phosphorus Accumulation in Paddy Soils with Long-Term Inorganic Fertilization. Commun. Soil Sci. Plant Anal. 2012, 43, 1823–1832. [Google Scholar] [CrossRef]
- Wang, B.; Liu, H.; Wang, X.H.; Li, J.M.; Ma, Y.B.; Ma, X.W. Soil phosphorus accumulation model for an arid area of north-western China with 3-year rotation of wheat, maize and cotton. J. Agric. Sci. 2014, 153, 1247–1256. [Google Scholar] [CrossRef]
- Sims, J.T.; Sharpley, A.N. Phosphorus: Agriculture and the Environment; American Society of Agronomy: Madison, WI, USA, 2005. [Google Scholar]
- Johnston, A.E.; Syers, J.K. Changes in Understanding the Behaviour of Soil and Fertiliser Phoshorus: Implications for Their Efficient Use in Agriculture. In Proceedings of the International Fertiliser Society, York, UK, 14 December 2006. [Google Scholar]
- Rowe, H.; Withers, P.J.A.; Baas, P.; Chan, N.I.; Doody, D.; Holiman, J.; Jacobs, B.; Li, H.; MacDonald, G.K.; McDowell, R.; et al. Integrating legacy soil phosphorus into sustainable nutrient management strategies for future food, bioenergy and water security. Nutr. Cycl. Agroecosys. 2016, 104, 393–412. [Google Scholar] [CrossRef]
- Cadot, S.; Bélanger, G.; Ziadi, N.; Morel, C.; Sinaj, S. Critical plant and soil phosphorus for wheat, maize, and rapeseed after 44 years of P fertilization. Nutr. Cycl. Agroecosys. 2018, 112, 417–433. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Ma, Y.; Hao, X.; Li, X.; Li, J.; Huang, S.; Yang, X. Determining critical values of soil Olsen-P for maize and winter wheat from long-term experiments in China. Plant Soil. 2009, 323, 143–151. [Google Scholar] [CrossRef]
- Mallarino, A.P.; Blackmer, A.M. Comparison of Methods for Determining Critical Concentrations of Soil Test Phosphorus for Corn. Agron. J. 1992, 84, 850–856. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Liu, H.; Hao, X.Y.; Wang, X.H.; Sun, J.S.; Li, J.M.; Ma, Y.B. Agronomic threshold of soil available phosphorus in grey desert soils in Xinjiang, China. IOP Conf. Ser. Earth Environ. Sci. 2016, 41, 012010. [Google Scholar] [CrossRef] [Green Version]
- Brennan, R.F.; Bolland, M.D.A. Soil and tissue tests to predict the sulfur requirements of canola in south-western Australia. Aust. J. Exp. Agric. 2016, 46, 1061–1068. [Google Scholar] [CrossRef]
- Chen, W.; Bell, R.W.; Brennan, R.F.; Bowden, J.W.; Dobermann, A.; Rengel, Z.; Porter, W. Key crop nutrient management issues in the Western Australia grains industry: A review. Soil Res. 2009, 47, 1–18. [Google Scholar] [CrossRef]
- Silveira, M.L.; Obour, A.K.; Vendramini, J.M.; Sollenberger, L.E. Using tissue analysis as a tool to predict bahiagrass phoshorus fertilization requirement. J. Plant Nutr. 2011, 34, 2193–2205. [Google Scholar] [CrossRef]
- Campbell, R. Reference Sufficiency Ranges for Plant Analysis in the Southern Region of the United States; North Carolina Department of Agriculture and Consumer Services Agronomic Division: Raleigh, NC, USA, 2000. [Google Scholar]
- Stammer, A.J.; Antonio, P.M. Plant tissue analysis to assess phosphorus and potassium nutritional status of corn and soybean. Soil. Sci. Soc. Am. J. 2018, 82, 260–270. [Google Scholar] [CrossRef] [Green Version]
- Eissa, M.A.; Nafady, M.; Ragheb, H.; Attia, K. Effect of soil moisture and forms of phosphorus fertilizers on corn production under sandy calcareous soil. World Appl. Sci. J. 2013, 26, 540–547. [Google Scholar]
- FAO. Guidelines for Soil Description, 4th ed.; Viale delle Terme di Caracalla: Rome, Italy, 2006. [Google Scholar]
- Parkinson, J.A.; Allen, S.E. A wet oxidation procedure suitable for the determination of nitrogen andmineral nutrients in biological material. Commun. Soil Sci. Plant Anal. 1975, 6, 1–11. [Google Scholar] [CrossRef]
- Burt, R. Soil Survey Laboratory Methods Manual. Soil Survey Investigations Report No. 42, Version 4.0, Natural Esources Conservation Service; United States Department of Agriculture: Lincoln, NE, USA, 2004.
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: New York, NY, USA, 1954.
- Blombäck, K.; Bolster, C.H.; Lindsjö, A.; Hesse, K.; Linefur, H.; Parvage, M.M. Comparing measures for determination of phosphorus saturation as a method to estimate dissolved P in soil solution. Geoderma 2021, 383, 114708. [Google Scholar] [CrossRef]
- Eissa, M.A. Efficiency of P fertigation for drip-irrigated potato grown on calcareous sandy soils. Potato Res. 2019, 62, 97–108. [Google Scholar] [CrossRef]
- Jalali, M.; Jalali, M. Relation between various soil phosphorus extraction methods and sorption parameters in calcareous soils with di_erent texture. Sci. Total Environ. 2016, 566, 1080–1093. [Google Scholar] [CrossRef]
- Colomb, B.; Debaeke, P.; Jouany, C.; Nolot, J.M. Phosphorus management in low input stockless cropping systems: Crop and soil responses to contrasting P regimes in a 36-year experiment in southern France. Eur. J. Agron. 2007, 26, 154–165. [Google Scholar] [CrossRef]
- Manschadi, A.M.; Kaul, H.P.; Vollmann, J.; Eitzinger, J.; Wenzel, W. Developing phosphorus-efficient crop varieties—An interdisciplinary research framework. Field Crop. Res. 2014, 162, 87–98. [Google Scholar] [CrossRef]
- Naumann, M.; Koch, M.; Thiel, H.; Gransee, A.; Pawelzik, E. The importance of nutrient management for potato production part II: Plant nutrition and tuber quality. Potato Res. 2020, 63, 121–137. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Zhou, Z.; Lin, X.; Zhao, F.; Wang, B.; Lin, F.; Ge, Y.; Eissa, M.A. Biochar impacts on NH3-volatilization kinetics and growth of sweet basil (Ocimum basilicum L.) under saline conditions. Ind. Crop. Prod. 2020, 157, 11290–12903. [Google Scholar] [CrossRef]
- Eissa, M.A.; Abeed, A.H. Growth and biochemical changes in quail bush (Atriplex lentiformis (Torr.) S.Wats) under Cd stress. Environ. Sci. Pollut. Res. 2019, 26, 628–635. [Google Scholar] [CrossRef]
- Abou-Zaid, E.A.; Eissa, M.A. Thompson seedless grapevines growth and quality as affected by glutamic acid, vitamin b, and algae. J. Soil Sci. Plant Nutr. 2019, 19, 725–733. [Google Scholar] [CrossRef]
- Ali, A.M.; Awad, M.Y.; Hegab, S.A.; Gawad, A.M.A.E.; Eissa, M.A. Effect of potassium solubilizing bacteria (Bacillus cereus) on growth and yield of potato. J. Plant Nutr. 2021, 44, 411–420. [Google Scholar] [CrossRef]
- Al-Sayed, H.; Hegab, S.A.; Youssef, M.; Khalafalla, M.; Almaroai, Y.A.; Ding, Z.; Eissa, M.A. Evaluation of quality and growth of roselle (Hibiscus sabdariffa L.) as affected by bio-fertilizers. J. Plant Nutr. 2021, 43, 1025–1035. [Google Scholar] [CrossRef]
- Agegnehu, G.; Ghizaw, A.; Sinebo, W. Yield potential and land-use efficiency of wheat and faba bean mixed intercropping. Agron. Sustain. Dev. 2008, 28, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Teng, W.; Tong, Y.P.; Chen, X.P.; Zou, C.Q. Phosphorus Efficiency Mechanisms of Two Wheat Cultivars as Affected by a Range of Phosphorus Levels in the Field. Front. Plant Sci. 2018, 9, 1614. [Google Scholar] [CrossRef]
- Shepherd, M.A.; Withers, P.J. Applications of poultry litter and triple superphosphate fertilizer to a sandy soil: Effects on soil phosphorus status and profile distribution. Nutr. Cycl. Agroecosys. 1999, 54, 233–242. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Garg, A.K.; Kabba, B.S. Phosphorus accumulation, leaching and residual effects on crop yields from long-term applications in the subtropics. Soil Use Manag. 2007, 23, 417–427. [Google Scholar] [CrossRef]
- Johnston, A.E.; Poulton, P.R.; White, R.P. Plant-available soil phosphorus. Part II: The response of arable crops to Olsen P on a sandy clay loam and a silty clay loam. Soil Use Manag. 2013, 29, 12–21. [Google Scholar] [CrossRef]
- Poulton, P.R.; Johnston, A.E.; White, R.P. Plant-available soil phosphorus. Part I: The response of winter wheat and spring barley to Olsen P on a silty clay loam. Soil Use Manag. 2013, 29, 4–11. [Google Scholar] [CrossRef]
- Jordan-Meille, L.; Rubæk, G.H.; Ehlert, P.A.I.; Genot, V.; Hofman, G.; Goulding, K.; Recknagel, J.; Provolo, G.; Barraclough, P. An overview of fertilizer-P recommendations in Europe: Soil testing, calibration and fertilizer recommendations. Soil Use Manag. 2012, 28, 419–435. [Google Scholar] [CrossRef]
- Bollons, H.M.; Barraclough, P.B. Assessing the phosphorus status of winter wheat crops: Inorganic orthophosphate in whole shoots. J. Agric. Sci. 1999, 133, 285–295. [Google Scholar] [CrossRef]
- Bai, Z.; Li, H.; Yang, X.; Zhou, B.; Shi, X.; Wang, B.; Li, D.; Shen, J.; Chen, Q.; Qin, W.; et al. The critical soil P levels for crop yield, soil fertility and environmental safety in different soil types. Plant Soil. 2013, 372, 27–37. [Google Scholar] [CrossRef]
- Holmes, M.R.J. Nutrition of the Oilseed Rape Crop; Applied Science Publishers: London, UK, 1980. [Google Scholar]
- Cate, R.B.; Nelson, L.A. A Simple Statistical Procedure for Partitioning Soil Test Correlation Data into Two Classes1. Soil Sci. Soc. Am. J. 1971, 35, 658–660. [Google Scholar] [CrossRef]
- Eissa, M.A.; Ghoneim, M.F.; Elgharably, G.A.; Abd El-Razek, M. Phytoextraction of nickel, lead and cadmium from metal contaminated soils using different field. World Appl. Sci. J. 2014, 32, 1045–1052. [Google Scholar]
- Eissa, M.A. Impact of compost on metals phytostabilization potential of two halophytes species. Int. J. Phytorem. 2015, 17, 662–668. [Google Scholar] [CrossRef]
- Eissa, M.A.; Roshdy, N.M. Nitrogen fertilization: Effect on Cd-phytoextraction by the halophytic plant quail bush [Atriplex lentiformis (Torr.) S. Wats]. S. Afr. Bot. 2018, 115, 126–131. [Google Scholar] [CrossRef]
- Eissa, M.A.; Ahmed, E.M. Nitrogen and phosphorus fertilization for some Atriplex plants grown on metal-contaminated soils. Soil Sediment Contam. 2016, 25, 431–442. [Google Scholar] [CrossRef]
- Johnston, A.E.; Lane, P.W.; Mattingly, G.E.G.; Poulton, P.R.; Hewitt, M.V. Effects of soil and fertilizer P on yields of potatoes, sugar beet, barley and winter wheat on a sandy clay loam soil at Saxmundham, Suffolk. J. Agric. Sci. 2009, 106, 155–167. [Google Scholar] [CrossRef]
- Cox, F.R. Range in soil phosphorus critical levels with time. Soil Sci. Soc. Am. J. 1992, 56, 1504–1509. [Google Scholar] [CrossRef]
- Jalali, M.; Jalali, M. Assessment risk of phosphorus leaching from calcareous soils using soil test phosphorus. Chemosphere 2017, 171, 106–117. [Google Scholar] [CrossRef]
- Abboud, F.Y.; Favaretto, N.; Motta, A.C.V.; Barth, G.; Goularte, G.D. Phosphorus mobility and degree of saturation in Oxisol under no-tillage after long-term dairy liquid manure application. Soil Tillage Res. 2018, 177, 45–53. [Google Scholar] [CrossRef]
- Pizzeghello, D.; Berti, A.; Nardi, S.; Morari, F. Phosphorus forms and P sorption in three alkaline soils after long-term mineral and manure applications. Agric. Ecosyst. Environ. 2011, 141, 58–66. [Google Scholar] [CrossRef]
- Wang, Y.T.; Zhang, T.Q.; O’Halloran, I.P.; Tan, C.S.; Hu, Q.C.; Reid, D.K. Soil tests as risk indicators for leaching of dissolved phosphorus from agricultural soils in Ontario. Soil Sci. Soc. Am. J. 2012, 76, 220–229. [Google Scholar] [CrossRef]
| Properties | 0–20 cm |
|---|---|
| Sand (%) | 86 |
| Silt (%) | 10 |
| Clay (%) | 4 |
| Texture | Sandy |
| Field capacity (v%) | 16 |
| Witling point (v%) | 10 |
| CaCO3 (%) | 18 |
| pH (1:2 suspension) | 8.1 |
| ECe (dS m−1) | 3.5 |
| Organic matter (g kg−1) | 4.0 |
| Total N (mg kg−1) | 200 |
| Available N (mg kg−1) | 20 |
| Available Olsen P (mg kg−1) | 5.0 |
| Available K (mg kg−1) | 200 |
| Month | Tmax | Tmin | RH (%) | WS (km h−1) | ETo (mm) |
|---|---|---|---|---|---|
| December | 19 | 7 | 40 | 3.8 | 2.9 |
| January | 17 | 6 | 45 | 5.2 | 3.2 |
| February | 21 | 7 | 50 | 6.6 | 4.0 |
| March | 26 | 14 | 40 | 5.0 | 5.5 |
| April | 30 | 18 | 45 | 4.4 | 7.0 |
| Season | P Rate (kg ha−1) | Soil Olsen
P (mg kg−1) | P in Plant (g kg−1) | Grain Yield (kg ha−1) | Straw Yield (kg ha−1) |
|---|---|---|---|---|---|
| 2017 | 0 | 5.00 ± 1.41 G | 3.37 ± 0.07 I | 3842 ± 247 C | 5175 ± 354 A |
| 15 | 8.75 ± 3.65 G | 4.08 ± 0.46 H | 4427 ± 126 B | 5750 ± 233 B | |
| 30 | 13.50 ± 5.09 GF | 4.55 ± 0.42 GH | 5025 ± 483 B | 6300 ± 460 AB | |
| 45 | 17.75 ± 6.69 F | 5.16 ± 0.31 FG | 5480 ± 656 A | 6425 ± 183 AB | |
| 60 | 21.00 ± 7.36 EF | 5.63 ± 0.20 F | 5505 ± 678 A | 6150 ± 489 AB | |
| 75 | 25.50 ± 8.54 ED | 6.40 ± 0.29 E | 5475 ± 779 A | 6525 ± 672 A | |
| 90 | 27.25 ± 8.84 D | 7.20 ± 0.53 DE | 5485 ± 741 A | 6650 ± 638 A | |
| 105 | 32.50 ± 11.91 CD | 7.94 ± 0.20 CD | 5335 ± 736 A | 6275 ± 720 A | |
| 120 | 35.25 ± 10.76 BC | 8.48 ± 0.15 BC | 5547 ± 622 A | 6400 ± 820 A | |
| 135 | 38.50 ± 14.33 B | 8.99 ± 0.18 AB | 5545 ± 758 A | 6150 ± 845 AB | |
| 150 | 44.50 ± 15.77 A | 9.49 ± 0.16 A | 5632 ± 659 A | 6550 ± 620 A | |
| 2018 | 0 | 4.25 ± 0.50 H | 3.38 ± 0.12 G | 3875 ± 126 D | 4300 ± 141 DE |
| 15 | 9.25 ± 0.96 G | 4.03 ± 0.26 FG | 4400 ± 141 C | 4625 ± 236 D | |
| 30 | 14.25 ± 1.26 F | 4.29 ± 0.08 F | 4963 ± 281 B | 5375 ± 519 C | |
| 45 | 18.00 ± 0.82 E | 5.08 ± 0.21 EF | 5385 ± 87 A | 6250 ± 289 AB | |
| 60 | 24.25 ± 4.57 D | 5.87 ± 0.09 E | 5498 ± 87 A | 6100 ± 115 B | |
| 75 | 27.25 ± 2.06 D | 6.48 ± 0.38 DE | 5592 ± 82 A | 6500 ± 141 AB | |
| 90 | 27.50 ± 0.58 D | 7.10 ± 0.71 D | 5558 ± 128 A | 6375 ± 222 AB | |
| 105 | 33.25 ± 2.87 C | 7.97 ± 0.70 BC | 5605 ± 110 A | 6675 ± 189 AB | |
| 120 | 36.00 ± 1.83 C | 8.69 ± 0.18 AB | 5558 ± 51 A | 6600 ± 183 AB | |
| 135 | 39.75 ± 4.50 B | 9.10 ± 0.09 A | 5610 ± 66 A | 6725 ± 206 A | |
| 150 | 44.00 ± 2.83 A | 9.39 ± 0.09 A | 5573 ± 152 A | 6800 ± 141 A | |
| 2019 | 0 | 4.00 ± 1.15 I | 3.18 ± 0.24 F | 3175 ± 236 D | 3900 ± 115 E |
| 15 | 10.00 ± 1.63 H | 4.05 ± 0.74 E | 4250 ± 191 C | 5075 ± 96 D | |
| 30 | 15.00 ± 2.94 G | 4.15 ± 0.66 E | 4950 ± 100 B | 5225 ± 330 D | |
| 45 | 16.00 ± 3.77 G | 4.77 ± 0.94 E | 5533 ± 238 A | 5800 ± 50 BC | |
| 60 | 18.75 ± 3.51 F | 5.34 ± 0.66 DE | 5308 ± 216 B | 5775 ± 100 BC | |
| 75 | 24.75 ± 1.89 E | 5.75 ± 0.96 CD | 5475 ± 96 A | 5725 ± 50 BC | |
| 90 | 31.25 ± 1.50 D | 7.35 ± 0.93 B | 5485 ± 60 A | 5475 ± 320 BD | |
| 105 | 33.00 ± 2.16 CD | 7.53 ± 0.22 B | 5300 ± 115 A | 6225 ± 171 AC | |
| 120 | 35.25 ± 1.71 BC | 8.23 ± 0.26 AB | 5450 ± 208 A | 6525 ± 310 AC | |
| 135 | 38.75 ± 1.50 B | 8.55 ± 1.05 A | 5575 ± 96 A | 6400 ± 245 A | |
| 150 | 43.25 ± 1.50 A | 8.90 ± 0.81 A | 5500 ± 141 A | 6500 ± 377 A | |
| 2020 | 0 | 3.75 ± 0.96 H | 3.20 ± 0.24 E | 3000 ± 141 E | 3325 ± 236 E |
| 15 | 10.50 ± 3.70 G | 4.35 ± 0.70 D | 4025 ± 330 D | 4450 ± 412 D | |
| 30 | 13.00 ± 2.58 F | 4.43 ± 0.81 D | 4425 ± 386 C | 5350 ± 443 D | |
| 45 | 18.50 ± 3.11 E | 4.88 ± 0.63 D | 5275 ± 411 A | 6175 ± 418 C | |
| 60 | 24.75 ± 3.77 D | 5.38 ± 0.67 CD | 5475 ± 206 A | 6425 ± 386 C | |
| 75 | 26.00 ± 4.08 D | 5.88 ± 0.81 C | 5000 ± 141 B | 5975 ± 314 C | |
| 90 | 32.75 ± 2.06 C | 7.50 ± 0.71 B | 5475 ± 222 A | 6350 ± 243 A | |
| 105 | 34.00 ± 1.41 C | 7.83 ± 0.17 B | 5450 ± 208 A | 6825 ± 126 A | |
| 120 | 38.00 ± 3.56 B | 8.33 ± 0.41 AB | 5425 ± 310 A | 6250 ± 289 B | |
| 135 | 41.25 ± 3.40 AB | 8.60 ± 1.43 AB | 5575 ± 96 A | 6825 ± 356 A | |
| 150 | 42.25 ± 8.58 A | 8.95 ± 0.74 A | 5575 ± 386 A | 6850 ± 243 A |
| Source of Variance | p-Value (Significance Level) | |||||
|---|---|---|---|---|---|---|
| Soil Olsen P | P in Plant | Grain Yield | Straw Yield | PUE | DPS | |
| Year | * | * | * | ** | ** | ** |
| P rate | ** | ** | ** | ** | ** | ** |
| Model | Parameters | Formulas | R2 | Critical P Value |
|---|---|---|---|---|
| LL | SOP—RGY | 0.84 | 21.11 | |
| SOP—RSY | 0.76 | 25.71 | ||
| PiP—RGY | 0.71 | 6.40 | ||
| PiP—RSY | 0.69 | 7.36 | ||
| SOP—DPS | 0.86 | 29.11 | ||
| sExp | SOP—RGY | 0.59 | 25.04 | |
| SOP—RSY | 0.57 | 31.60 | ||
| PiP—RGY | 0.62 | 6.45 | ||
| PiP—RSY | 0.60 | 7.49 | ||
| SOP—DPS | 0.73 | 36.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Ding, Z.; Al-Yasi, H.M.; Ali, E.F.; Eissa, M.A.; Abou-Elwafa, S.F.; Sayed, M.A.; Said, M.T.; Said, A.A.; Ibrahim, K.A.M.; et al. Modeling of Phosphorus Nutrition to Obtain Maximum Yield, High P Use Efficiency and Low P-Loss Risk for Wheat Grown in Sandy Calcareous Soils. Agronomy 2021, 11, 1950. https://doi.org/10.3390/agronomy11101950
Hu Z, Ding Z, Al-Yasi HM, Ali EF, Eissa MA, Abou-Elwafa SF, Sayed MA, Said MT, Said AA, Ibrahim KAM, et al. Modeling of Phosphorus Nutrition to Obtain Maximum Yield, High P Use Efficiency and Low P-Loss Risk for Wheat Grown in Sandy Calcareous Soils. Agronomy. 2021; 11(10):1950. https://doi.org/10.3390/agronomy11101950
Chicago/Turabian StyleHu, Zhanyao, Zheli Ding, Hatim M. Al-Yasi, Esmat F. Ali, Mamdouh A. Eissa, Salah F. Abou-Elwafa, Mohammed Abdelaziz Sayed, Mohamed Tharwat Said, Alaa A. Said, Khaled A. M. Ibrahim, and et al. 2021. "Modeling of Phosphorus Nutrition to Obtain Maximum Yield, High P Use Efficiency and Low P-Loss Risk for Wheat Grown in Sandy Calcareous Soils" Agronomy 11, no. 10: 1950. https://doi.org/10.3390/agronomy11101950
APA StyleHu, Z., Ding, Z., Al-Yasi, H. M., Ali, E. F., Eissa, M. A., Abou-Elwafa, S. F., Sayed, M. A., Said, M. T., Said, A. A., Ibrahim, K. A. M., & Hamada, A. (2021). Modeling of Phosphorus Nutrition to Obtain Maximum Yield, High P Use Efficiency and Low P-Loss Risk for Wheat Grown in Sandy Calcareous Soils. Agronomy, 11(10), 1950. https://doi.org/10.3390/agronomy11101950










