Enhancing Grain Yield and Nitrogen Accumulation in Wheat Plants Grown under a Mediterranean Arid Environment by Foliar Spray with Papain-Released Whey Peptides
Abstract
1. Introduction
2. Materials and Methods
2.1. Whey Protein Hydrolysates Preparation
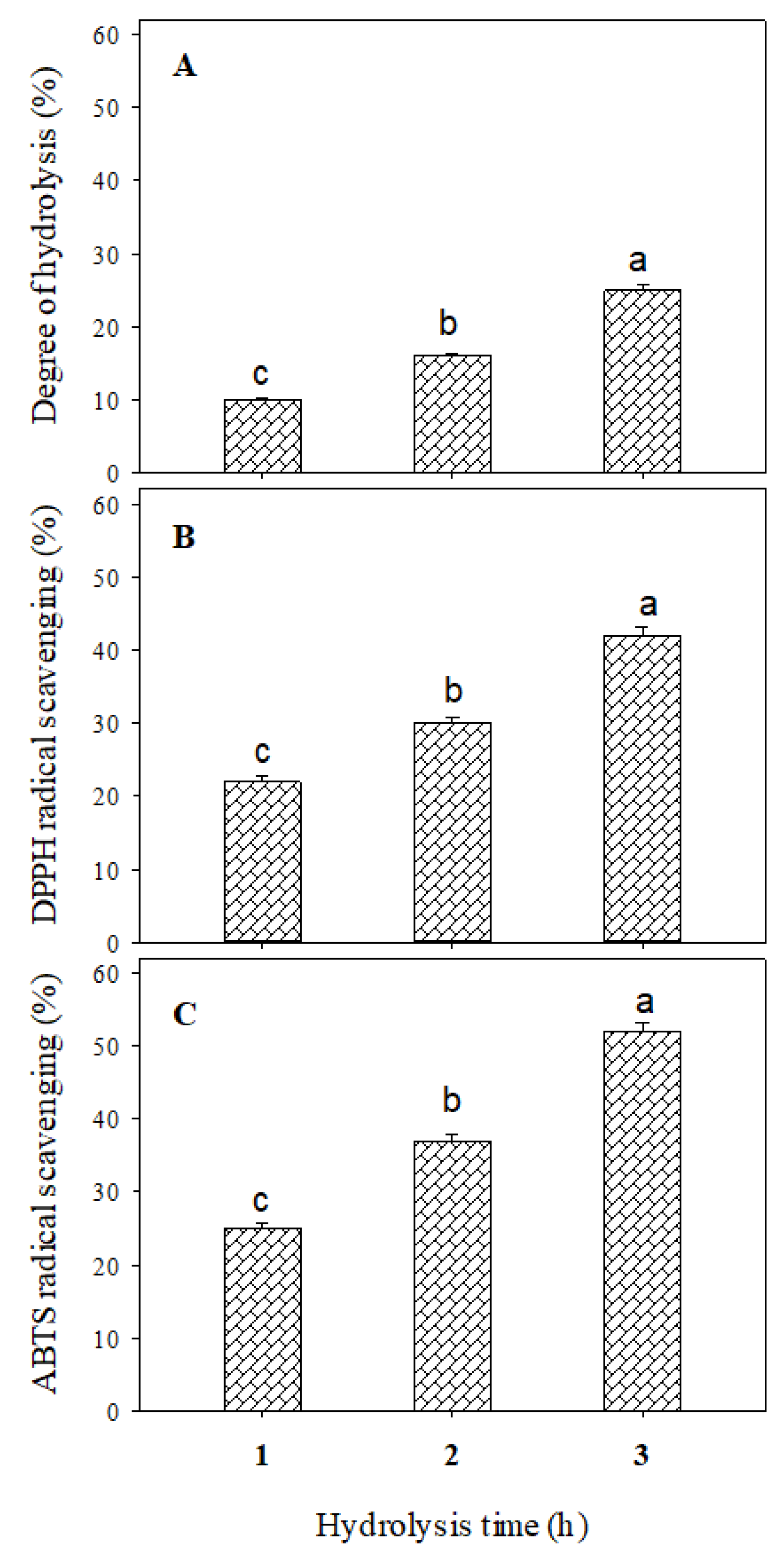
2.2. Antioxidant Activity Estimation
2.2.1. DPPH-Assay
2.2.2. ABTS-Assay
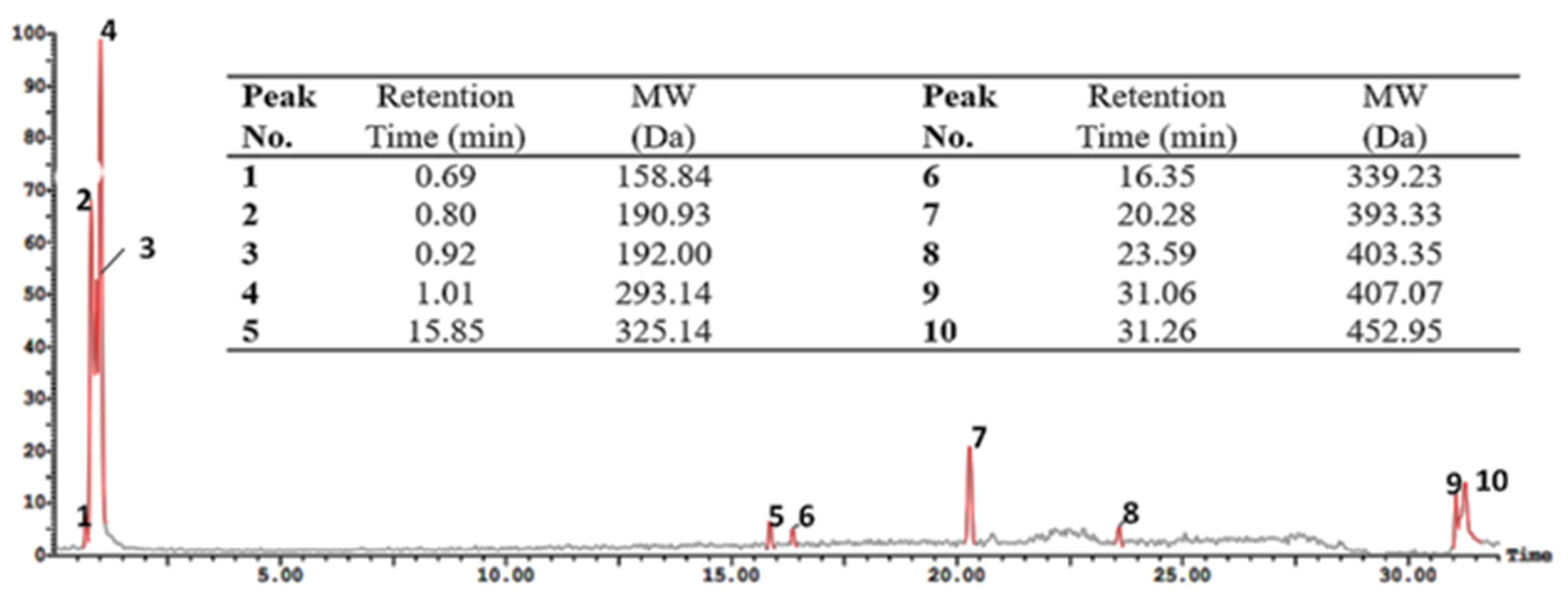
2.3. Electro-Spray-Ionization-Mass-Spectrometry (ESI-MS) of Protein Hydrolysates
2.4. Free Amino Acids Estimation
2.5. Field Experiments
2.5.1. Site Description
2.5.2. Experimental Design and Treatments
2.5.3. Crop Management
2.5.4. Field Measurements
2.5.5. N Accumulation Measurements
2.5.6. Statistical Analysis
3. Results
3.1. Whey Protein Hydrolysate Characterization
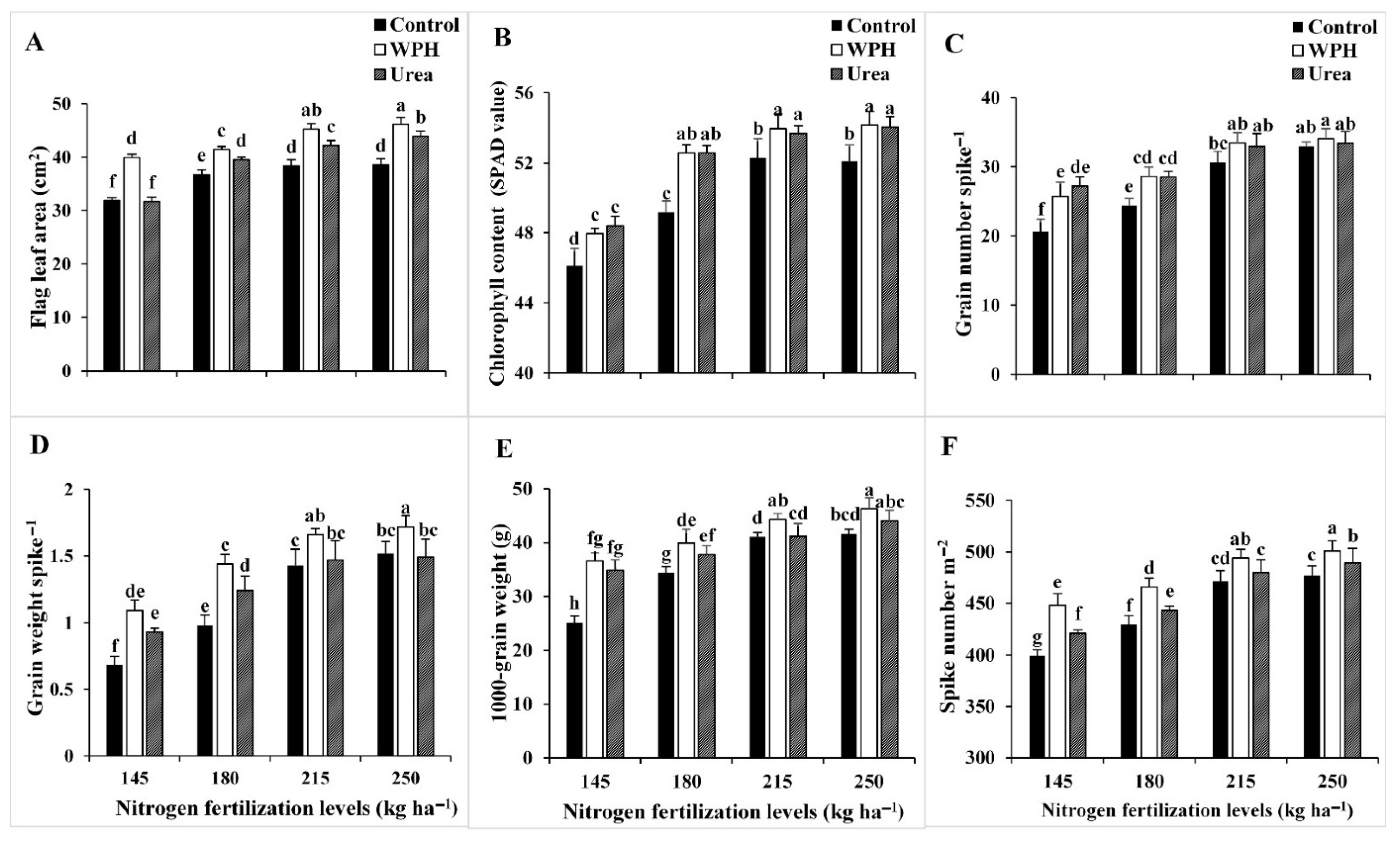
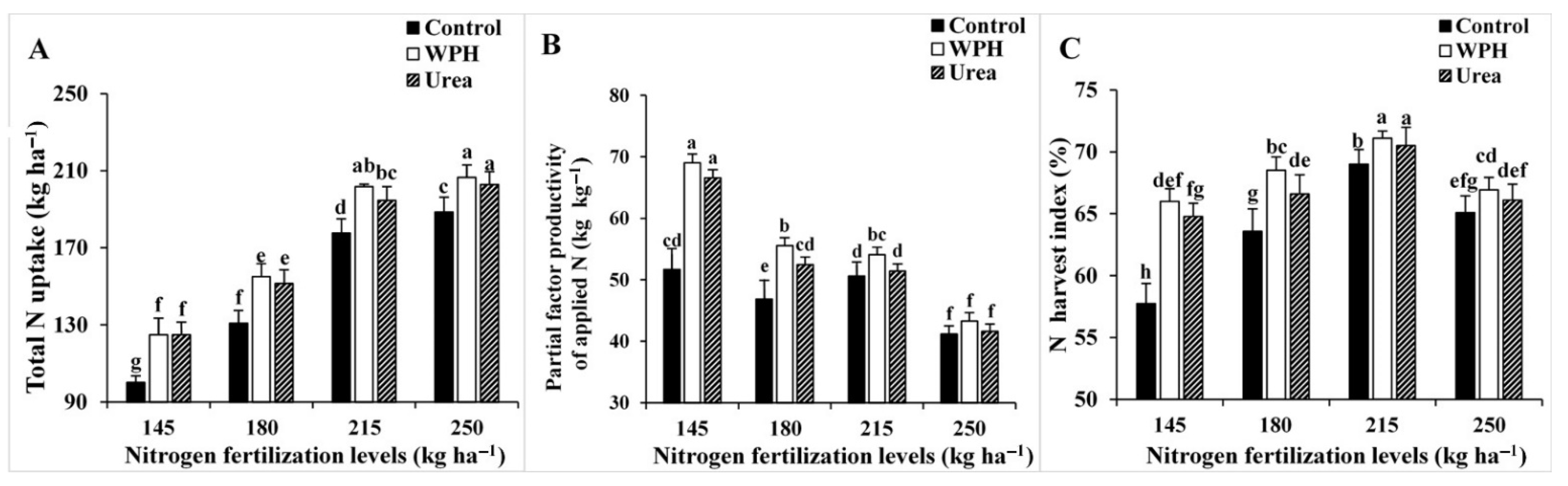
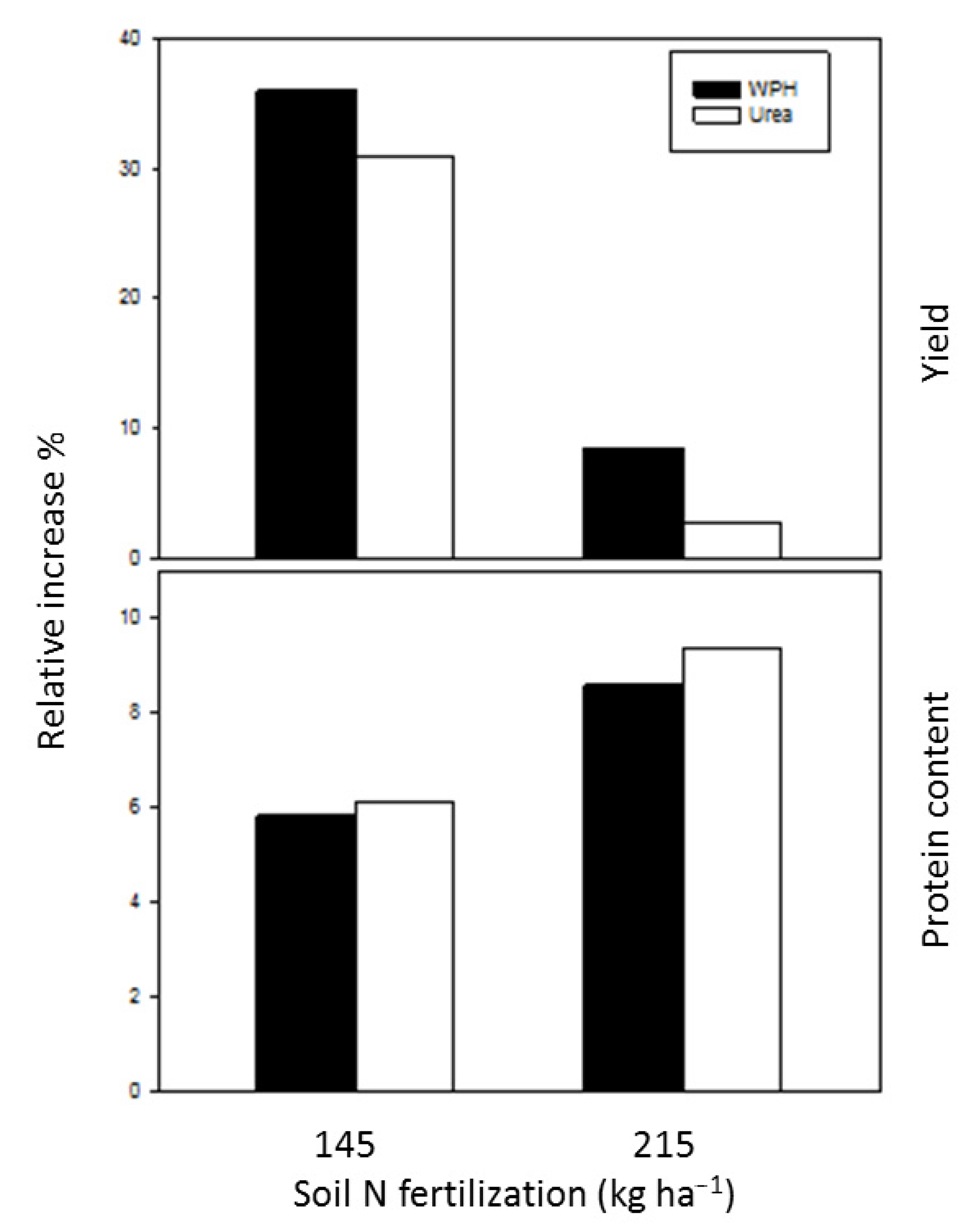
3.2. Field and N Accumulation Measurements
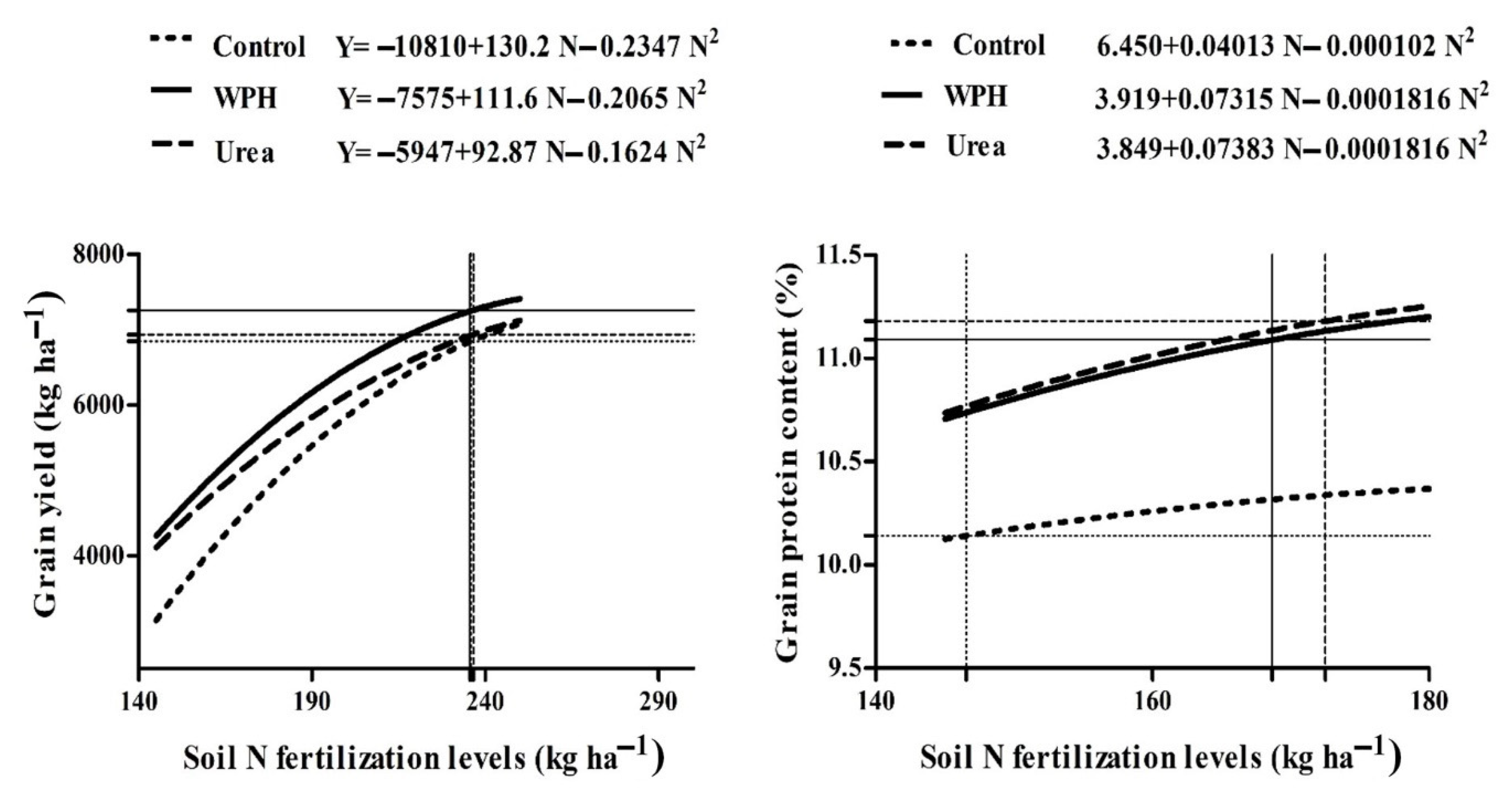
3.3. Response of Grain Yield and Grain Protein Content to Nitrogen Fertilization Levels
3.4. Interrelationship among Measured Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tabak, M.; Lepiarczyk, A.; Filipek-Mazur, B.; Lisowska, A. Efficiency of nitrogen fertilization of winter wheat depending on sulfur fertilization. Agronomy 2020, 10, 1304. [Google Scholar] [CrossRef]
- Xu, A.; Li, L.; Xie, J.; Wang, X.; Coulter, J.A.; Liu, C.; Wang, L. Effect of long-term nitrogen addition on wheat yield, nitrogen use efficiency, and residual soil nitrate in a semiarid area of the loess plateau of China. Sustainability 2020, 12, 1735. [Google Scholar] [CrossRef]
- Arzani, A.; Ashraf, M. Cultivated ancient wheats (Triticum spp.): A potential source of health-beneficial food products. Compr. Rev. Food Sci. Food Saf. 2017, 16, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Ma, B. Integrated nutrient management (INM) for sustaining crop productivity and reducing environmental impact: A review. Sci. Total Environ. 2015, 512, 415–427. [Google Scholar] [CrossRef]
- Hawkesford, M.J. Reducing the reliance on nitrogen fertilizer for wheat production. J. Cereal Sci. 2014, 59, 276–283. [Google Scholar] [CrossRef]
- Randive, K.; Raut, T.; Jawadand, S. An overview of the global fertilizer trends and India’s position in 2020. Miner. Econ. 2021, 34, 371–384. [Google Scholar] [CrossRef]
- Mansour, E.; Merwad, A.M.A.; Yasin, M.A.T.; Abdul-Hamid, M.I.E.; El-Sobky, E.E.A.; Oraby, H.F. Nitrogen use efficiency in spring wheat: Genotypic variation and grain yield response under sandy soil conditions. J. Agric. Sci. 2017, 155, 1407–1423. [Google Scholar] [CrossRef]
- Omara, P.; Aula, L.; Oyebiyi, F.; Raun, W.R. World cereal nitrogen use efficiency trends: Review and current knowledge. Agrosyst. Geosci. Environ. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- López-Bellido, L.; López-Bellido, R.J.; López-Bellido, F.J. Fertilizer nitrogen efficiency in durum wheat under rainfed Mediterranean conditions: Effect of split application. Agron. J. 2006, 98, 55–62. [Google Scholar] [CrossRef]
- Ma, B.; Qian, W.; Yuan, C.; Yuan, Z.; Peng, Y. Achieving mainstream nitrogen removal through coupling anammox with denitratation. Environ. Sci. Technol. 2017, 51, 8405–8413. [Google Scholar] [CrossRef]
- Dobermann, A.R. Nitrogen use efficiency-state of the art. Agron. Fac. Publ. 2005, 316. Available online: https://digitalcommons.unl.edu/agronomyfacpub/316 (accessed on 25 August 2021).
- Barut, H. Effects of foliar urea, potassium and zinc sulphate treatments before and after flowering on grain yield, technological quality and nutrient concentrations of wheat. Appl. Ecol. Environ. Res. 2019, 17, 4325–4342. [Google Scholar] [CrossRef]
- Rosecrance, R.; Johnson, R.; Weinbaum, S. The effect of timing of post-harvest foliar urea sprays on nitrogen absorption and partitioning in peach and nectarine trees. J. Hortic. Sci. Biotechnol. 1998, 73, 856–861. [Google Scholar] [CrossRef]
- Khan, P.; Memon, M.Y.; Imtiaz, M.; Aslam, M. Response of wheat to foliar and soil application of urea at different growth stages. Pak. J. Bot. 2009, 41, 1197–1204. [Google Scholar]
- Wagan, Z.A.; Buriro, M.; Wagan, T.A.; Wagan, Z.A.; Jamro, S.A.; Memon, Q.U.A.; Wagan, S.A. Effect of foliar applied urea on growth and yield of wheat (Triticum aestivium L.). Int. J. Bioorg. Chem. 2017, 2, 185–191. [Google Scholar]
- Pospišil, A.; Varga, B.; Svečnjak, Z.; Carović, K. Influence of cropping system intensity on dry matter yield and nitrogen concentration in different parts of soybean plant. Agric. Conspec. Sci. 2006, 71, 51–57. [Google Scholar]
- Saleem, I.; Javid, S.; Sial, R.A.; Ehsan, S.; Ahmad, Z.A. Substitution of soil application of urea with foliar application to minimize the wheat yield losses. Soil Environ. 2013, 32, 141–145. [Google Scholar]
- Emam, Y.; Borjian, A. Yield and yield components of two winter wheat (Triticum aestivum L.) cultivars in response to rate and time of foliar urea application. J. Agric. Sci. Technol. 2000, 2, 263–270. [Google Scholar]
- Görgüç, A.; Gençdağ, E.; Yılmaz, F.M. Bioactive peptides derived from plant origin by-products: Biological activities and techno-functional utilizations in food developments–A review. Food Res. Int. 2020, 136, 109504. [Google Scholar] [CrossRef]
- Osman, A.; Merwad, A.R.M.; Mohamed, A.H.; Sitohy, M. Foliar Spray with Pepsin-and Papain-Whey Protein Hydrolysates Promotes the Productivity of Pea Plants Cultivated in Clay Loam Soil. Molecules 2021, 26, 2805. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Desoky, E.S.M.; Osman, A.; Rady, M.M. Pumpkin seed protein hydrolysate treatment alleviates salt stress effects on Phaseolus vulgaris by elevating antioxidant capacity and recovering ion homeostasis. Sci. Hortic. 2020, 271, 109495. [Google Scholar] [CrossRef]
- Osman, A.; Imbabi, T.A.; El-Hadary, A.; Sabeq, I.I.; Edris, S.N.; Merwad, A.; Azab, E.; Gobouri, A.; Mohammadein, A.; Sitohy, M. Health Aspects, Growth Performance, and Meat Quality of Rabbits Receiving Diets Supplemented with Lettuce Fertilized with Whey Protein Hydrolysate Substituting Nitrate. Biomolecules 2021, 11, 835. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Reynaud, H.; Canaguier, R.; Trtílek, M.; Panzarová, K.; et al. Understanding the biostimulant action of vegetal-derived protein hydrolysates by high-throughput plant phenotyping and metabolomics: A case study on tomato. Front. Plant Sci. 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Goda, H.A.; Abdel-Hamid, M.; Badran, S.M.; Otte, J. Antibacterial peptides generated by Alcalase hydrolysis of goat whey. LWT Food Sci. Technol. 2016, 65, 480–486. [Google Scholar] [CrossRef]
- Sitohy, M.; Taha, S.; Osman, A.; Abdel-Hamid, M.; Hamed, A.; Abdelbacki, A. Antiviral action of native and methylated lactoferrin and β-Lactoglobulin against potato virus Y (PVY) infected into potato plants grown in an open field. Antibiotics 2020, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Sitohy, M.; Chobert, J.-M.; Haertlé, T. Esterified whey proteins can protect Lactococcus lactis against bacteriophage infection. Comparison with the effect of native basic proteins and L-polylysines. J. Agric. Food Chem. 2005, 53, 3727–3734. [Google Scholar] [CrossRef]
- Sitohy, M.; Dalgalarrondo, M.; Nowoczin, M.; Besse, B.; Billaudel, S.; Haertlé, T.; Chobert, J.M. The effect of bovine whey proteins on the ability of poliovirus and Coxsackie virus to infect Vero cell cultures. Int. Dairy J. 2008, 18, 658–668. [Google Scholar] [CrossRef]
- Sitohy, M.; Besse, B.; Billaudel, S.; Haertlé, T.; Chobert, J.M. Antiviral Action of Methylated β-Lactoglobulin on the Human Influenza Virus A Subtype H3N2. Probiotics Antimicrob. Proteins 2010, 2, 104–111. [Google Scholar] [CrossRef]
- Abdelbacki, A.M.; Taha, S.H.; Sitohy, M.Z.; Abou Dawood, A.I.; Abd-El Hamid, M.M.; Rezk, A.A. Inhibition of tomato yellow leaf curl virus (TYLCV) using whey proteins. Virol. J. 2010, 7, 26. [Google Scholar] [CrossRef]
- Taha, S.H.; Mehrez, M.A.; Sitohy, M.Z.; Abou Dawood, A.G.I.; Abd-El Hamid, M.M.; Kilany, W.H. Effectiveness of esterified whey proteins fractions against Egyptian Lethal Avian Influenza A (H5N1). Virol. J. 2010, 7, 330. [Google Scholar] [CrossRef]
- Otte, J.; Abdel-Hamid, M.; Osman, A. Comparative assessment of peptide concentration in milk protein hydrolysates and fractions. Int. J. Dairy Sci. 2015, 10, 228–235. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Otte, J.; De Gobba, C.; Osman, A.; Hamad, E. Angiotensin I-converting enzyme inhibitory activity and antioxidant capacity of bioactive peptides derived from enzymatic hydrolysis of buffalo milk proteins. Int. Dairy J. 2017, 66, 91–98. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Goda, H.A.; De Gobba, C.; Jenssen, H.; Osman, A. Antibacterial activity of papain hydrolysed camel whey and its fractions. Int. Dairy J. 2016, 61, 91–98. [Google Scholar] [CrossRef]
- El-Zahar, K.; Sitohy, M.; Choiset, Y.; Metro, F.; Haertle, T.; Chobert, J.M. Antimicrobial activity of ovine whey protein and their peptic hydrolysates. Milchwissenschaft 2004, 59, 653–656. [Google Scholar]
- EL-Zahar, K.; Chobert, J.M.; Dalgalarrondo, M.; Sitohy, M.; Haertlé, T. Proteolysis of ewe’s caseins and whey proteins during fermentation of yogurt and storage. Effect of the starters used. J. Food Biochem. 2004, 28, 319–335. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Osman, A.; El-Hadary, A.; Romeih, E.; Sitohy, M.; Li, L. Hepatoprotective action of papain-hydrolyzed buffalo milk protein on carbon tetrachloride oxidative stressed albino rats. J. Dairy Sci. 2020, 103, 1884–1893. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Romeih, E.; Saporito, P.; Osman, A.; Mateiu, R.V.; Mojsoska, B.; Jenssen, H. Camel milk whey hydrolysate inhibits growth and biofilm formation of Pseudomonas aeruginosa PAO1 and methicillin-resistant Staphylococcus aureus. Food Control 2020, 111, 107056. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F.; Osman, A.; El-Akad, H. Total antioxidant potential of juices and beverages-Screening by DPPH in vitro assay. Wiss. Verl. Stuttg. 2008, 104, 235–239. [Google Scholar]
- Osman, A.; El-Hadary, A.; Korish, A.A.; AlNafea, H.M.; Alhakbany, M.A.; Awad, A.A.; Abdel-Hamid, M. Angiotensin-I converting enzyme inhibition and antioxidant activity of papain-hydrolyzed camel whey protein and its hepato-renal protective effects in thioacetamide-induced toxicity. Foods 2021, 10, 468. [Google Scholar] [CrossRef]
- Al-Mohammadi, A.-R.; Osman, A.; Enan, G.; Abdel-Shafi, S.; El-Nemer, M.; Sitohy, M.; Taha, M.A. Powerful antibacterial peptides from egg albumin hydrolysates. Antibiotics 2020, 9, 901. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Voldeng, H.; Simpson, G. The relationship between photosynthetic area and grain yield per plant in wheat. Can. J. Plant Sci. 1967, 47, 359–365. [Google Scholar] [CrossRef]
- FAO. World Fertilizer Trends and Outlook to 2022; FAO: Rome, Italy, 2019. [Google Scholar]
- Daba, N.A. Influence of nitrogen fertilizer application on grain yield, nitrogen uptake efficiency and nitrogen use efficiency of bread wheat (Triticum aestivum L.) cultivars in Eastern Ethiopia. J. Agric. Sci. 2017, 9, 202–217. [Google Scholar] [CrossRef][Green Version]
- Wang, D.; Xu, Z.; Zhao, J.; Wang, Y.; Yu, Z. Excessive nitrogen application decreases grain yield and increases nitrogen loss in a wheat—Soil system. Acta Agric. Scand. Sect. B Soil Plant Sci. 2011, 61, 681–692. [Google Scholar] [CrossRef]
- Kong, L.; Xie, Y.; Hu, L.; Si, J.; Wang, Z. Excessive nitrogen application dampens antioxidant capacity and grain filling in wheat as revealed by metabolic and physiological analyses. Sci. Rep. 2017, 7, 43363. [Google Scholar] [CrossRef] [PubMed]
- Yassen, A.; Abou El-Nour, E.; Shedeed, S. Response of wheat to foliar spray with urea and micronutrients. J. Am. Sci. 2010, 6, 14–22. [Google Scholar]
- Schiavon, M.; Ertani, A.; Nardi, S. Effects of an alfalfa protein hydrolysate on the gene expression and activity of enzymes of the tricarboxylic acid (TCA) cycle and nitrogen metabolism in Zea mays L. J. Agric. Food Chem. 2008, 56, 11800–11808. [Google Scholar] [CrossRef]
- Sestili, F.; Rouphael, Y.; Cardarelli, M.; Pucci, A.; Bonini, P.; Canaguier, R.; Colla, G. Protein hydrolysate stimulates growth in tomato coupled with N-dependent gene expression involved in N assimilation. Front. Plant Sci. 2018, 9, 1233. [Google Scholar] [CrossRef]
- Youldash, K.M.; Barutcular, C.; El Sabagh, A.; Toptas, I.; Kayaalp, G.T.; Hossain, A.; Alharby, H.; Bamagoos, A.; Saneoka, H.; Farooq, M. Evaluation of grain yield in fifty-eight spring bread wheat genotypes grown under heat stress. Pak. J. Bot. 2020, 52, 33–42. [Google Scholar] [CrossRef]
- Gurav, R.G.; Jadhav, J.P. A novel source of biofertilizer from feather biomass for banana cultivation. Environ. Sci. Pollut. Res. 2013, 20, 4532–4539. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.; Van Beek, T. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Foh, M.B.K.; Amadou, I.; Foh, B.M.; Kamara, M.T.; Xia, W. Functionality and antioxidant properties of tilapia (Oreochromis niloticus) as influenced by the degree of hydrolysis. Int. J. Mol. Sci. 2010, 11, 1851–1869. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Cui, C.; Zhao, H.; Yang, B. Effect of degree of hydrolysis on the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates. Innov. Food Sci. Emerg. Technol. 2009, 10, 235–240. [Google Scholar] [CrossRef]
- Moreno-Hernández, J.M.; Benítez-García, I.; Mazorra-Manzano, M.A.; Ramírez-Suárez, J.C.; Sánchez, E. Strategies for production, characterization and application of protein-based biostimulants in agriculture: A review. Chil. J. Agric. Res. 2020, 80, 274–289. [Google Scholar] [CrossRef]
- Fageria, N. Nitrogen harvest index and its association with crop yields. J. Plant Nutr. 2014, 37, 795–810. [Google Scholar] [CrossRef]
- Triboi, E.; Martre, P.; Girousse, C.; Ravel, C.; Triboi-Blondel, A.M. Unravelling environmental and genetic relationships between grain yield and nitrogen concentration for wheat. Eur. J. Agron. 2006, 25, 108–118. [Google Scholar] [CrossRef]


| Month | 2017–2018 | 2018–2019 | 20-yr Average (2001–2019) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Max. T | Min. T | RH | P | Max. T | Min. T | RH | P | Max. T | Max. T | RH | P | |
| --------°C-------- | % | mm | --------°C-------- | % | mm | --------°C-------- | % | mm | ||||
| November | 24.20 | 16.23 | 60.98 | 1.02 | 25.46 | 15.70 | 44.57 | 3.00 | 25.10 | 14.32 | 65.00 | 3.00 |
| December | 22.29 | 14.03 | 64.02 | 0.25 | 21.12 | 10.90 | 47.93 | 3.00 | 20.65 | 10.61 | 61.00 | 3.00 |
| January | 19.38 | 11.45 | 60.00 | 5.33 | 19.41 | 7.74 | 59.28 | 6.90 | 18.16 | 8.46 | 63.60 | 7.00 |
| February | 23.75 | 13.67 | 55.31 | 13.46 | 20.39 | 11.50 | 55.55 | 3.01 | 19.64 | 9.60 | 58.70 | 3.00 |
| March | 27.96 | 16.32 | 45.65 | 2.03 | 26.61 | 13.58 | 53.13 | 2.00 | 24.27 | 12.99 | 51.30 | 2.00 |
| April | 29.26 | 18.20 | 44.09 | 6.10 | 28.50 | 12.63 | 48.52 | 1.00 | 28.20 | 15.43 | 46.90 | 1.00 |
| Soil Depth (cm) | Soil Particles Distribution | Textural Class | Field Capacity (%) | Wilting Point (%) | Bulk Density (g cm−3) | Calcium Carbonate (%) | Organic Matter (%) | pH * | EC (dS m−1) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand (%) | Silt (%) | Clay (%) | |||||||||
| 0–30 | 48.19 | 13.83 | 37.98 | Sandy clay | 13.50 | 6.75 | 1.48 | 0.41 | 0.46 | 7.96 | 1.63 |
| 30–60 | 47.99 | 13.76 | 38.25 | Sandy clay | 12.31 | 6.15 | 1.51 | 0.41 | 0.34 | 7.90 | 1.60 |
| 60–90 | 48.02 | 13.69 | 38.29 | Sandy clay | 12.20 | 6.10 | 1.53 | 0.40 | 0.32 | 7.90 | 1.56 |
| Soil Depth (cm) | Soluble Cations and Anions (mmolc L−1) ** | Available Nutrient (mg kg−1 Soil) | |||||||||
| Calcium | Magnesium | Sodium | Potassium | Carbonate | Bicarbonate | Chloride | Sulphate | Nitrogen | Phosphorus | Potassium | |
| 0–30 | 5.54 | 4.65 | 3.07 | 2.90 | 0 | 6.57 | 3.94 | 5.65 | 20.02 | 11.00 | 170.00 |
| 30–60 | 5.53 | 4.58 | 3.09 | 2.70 | 0 | 6.33 | 4.97 | 4.60 | 17.50 | 9.25 | 154.00 |
| 60–90 | 5.47 | 4.40 | 3.11 | 2.68 | 0 | 6.07 | 4.98 | 4.61 | 16.70 | 9.00 | 143.20 |
| Amino Acid | Conc (g/100 g) | Relative Content |
|---|---|---|
| Basic amino acids | 35.35% | |
| Lysine | 2.2 | |
| Arginine | 19.4 | |
| Histidine | 11.7 | |
| Total | 33.3 | |
| Acidic amino acids | 14.22% | |
| Aspartic | 10 | |
| Glutamic | 3.4 | |
| Total | 13.4 | |
| Aromatic amino acids | 4.45% | |
| Tyrosine | 2.1 | |
| Phenylalanine | 2.1 | |
| Total | 4.2 | |
| Hydrophobic amino acids | 19.00% | |
| Glycine | 3.5 | |
| Alanine | 2.4 | |
| Valine | 2.2 | |
| Leucine | 2.5 | |
| Isoleucine | 2.3 | |
| Tyrosine | 2.1 | |
| Phenylalanine | 2.1 | |
| Methionine | 0.8 | |
| Total | 17.9 | |
| Hydrophilic amino acids | 31.42% | |
| Serine | 3.4 | |
| Threonine | 3 | |
| Asparagine | 20 | |
| Glutamine | 3.2 | |
| Total | 29.6 |
| Studied Factors | Flag Leaf Area (cm2) | Total Chlorophyll Content (SPAD Value) | Fertile Spikelets Number Spike−1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | ||||||||
| Spraying treatments (S) | |||||||||||||
| Control | 34.63 | C | 38.12 | C | 49.54 | B | 50.30 | B | 17.05 | 14.57 | |||
| WPH solution | 41.61 | A | 44.79 | A | 51.19 | AB | 52.91 | A | 18.34 | 15.08 | |||
| Urea solution | 38.20 | B | 40.41 | B | 51.44 | A | 52.88 | A | 17.61 | 15.01 | |||
| Nitrogen fertilization levels (N) | |||||||||||||
| 145 kg N ha−1 | 33.63 | c | 35.38 | c | 47.36 | b | 47.33 | c | 16.15 | b | 12.88 | c | |
| 180 kg N ha−1 | 38.10 | b | 40.32 | b | 51.24 | a | 51.61 | b | 17.51 | ab | 14.19 | b | |
| 215 kg N ha−1 | 39.89 | a | 43.94 | a | 52.14 | a | 54.47 | a | 18.30 | a | 16.20 | a | |
| 250 kg N ha−1 | 40.96 | a | 44.78 | a | 52.14 | a | 54.72 | a | 18.71 | a | 16.28 | a | |
| ANOVA | df | Mean Square (MS) and p-Value of Main Effects and Their Interaction (p-Value) | |||||||||||
| MS | p-Value | MS | p-Value | MS | p-Value | MS | p-Value | MS | p-Value | MS | p-Value | ||
| S | 2 | 146.05 | 0.005 | 137.74 | <0.001 | 12.79 | 0.042 | 26.87 | 0.077 | 5.03 | 0.356 | 0.91 | 0.202 |
| N | 3 | 94.00 | <0.001 | 164.74 | <0.001 | 46.73 | <0.001 | 106.42 | <0.001 | 11.40 | 0.005 | 24.54 | <0.001 |
| Linear | 1 | 254.31 | <0.001 | 455.50 | <0.001 | 104.42 | <0.001 | 282.00 | <0.001 | 32.19 | 0.002 | 67.10 | <0.001 |
| Quadratic | 1 | 25.93 | 0.002 | 37.76 | <0.001 | 33.83 | 0.007 | 36.60 | <0.001 | 2.01 | 0.355 | 3.42 | 0.197 |
| Deviations | 1 | 1.76 | 0.327 | 0.96 | 0.352 | 1.94 | 0.450 | 0.66 | 0.310 | 0.01 | 0.932 | 3.12 | 0.217 |
| S × N | 6 | 11.93 | <0.001 | 6.80 | 0.001 | 0.142 | 0.039 | 3.42 | 0.02 | 1.85 | 0.472 | 0.89 | 0.704 |
| Studied Factors | Grain Number Spike−1 | Grain Weight Spike−1 (g) | 1000-Grain Weight (g) | ||||||||||
| 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | ||||||||
| Spraying Treatments (S) | |||||||||||||
| Control | 27.79 | B | 26.48 | B | 1.14 | B | 1.21 | B | 33.83 | B | 37.35 | B | |
| WPH solution | 30.00 | A | 30.38 | A | 1.34 | A | 1.61 | A | 39.01 | A | 44.53 | A | |
| Urea solution | 30.25 | A | 30.78 | A | 1.25 | AB | 1.29 | B | 36.38 | AB | 42.55 | A | |
| Nitrogen Fertilization Levels (N) | |||||||||||||
| 145 kg N ha−1 | 27.33 | c | 20.96 | c | 1.00 | c | 0.80 | c | 30.03 | c | 34.35 | c | |
| 180 kg N ha−1 | 28.12 | bc | 26.2 | b | 1.17 | b | 1.27 | b | 33.64 | b | 41.11 | b | |
| 215 kg N ha−1 | 30.38 | ab | 34.32 | a | 1.38 | a | 1.69 | a | 39.71 | a | 44.70 | a | |
| 250 kg N ha−1 | 31.56 | a | 35.36 | a | 1.42 | a | 1.73 | a | 42.25 | a | 45.75 | a | |
| ANOVA | df | Mean Square (MS) and p-Value of Main Effects and Their Interaction (p-Value) | |||||||||||
| MS | p-Value | MS | p-Value | MS | p-Value | MS | p-Value | MS | p-Value | MS | p-Value | ||
| S | 2 | 21.88 | 0.025 | 67.802 | 0.038 | 0.12 | 0.028 | 1.71 | 0.014 | 80.37 | 0.015 | 164.97 | 0.002 |
| N | 3 | 34.48 | 0.004 | 423.12 | <0.001 | 0.34 | <0.001 | 0.73 | <0.001 | 280.19 | <0.001 | 238.74 | <0.001 |
| Linear | 1 | 100.20 | <0.001 | 1185.03 | <0.001 | 0.97 | <0.001 | 0.43 | <0.001 | 821.88 | <0.001 | 642.59 | <0.001 |
| Quadratic | 1 | 0.34 | 0.809 | 39.79 | 0.030 | 0.03 | 0.277 | 0.05 | <0.001 | 2.53 | 0.582 | 73.47 | 0.002 |
| Deviations | 1 | 2.91 | 0.482 | 44.55 | 0.023 | 0.02 | 0.388 | 1.71 | 0.133 | 16.17 | 0.179 | 0.17 | 0.852 |
| S × N | 6 | 7.37 | 0.031 | 9.75 | 0.046 | 0.01 | 0.049 | 0.10 | 0.005 | 20.30 | 0.098 | 16.75 | 0.004 |
| Studied Factors | Spike Number m−2 | Grain Yield (kg ha−1) | Straw Yield (kg ha−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | ||||||||
| Spraying treatments (S) | |||||||||||||
| Control | 462.83 | B | 425.58 | C | 5494.0 | C | 5293.6 | C | 8155.3 | 8272.7 | |||
| WPH solution | 491.25 | A | 463.25 | A | 6097.9 | A | 6078.0 | A | 8318.8 | 8392.2 | |||
| Urea solution | 475.42 | B | 441.42 | B | 5832.8 | B | 5791.5 | B | 8285.7 | 8390.8 | |||
| Nitrogen fertilization levels (N) | |||||||||||||
| 145 kg N ha−1 | 434.11 | c | 411.78 | c | 4264.5 | c | 3651.1 | c | 7034.3 | d | 6665.0 | ||
| 180 kg N ha−1 | 459.22 | b | 433.00 | b | 5152.8 | b | 5005.4 | b | 7578.2 | c | 7938.5 | ||
| 215 kg N ha−1 | 502.78 | a | 460.78 | a | 6841.5 | a | 7044.5 | a | 8750.6 | b | 9054.7 | ||
| 250 kg N ha−1 | 509.89 | a | 468.11 | a | 6974.1 | a | 7183 | a | 9649.9 | a | 9749.4 | ||
| ANOVA | df | Mean Square (MS) and p-Value of Main Effects and Their Interaction (p-Value) | |||||||||||
| MS | p-Value | MS | p-Value | MS | p-Value | MS | p-Value | MS | p-Value | MS | p-Value | ||
| S | 2 | 2433.08 | 0.009 | 4292.33 | 0.001 | 1,099,726 | 0.001 | 1,890,618 | 0.004 | 89,733 | 0.785 | 56445 | 0.712 |
| N | 3 | 11,702.05 | <0.001 | 6062.25 | <0.001 | 15,718,418 | <0.001 | 26,057,327 | <0.001 | 12,418,396 | <0.001 | 16,390,733 | <0.001 |
| Linear | 1 | 33,021.40 | <0.001 | 17,424.67 | <0.001 | 43,371,168 | <0.001 | 71,837,979 | <0.001 | 36,605,001 | <0.001 | 48,387,202 | <0.001 |
| Quadratic | 1 | 729.00 | 0.016 | 434.03 | <0.001 | 1,285,100 | <0.001 | 3,326,111 | <0.001 | 284,172 | 0.176 | 753,547 | 0.103 |
| Deviations | 1 | 1355.76 | 0.002 | 328.05 | <0.001 | 2,498,986 | <0.001 | 3,007,892 | <0.001 | 366,016 | 0.129 | 31,450 | 0.725 |
| S × N | 6 | 400.79 | 0.011 | 106.89 | 0.001 | 79,750 | 0.060 | 37,972 | 0.001 | 49,579 | 0.914 | 133,285 | 0.671 |
| Studied Factors | Harvest index (%) | Straw N content (mg g−1) | Grain N content (mg g−1) | ||||||||||
| 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | ||||||||
| Spraying treatments (S) | |||||||||||||
| Control | 39.84 | 37.84 | B | 6.26 | C | 6.45 | C | 17.40 | B | 18.49 | B | ||
| WPH solution | 42.06 | 41.68 | A | 6.40 | B | 6.55 | B | 19.55 | A | 19.00 | A | ||
| Urea solution | 41.11 | 40.48 | A | 6.50 | A | 6.67 | A | 18.81 | A | 19.10 | A | ||
| Nitrogen fertilization levels (N) | |||||||||||||
| 145 kg N ha−1 | 37.71 | d | 35.26 | c | 6.17 | c | 6.32 | c | 18.53 | b | 18.62 | b | |
| 180 kg N ha−1 | 40.48 | c | 38.56 | b | 6.24 | bc | 6.38 | b | 19.01 | ab | 19.03 | a | |
| 215 kg N ha−1 | 43.87 | a | 43.77 | a | 6.28 | b | 6.44 | b | 19.55 | a | 19.21 | a | |
| 250 kg N ha−1 | 41.94 | b | 42.42 | a | 6.85 | a | 7.08 | a | 18.53 | b | 18.59 | b | |
| ANOVA | df | Mean square (MS) and p-value of main effects and their interaction (p-value) | |||||||||||
| MS | p-Value | MS | p-Value | MS | p-Value | MS | p-Value | MS | p-Value | MS | p-Value | ||
| S | 2 | 14.90 | 0.165 | 46.26 | 0.003 | 0.17 | <0.001 | 0.13 | 0.011 | 21.12 | 0.018 | 1.29 | <0.001 |
| N | 3 | 60.93 | <0.001 | 133.81 | <0.001 | 0.87 | <0.001 | 1.10 | <0.001 | 1.98 | 0.046 | 0.85 | <0.001 |
| Linear | 1 | 116.98 | <0.001 | 320.49 | <0.001 | 1.94 | <0.001 | 2.42 | <0.001 | 1.36 | <0.001 | 0.12 | 0. 049 |
| Quadratic | 1 | 49.95 | <0.001 | 48.66 | <0.001 | 0.57 | <0.001 | 0.75 | <0.001 | 4.57 | <0.001 | 2.43 | <0.001 |
| Deviations | 1 | 15.869 | 0.027 | 32.29 | 0.003 | 0.12 | <0.001 | 0.15 | <0.001 | 0.01 | 0.177 | 0.003 | 0.613 |
| S × N | 6 | 1.811 | 0.034 | 21.99 | <0.001 | 0.002 | 0.034 | 0.001 | 0.048 | 0.16 | 0.041 | 0.01 | 0.0306 |
| Studied Factors | Grain Protein Content (%) | Grain N Uptake (kg ha−1) | Total N Uptake (kg ha−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | ||||||||
| Spraying treatments (S) | |||||||||||||
| Control | 9.93 | B | 10.55 | B | 95.57 | B | 98.04 | C | 146.92 | B | 151.69 | B | |
| WPH solution | 11.16 | A | 10.85 | A | 119.59 | A | 115.61 | A | 173.11 | A | 170.96 | A | |
| Urea solution | 11.31 | A | 10.91 | A | 115.83 | A | 110.71 | B | 169.95 | A | 166.95 | A | |
| Nitrogen fertilization levels (N) | |||||||||||||
| 145 kg N ha−1 | 10.58 | b | 10.63 | b | 79.41 | c | 68.13 | c | 122.90 | c | 110.30 | d | |
| 180 kg N ha−1 | 10.85 | ab | 10.87 | ab | 98.13 | b | 95.44 | b | 145.43 | b | 146.15 | c | |
| 215 kg N ha−1 | 11.16 | a | 10.97 | a | 133.93 | a | 135.38 | a | 188.95 | a | 193.74 | b | |
| 250 kg N ha−1 | 10.62 | b | 10.61 | b | 129.87 | a | 133.54 | a | 196.03 | a | 202.61 | a | |
| ANOVA | df | Mean Square (MS) and p-Value of Main Effects and Their Interaction (p-Value) | |||||||||||
| MS | p-Value | MS | p-Value | MS | p-Value | MS | p-Value | MS | p-Value | MS | p-Value | ||
| S | 2 | 6.88 | 0.042 | 0.42 | <0.001 | 2002.81 | 0.003 | 986.84 | <0.001 | 2452.27 | 0.004 | 1239.33 | 0.002 |
| N | 3 | 0.64 | <0.001 | 0.28 | <0.001 | 6131.06 | <0.001 | 9446.82 | <0.001 | 11,042.86 | <0.001 | 16,724.94 | <0.001 |
| Linear | 1 | 0.98 | 0.041 | 0.24 | 0.024 | 15,767.82 | <0.001 | 25,096.65 | <0.001 | 31,106.57 | <0.001 | 47,390.6 | <0.001 |
| Quadratic | 1 | 0.85 | 0.042 | 0.59 | <0.001 | 1166.53 | <0.001 | 1912.05 | <0.001 | 537.38 | 0.004 | 31,638.06 | <0.001 |
| Deviations | 1 | 0.10 | 0.177 | 0.01 | <0.001 | 1458.85 | <0.001 | 1331.77 | <0.001 | 1484.63 | <0.001 | 1146.17 | <0.001 |
| S × N | 6 | 0.05 | 0.039 | 0.003 | 0.041 | 18.60 | 0.032 | 120.94 | <0.001 | 31.56 | 0.041 | 94.26 | 0.028 |
| Studied Factors | N Utilization Efficiency (kg kg−1) | N Harvest Index (%) | Partial Factor Productivity of Applied N (kg kg−1) | ||||||||||
| 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | 2017–2018 | 2018–2019 | ||||||||
| Spraying treatments (S) | |||||||||||||
| Control | 37.19 | 34.15 | 64.68 | B | 63.19 | B | 49.56 | B | 45.61 | B | |||
| WPH solution | 35.24 | 35.48 | 68.86 | A | 67.43 | A | 55.05 | A | 54.14 | A | |||
| Urea solution | 34.34 | 34.56 | 67.95 | A | 66.03 | A | 42.84 | C | 42.00 | B | |||
| Nitrogen fertilization levels (N) | |||||||||||||
| 145 kg N ha−1 | 34.77 | 32.89 | d | 64.37 | c | 61.30 | c | 60.17 | a | 51.12 | a | ||
| 180 kg N ha−1 | 35.55 | 34.19 | c | 67.35 | b | 65.11 | b | 48.47 | b | 47.18 | b | ||
| 215 kg N ha−1 | 36.38 | 36.38 | a | 70.76 | a | 69.88 | a | 48.44 | b | 49.86 | b | ||
| 250 kg N ha−1 | 35.66 | 35.46 | b | 66.16 | b | 65.92 | b | 39.53 | c | 40.71 | c | ||
| ANOVA | df | Mean Square (MS) and p-Value of Main Effects and Their Interaction (p-Value) | |||||||||||
| MS | p-Value | MS | p-Value | MS | p-Value | MS | p-Value | MS | p-Value | MS | p-Value | ||
| S | 2 | 25.39 | 0.076 | 5.55 | 0.034 | 57.73 | 0.0112 | 55.88 | 0.003 | 448.48 | 0.002 | 458.05 | 0.009 |
| N | 3 | 3.84 | 0.108 | 20.78 | <0.001 | 65.40 | <0.001 | 111.53 | <0.001 | 644.86 | <0.001 | 194.05 | <0.001 |
| Linear | 1 | 5.95 | 0.005 | 43.98 | <0.001 | 34.65 | 0.006 | 156.16 | <0.001 | 1726.71 | <0.001 | 367.41 | <0.001 |
| Quadratic | 1 | 5.46 | 0.006 | 11.12 | 0.001 | 129.44 | <0.001 | 136.08 | <0.001 | 17.55 | 0.051 | 61.19 | 0.009 |
| Deviations | 1 | 0.11 | 0.376 | 7.24 | 0.005 | 32.12 | 0.008 | 42.35 | <0.001 | 190.32 | <0.001 | 153.55 | <0.001 |
| S × N | 6 | 1.87 | 0.305 | 7.51 | 0.061 | 1.06 | 0.041 | 25.81 | <0.001 | 33.66 | <0.001 | 58.34 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sanatawy, A.M.; Ash-Shormillesy, S.M.A.I.; El-Yazied, A.A.; El-Gawad, H.G.A.; Azab, E.; Gobouri, A.A.; Sitohy, M.; Osman, A. Enhancing Grain Yield and Nitrogen Accumulation in Wheat Plants Grown under a Mediterranean Arid Environment by Foliar Spray with Papain-Released Whey Peptides. Agronomy 2021, 11, 1913. https://doi.org/10.3390/agronomy11101913
El-Sanatawy AM, Ash-Shormillesy SMAI, El-Yazied AA, El-Gawad HGA, Azab E, Gobouri AA, Sitohy M, Osman A. Enhancing Grain Yield and Nitrogen Accumulation in Wheat Plants Grown under a Mediterranean Arid Environment by Foliar Spray with Papain-Released Whey Peptides. Agronomy. 2021; 11(10):1913. https://doi.org/10.3390/agronomy11101913
Chicago/Turabian StyleEl-Sanatawy, AbdAllah M., Salwa M. A. I. Ash-Shormillesy, Ahmed Abou El-Yazied, Hany G. Abd El-Gawad, Ehab Azab, Adil A. Gobouri, Mahmoud Sitohy, and Ali Osman. 2021. "Enhancing Grain Yield and Nitrogen Accumulation in Wheat Plants Grown under a Mediterranean Arid Environment by Foliar Spray with Papain-Released Whey Peptides" Agronomy 11, no. 10: 1913. https://doi.org/10.3390/agronomy11101913
APA StyleEl-Sanatawy, A. M., Ash-Shormillesy, S. M. A. I., El-Yazied, A. A., El-Gawad, H. G. A., Azab, E., Gobouri, A. A., Sitohy, M., & Osman, A. (2021). Enhancing Grain Yield and Nitrogen Accumulation in Wheat Plants Grown under a Mediterranean Arid Environment by Foliar Spray with Papain-Released Whey Peptides. Agronomy, 11(10), 1913. https://doi.org/10.3390/agronomy11101913









