Alternative Lime Pretreatment of Corn Stover for Second-Generation Bioethanol Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Analysis of the Raw Material
2.3. Lime Pretreatment of Corn Stover
2.4. Enzymatic Hydrolysis of Solids from Lime Pretreatments
2.5. Yeast Cultivation and Inoculum Preparation
2.6. Simultaneous Saccharification and Fermentation (SSF)
2.7. Fitting Data
3. Results and Discussion
3.1. Raw Material and Lime Pretreatment
3.2. Evaluation of Enzymatic Susceptibility of Lime-Pretreated Corn Stover
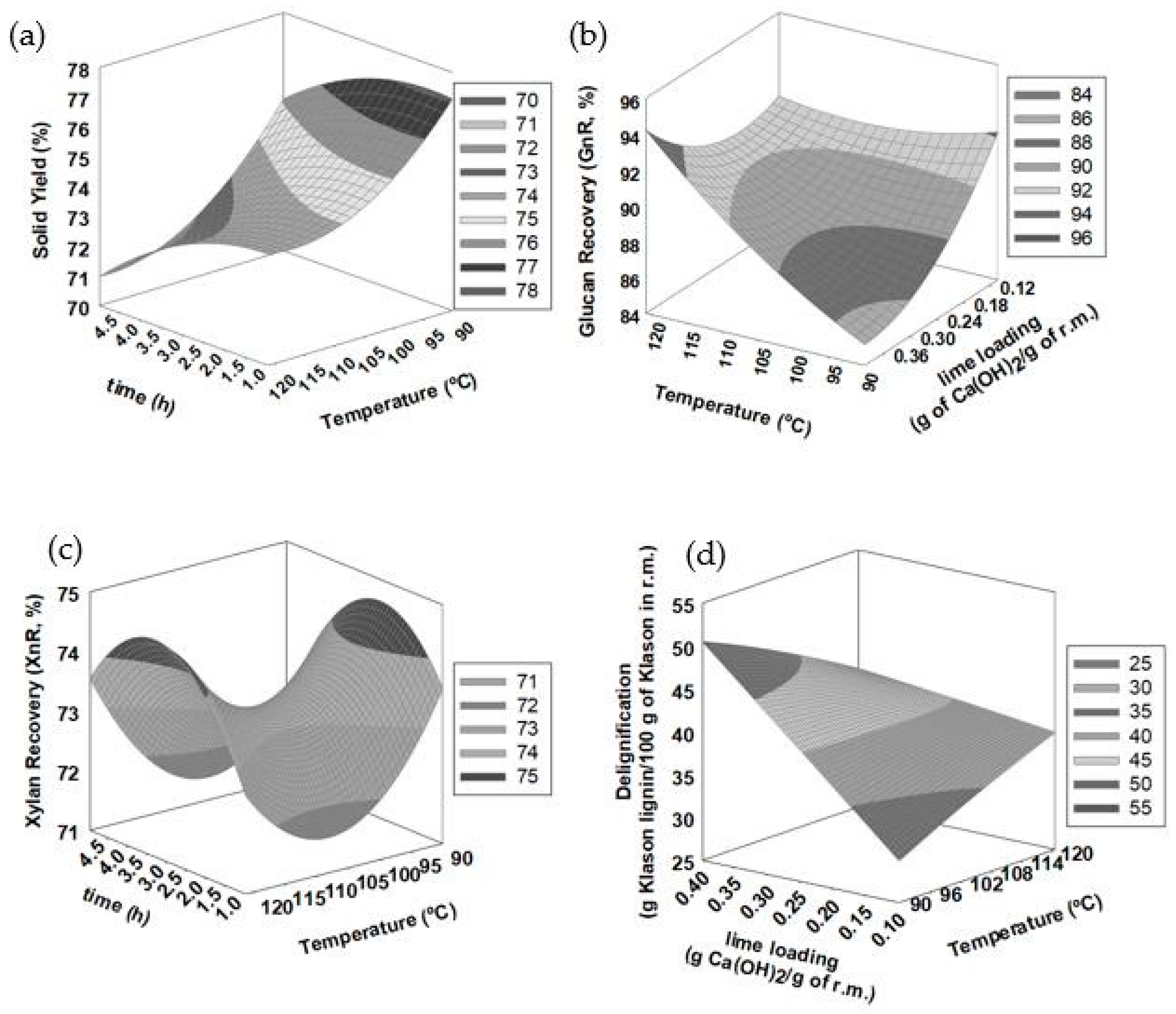
3.3. Response Surface Methodology (RSM) Assessment
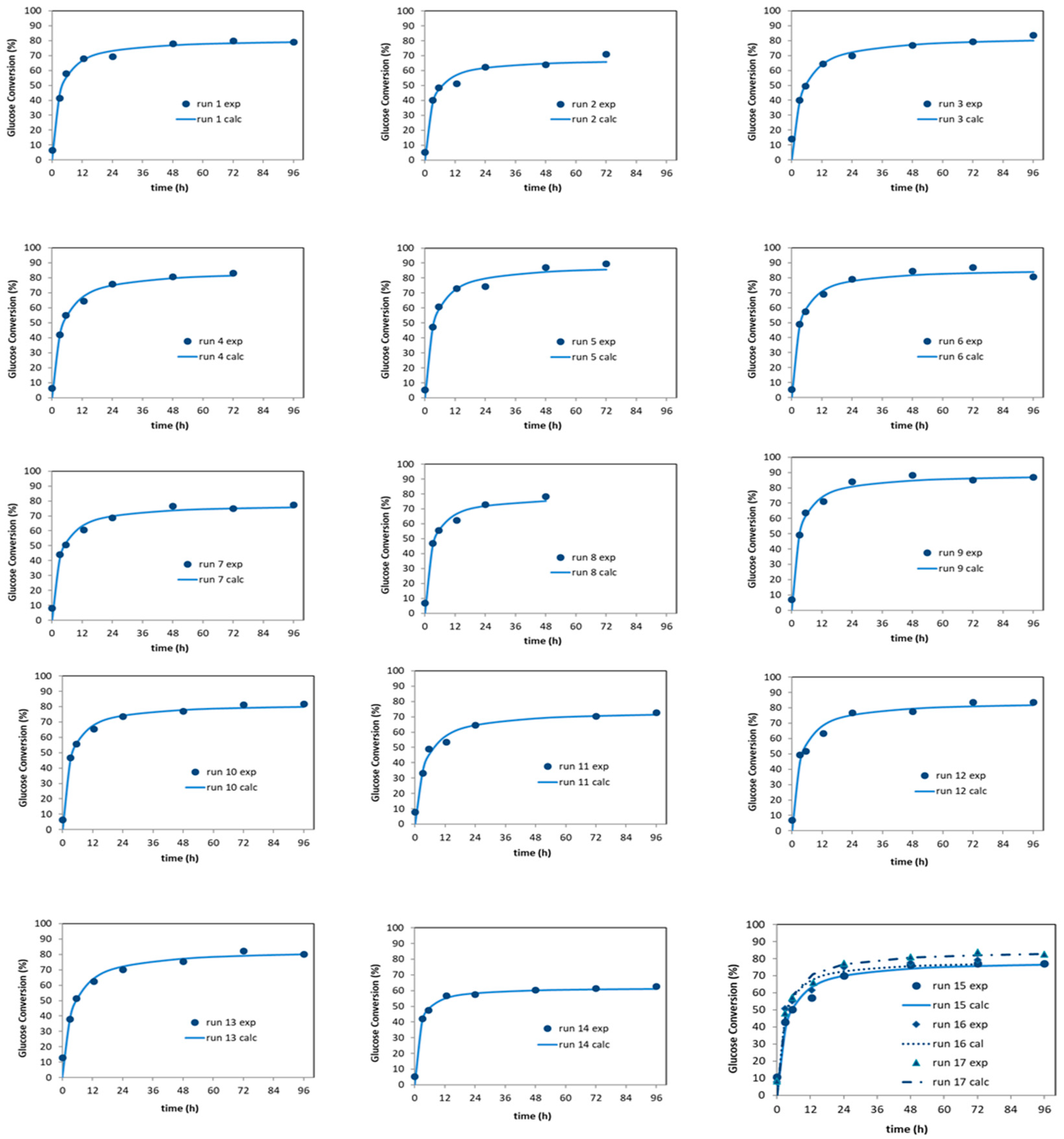
3.4. Simultaneous Saccharification and Fermentation (SSF) for Bioethanol Producction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Del Río, P.G.; Domínguez, V.D.; Domínguez, E.; Gullón, P.; Gullón, B.; Garrote, G.; Romaní, A. Comparative study of biorefinery processes for the valorization of fast-growing Paulownia wood. Bioresour. Technol. 2020, 314, 123722. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, E.; del Río, P.G.; Romaní, A.; Garrote, G.; Gullón, P.; de Vega, A. Formosolv pretreatment to fractionate paulownia wood following a biorefinery approach: Isolation and characterization of the lignin fraction. Agronomy 2020, 10, 1205. [Google Scholar] [CrossRef]
- Del Razola-Díaz, M.C.; Verardo, V.; Martín-García, B.; Díaz-De-Cerio, E.; García-Villanova, B.; Guerra-Hernández, E.J. Establishment of acid hydrolysis by box-behnken methodology as pretreatment to obtain reducing sugars from tiger nut byproducts. Agronomy 2020, 10, 477. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Espinosa, E.; Bascón-Villegas, I.; Pérez-Rodríguez, F.; Carrasco, E.; Rodríguez, A. Production of cellulose nanofibers from olive tree harvest–A residue with wide applications. Agronomy 2020, 10, 696. [Google Scholar] [CrossRef]
- Von Cossel, M.; Wagner, M.; Lask, J.; Magenau, E.; Bauerle, A.; Von Cossel, V.; Warrach-Sagi, K.; Elbersen, B.; Staritsky, I.; van Eupen, M.; et al. Prospects of Bioenergy Cropping Systems for a More Social-Ecologically Sound Bioeconomy. Agronomy 2019, 9, 605. [Google Scholar] [CrossRef]
- Momayez, F.; Karimi, K.; Taherzadeh, M.J. Energy recovery from industrial crop wastes by dry anaerobic digestion: A review. Ind. Crops Prod. 2019, 129, 673–687. [Google Scholar] [CrossRef]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Review of Second Generation Bioethanol Production from Residual Biomass. Food Technol. Biotechnol. 2018, 56, 174–187. [Google Scholar] [CrossRef]
- Rathmann, R.; Szklo, A.; Schaeffer, R. Land use competition for production of food and liquid biofuels: An analysis of the arguments in the current debate. Renew. Energy 2010, 35, 14–22. [Google Scholar] [CrossRef]
- Stöcker, M. Biofuels and biomass-to-liquid fuels in the biorefinery: Catalytic conversion of lignocellulosic biomass using porous materials. Angew. Chem. Int. Ed. 2008, 47, 9200–9211. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Krafft, M.J.; Bendler, M.; Schreiber, A.; Saake, B. Steam refining with subsequent alkaline lignin extraction as an alternative pretreatment method to enhance the enzymatic digestibility of corn stover. Agronomy 2020, 10, 811. [Google Scholar] [CrossRef]
- Mafa, M.S.; Malgas, S.; Bhattacharya, A.; Rashamuse, K. The effects of alkaline pretreatment on agricultural biomasses (corn cob and sweet sorghum bagasse) and their hydrolysis by a termite-derived enzyme cocktail. Agronomy 2020, 10, 1211. [Google Scholar] [CrossRef]
- Mkabayi, L.; Malgas, S.; Wilhelmi, B.S.; Pletscheke, B.I. Evaluating feruloyl esterase—Xylanase synergism for hydroxycinnamic zcid and xylo-oligosaccharide production from untreated, hydrothermally pre-treated and dilute-acid pre-treated corn cobs. Agronomy 2020, 10, 688. [Google Scholar] [CrossRef]
- David, K.; Ragauskas, A.J. Switchgrass as an energy crop for biofuel production: A review of its ligno-cellulosic chemical properties. Energy Environ. Sci. 2010, 3, 1182–1190. [Google Scholar] [CrossRef]
- Lynd, L.R.; Larson, E.; Greene, N.; Laser, M.; Sheehan, J.; Dale, B.E.; McLaughlin, S.; Wang, M. The role of biomass in America’s energy future: Framing the analysis. Biofuels Bioprod. Biorefin. 2009, 3, 113–123. [Google Scholar] [CrossRef]
- Jenkins, B.M. Global Agriculture: Industrial Feedstocks for Energy and Materials; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 3, ISBN 9780080931395. [Google Scholar]
- Tandzi, L.N.; Mutengwa, C.S. Estimation of maize (Zea mays L.) yield per harvest area: Appropriate methods. Agronomy 2020, 10, 29. [Google Scholar] [CrossRef]
- López-Malvar, A.; Djemel, A.; Santiago, R.; Revilla, P. Assessment of Algerian maize populations for saccharification and nutritive value. Agronomy 2020, 10, 646. [Google Scholar] [CrossRef]
- Zhao, Y.; Damgaard, A.; Christensen, T.H. Bioethanol from corn stover–A review and technical assessment of alternative biotechnologies. Prog. Energy Combust. Sci. 2018, 67, 275–291. [Google Scholar] [CrossRef]
- Akter, S.; Zabed, H.M.; Sahu, J.N.; Chowdhury, F.I.; Faruq, G.; Boyce, A.N.; Qi, X. Bioethanol production from water-soluble and structural carbohydrates of normal and high sugary corn stovers harvested at three growth stages. Energy Convers. Manag. 2020, 221, 113104. [Google Scholar] [CrossRef]
- Zhu, J.Q.; Zong, Q.J.; Li, W.C.; Chai, M.Z.; Xu, T.; Liu, H.; Fan, H.; Li, B.Z.; Yuan, Y.J. Temperature profiled simultaneous saccharification and co-fermentation of corn stover increases ethanol production at high solid loading. Energy Convers. Manag. 2020, 205, 112344. [Google Scholar] [CrossRef]
- del Río, P.G.; Gullón, P.; Rebelo, F.R.; Romaní, A.; Garrote, G.; Gullón, B. A whole-slurry fermentation approach to high-solid loading for bioethanol production from corn stover. Agronomy 2020, 10, 1790. [Google Scholar] [CrossRef]
- Domínguez, E.; Nóvoa, T.; del Río, P.G.; Garrote, G.; Romaní, A. Sequential two-stage autohydrolysis biorefinery for the production of bioethanol from fast-growing Paulownia biomass. Energy Convers. Manag. 2020, 226, 113517. [Google Scholar] [CrossRef]
- Wu, J.; Chandra, R.; Takada, M.; Del Rio, P.; Kim, K.H.; Kim, C.S.; Liu, L.Y.; Renneckar, S.; Saddler, J. Alkaline sulfonation and thermomechanical pulping pretreatment of softwood chips and pellets to enhance enzymatic hydrolysis. Bioresour. Technol. 2020, 315, 123789. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unloking low-cost cellulosic ethanol. Biofuels Bioprod. Biorefin. 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Pienkos, P.T.; Zhang, M. Role of pretreatment and conditioning processes on toxicity of lignocellulosic biomass hydrolysates. Cellulose 2009, 16, 743–762. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Zheng, Y.; Yu, C.W.; Dooley, T.M.; Jenkins, B.M.; Vandergheynst, J.S. Evaluation of high solids alkaline pretreatment of rice straw. Appl. Biochem. Biotechnol. 2010, 162, 1768–1784. [Google Scholar] [CrossRef]
- Romaní, A.; Tomaz, P.D.; Garrote, G.; Teixeira, J.A.; Domingues, L. Combined alkali and hydrothermal pretreatments for oat straw valorization within a biorefinery concept. Bioresour. Technol. 2016, 220, 323–332. [Google Scholar] [CrossRef]
- Rabelo, S.C.; Filho, R.M.; Costa, A.C. Lime pretreatment and fermentation of enzymatically hydrolyzed sugarcane bagasse. Appl. Biochem. Biotechnol. 2013, 169, 1696–1712. [Google Scholar] [CrossRef]
- Saha, B.C.; Cotta, M.A. Lime pretreatment, enzymatic saccharification and fermentation of rice hulls to ethanol. Biomass Bioenergy 2008, 32, 971–977. [Google Scholar] [CrossRef]
- Park, J.Y.; Shiroma, R.; Al-Haq, M.I.; Zhang, Y.; Ike, M.; Arai-Sanoh, Y.; Ida, A.; Kondo, M.; Tokuyasu, K. A novel lime pretreatment for subsequent bioethanol production from rice straw–Calcium capturing by carbonation (CaCCO) process. Bioresour. Technol. 2010, 101, 6805–6811. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cheng, J.J. Lime pretreatment of coastal bermudagrass for bioethanol production. Energy Fuels 2011, 25, 1830–1836. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples. Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, Colorado, 2008. Available online: https://doi.org/NREL/TP-510-42621 (accessed on 15 June 2019).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass. Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, Colorado, 2008. Available online: https://doi.org/NREL/TP-510-42619 (accessed on 15 June 2019).
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass. Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, Colorado, 2008. [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, Colorado, 2008. Available online: https://doi.org/NREL/TP-510-42618 (accessed on 15 June 2019).
- Ghose, T.K. Measurement fo cellulase activities. Pure Appl. Chem. 1987, 59, 695–702. [Google Scholar] [CrossRef]
- Paquot, P.M.; Thonart, P. Hydrolyse enzymatique de la ceilulose régénérée. Holzforschung 1982, 36, 177–181. [Google Scholar] [CrossRef]
- Garrote, G.; Yáñez, R.; Alonso, J.L.; Parajó, J.C. Coproduction of oligosaccharides and glucose from corncobs by hydrothermal processing and enzymatic hydrolysis. Ind. Eng. Chem. Res. 2008, 47, 1336–1345. [Google Scholar] [CrossRef]
- Romaní, A.; Garrote, G.; Alonso, J.L.; Parajó, J.C. Experimental assessment on the enzymatic hydrolysis of hydrothermally pretreated Eucalyptus globulus wood. Ind. Eng. Chem. Res. 2010, 49, 4653–4663. [Google Scholar] [CrossRef]
- Holtzapple, M.T.; Caram, H.S.; Humphrey, A.E. A comparison of two empirical models for the enzymatic hydrolysis of pretreated poplar wood. Biotechnol. Bioeng. 1984, 26, 936–941. [Google Scholar]
- Morales, A.; Gullón, B.; Dávila, I.; Eibes, G.; Labidi, J.; Gullón, P. Optimization of alkaline pretreatment for the co-production of biopolymer lignin and bioethanol from chestnut shells following a biorefinery approach. Ind. Crops Prod. 2018, 124, 582–592. [Google Scholar] [CrossRef]
- Dávila, I.; Gullón, B.; Labidi, J.; Gullón, P. Multiproduct biorefinery from vine shoots: Bio-ethanol and lignin production. Renew. Energy 2019, 142, 612–623. [Google Scholar] [CrossRef]
- Tan, M.; Ma, L.; Rehman, M.S.U.; Ahmed, M.A.; Sajid, M.; Xu, X.; Sun, Y.; Cui, P.; Xu, J. Screening of acidic and alkaline pretreatments for walnut shell and corn stover biorefining using two way heterogeneity evaluation. Renew. Energy 2019, 132, 950–958. [Google Scholar] [CrossRef]
- Barros-Ríos, J.; Romaní, A.; Peleteiro, S.; Garrote, G.; Ordas, B. Second-generation bioethanol of hydrothermally pretreated stover biomass from maize genotypes. Biomass Bioenergy 2016, 90, 42–49. [Google Scholar] [CrossRef]
- Chen, Y.; Stevens, M.A.; Zhu, Y.; Holmes, J.; Xu, H. Understanding of alkaline pretreatment parameters for corn stover enzymatic saccharification. Biotechnol. Biofuels 2013, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sharma-Shivappa, R.R.; Keshwani, D.; Chen, C. Potential of agricultural residues and hay for bioethanol production. Appl. Biochem. Biotechnol. 2007, 142, 276–290. [Google Scholar] [CrossRef]
- Silverstein, R.A.; Chen, Y.; Sharma-Shivappa, R.R.; Boyette, M.D.; Osborne, J. A comparison of chemical pretreatment methods for improving saccharification of cotton stalks. Bioresour. Technol. 2007, 98, 3000–3011. [Google Scholar] [CrossRef]
- Xu, J.; Cheng, J.J. Pretreatment of switchgrass for sugar production with the combination of sodium hydroxide and lime. Bioresour. Technol. 2011, 102, 3861–3868. [Google Scholar] [CrossRef]
- Sundin, J.; Hartler, N. Precipitation of kraft lignin by metal cations during pulp washing. Nord. Pulp Pap. Res. J. 2000, 15, 313–318. [Google Scholar] [CrossRef]
- Duong, T.D.; Hoang, M.; Nguyen, K.L. Sorption of Na+, Ca2+ ions from aqueous solution onto unbleached kraft fibers–Kinetics and equilibrium studies. J. Colloid Interface Sci. 2005, 287, 438–443. [Google Scholar] [CrossRef]

| Run | Dimensional, Independent Variables | Dimensionless Normalized Independent Variables | ||||
|---|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | Lime Loading (g Lime/g r.m) | x1 | x2 | x3 | |
| 1 | 121.0 | 5 | 0.40 | 1 | 1 | 1 |
| 2 | 121.0 | 5 | 0.10 | 1 | 1 | −1 |
| 3 | 121.0 | 1 | 0.40 | 1 | −1 | 1 |
| 4 | 121.0 | 1 | 0.10 | 1 | −1 | −1 |
| 5 | 90.0 | 5 | 0.40 | −1 | 1 | 1 |
| 6 | 90.0 | 1 | 0.40 | −1 | −1 | 1 |
| 7 | 90.0 | 1 | 0.10 | −1 | −1 | −1 |
| 8 | 90.0 | 5 | 0.10 | −1 | 1 | −1 |
| 9 | 121.0 | 3 | 0.25 | 1 | 0 | 0 |
| 10 | 90.0 | 3 | 0.25 | −1 | 0 | 0 |
| 11 | 105.5 | 5 | 0.25 | 0 | 1 | 0 |
| 12 | 105.5 | 1 | 0.25 | 0 | −1 | 0 |
| 13 | 105.5 | 3 | 0.40 | 0 | 0 | 1 |
| 14 | 105.5 | 3 | 0.10 | 0 | 0 | −1 |
| 15 | 105.5 | 3 | 0.25 | 0 | 0 | 0 |
| 16 | 105.5 | 3 | 0.25 | 0 | 0 | 0 |
| 17 | 105.5 | 3 | 0.25 | 0 | 0 | 0 |
| Run | SY (g/100 g) | Glucan (g/100 g) | Xylan (g/100 g) | Klason Lignin (g/100 g) | Glucan Recovery (g/100 g) | Xylan Recovery (g/100 g) | Delignification (g/100 g) | CGCMAX (g/100 g) |
|---|---|---|---|---|---|---|---|---|
| 1 | 77.4 | 46.4 | 20.9 | 16.0 | 95.6 | 75.7 | 31.4 | 79.9 |
| 2 | 78.8 | 44.3 | 22.3 | 17.4 | 92.9 | 82.0 | 23.8 | 71.1 |
| 3 | 73.2 | 45.4 | 21.1 | 16.9 | 88.5 | 72.2 | 31.3 | 83.8 |
| 4 | 77.4 | 47.4 | 22.1 | 17.9 | 97.7 | 79.7 | 22.9 | 83.3 |
| 5 | 74.9 | 45.6 | 21.5 | 15.3 | 90.8 | 75.2 | 36.4 | 89.5 |
| 6 | 76.3 | 45.2 | 21.6 | 15.5 | 91.9 | 77.1 | 34.4 | 87.0 |
| 7 | 76.7 | 45.3 | 22.0 | 16.3 | 92.4 | 78.8 | 30.6 | 77.5 |
| 8 | 74.6 | 45.0 | 21.3 | 16.3 | 89.4 | 74.4 | 32.4 | 78.5 |
| 9 | 71.2 | 47.5 | 21.5 | 15.5 | 90.1 | 71.6 | 38.5 | 86.9 |
| 10 | 73.0 | 46.1 | 22.0 | 15.6 | 89.7 | 75.0 | 36.6 | 81.9 |
| 11 | 75.2 | 46.2 | 21.3 | 15.7 | 92.4 | 74.6 | 34.4 | 72.7 |
| 12 | 73.7 | 46.1 | 21.8 | 15.7 | 90.4 | 75.1 | 35.9 | 83.8 |
| 13 | 73 | 45.0 | 20.8 | 14.7 | 87.6 | 71.1 | 40.2 | 82.4 |
| 14 | 74.7 | 45.1 | 21.8 | 16.0 | 89.7 | 76.1 | 33.6 | 62.8 |
| 15 | 71.9 | 46.4 | 21.7 | 14.9 | 88.9 | 72.9 | 40.7 | 77.0 |
| 16 | 74.0 | 45.9 | 21.4 | 15.0 | 90.3 | 74.1 | 38.4 | 79.2 |
| 17 | 73.1 | 45.8 | 21.7 | 14.6 | 89.1 | 74.2 | 40.7 | 82.7 |
| Coefficients | SY or y1 (g/100 g) | Glucan Recovery or y2 (g/100 g) | Xylan Recovery or y3 (g/100 g) | Delignification or y4 (g/100 g) | CGCMAX or y5 (g/100 g) |
|---|---|---|---|---|---|
| b0 | 72.607 | 89.124 | 73.161 | 39.783 | 78.430 |
| b1 | 0.235 | 1.043 c | 0.074 | −2.250 b | −0.959 |
| b2 | 0.360 | 0.025 | −0.091 | 0.330 | −2.371 |
| b3 | −0.748 c | −0.786 | −1.975 a | 3.040 a | 4.936 b |
| b11 | 1.131 b | 0.778 | 1.489 b | −0.350 | −2.447 |
| b22 | −0.691 c | −0.922 | −1.624 b | 1.025 | −1.399 |
| b33 | 0.434 | 1.740 b | 0.462 | −0.075 | 1.226 |
| b12 | −0.188 | 0.996 | 0.553 | −2.120 | 6.860 b |
| b13 | 2.110 b | 2.489 b | 2.152 b | −4.520 b | 0.756 |
| b23 | 1.553 b | −0.263 | 0.903 | −2.770 c | −4.942 |
| R2 | 0.900 | 0.830 | 0.910 | 0.930 | 0.790 |
| F | 6.660 | 3.690 | 7.640 | 9.730 | 2.880 |
| Significance level (%) | >98 | >95 | >99 | >99 | >91 |
| Substrate | Exp. | LSR (g/g) | ESR (FPU/g) | EMAX (g/L) | ECMAX (g E/100 g EPOT) |
|---|---|---|---|---|---|
| Lime-pretreated corn stover (121 °C, 0.1 g lime/g r.m., 1 h) | A1 | 4 | 15 | 27.48 | 49.0 |
| A2 | 5 | 15 | 30.61 | 65.9 | |
| A3 | 6 | 5 | 20.72 | 52.2 | |
| A4 | 6 | 10 | 23.88 | 60.2 | |
| A5 | 6 | 15 | 28.73 | 72.4 | |
| Lime-pretreated corn stover (90 °C; 0.4 g lime/g r.m.; 1 h) | B1 | 4 | 15 | 23.76 | 44.6 |
| B2 | 5 | 15 | 24.44 | 55.4 | |
| B3 | 6 | 5 | 16.90 | 44.8 | |
| B4 | 6 | 10 | 21.90 | 58.1 | |
| B5 | 6 | 15 | 24.87 | 66.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fírvida, I.; del Río, P.G.; Gullón, P.; Gullón, B.; Garrote, G.; Romaní, A. Alternative Lime Pretreatment of Corn Stover for Second-Generation Bioethanol Production. Agronomy 2021, 11, 155. https://doi.org/10.3390/agronomy11010155
Fírvida I, del Río PG, Gullón P, Gullón B, Garrote G, Romaní A. Alternative Lime Pretreatment of Corn Stover for Second-Generation Bioethanol Production. Agronomy. 2021; 11(1):155. https://doi.org/10.3390/agronomy11010155
Chicago/Turabian StyleFírvida, Iria, Pablo G. del Río, Patricia Gullón, Beatriz Gullón, Gil Garrote, and Aloia Romaní. 2021. "Alternative Lime Pretreatment of Corn Stover for Second-Generation Bioethanol Production" Agronomy 11, no. 1: 155. https://doi.org/10.3390/agronomy11010155
APA StyleFírvida, I., del Río, P. G., Gullón, P., Gullón, B., Garrote, G., & Romaní, A. (2021). Alternative Lime Pretreatment of Corn Stover for Second-Generation Bioethanol Production. Agronomy, 11(1), 155. https://doi.org/10.3390/agronomy11010155





