Volatile Profiling of Fifteen Willow Species and Hybrids and Their Responses to Giant Willow Aphid Infestation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Plant Material
2.2. Aphid Inoculation
2.3. VOCs Sampling
2.4. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
2.5. Statistical Analysis
3. Results
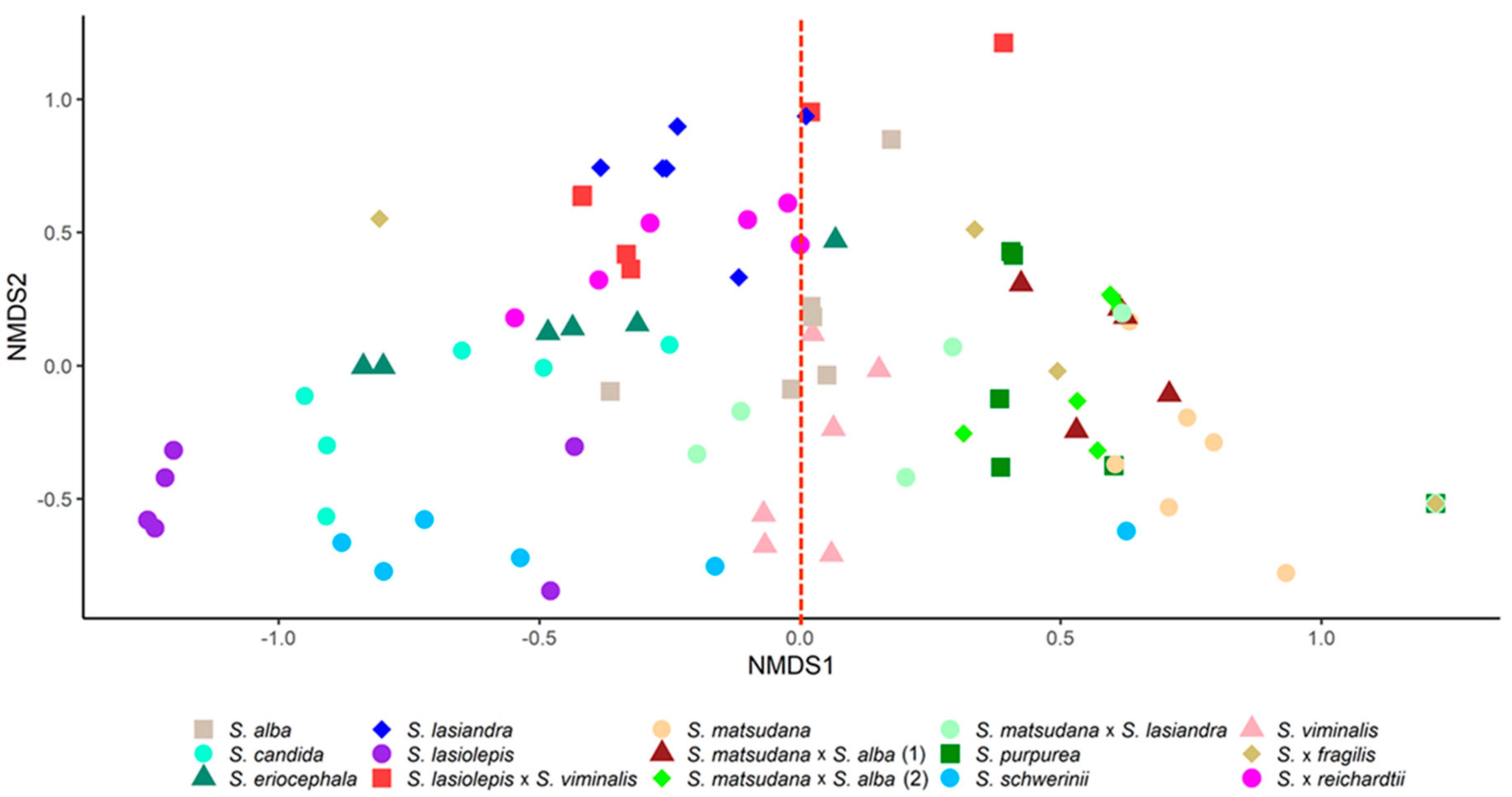
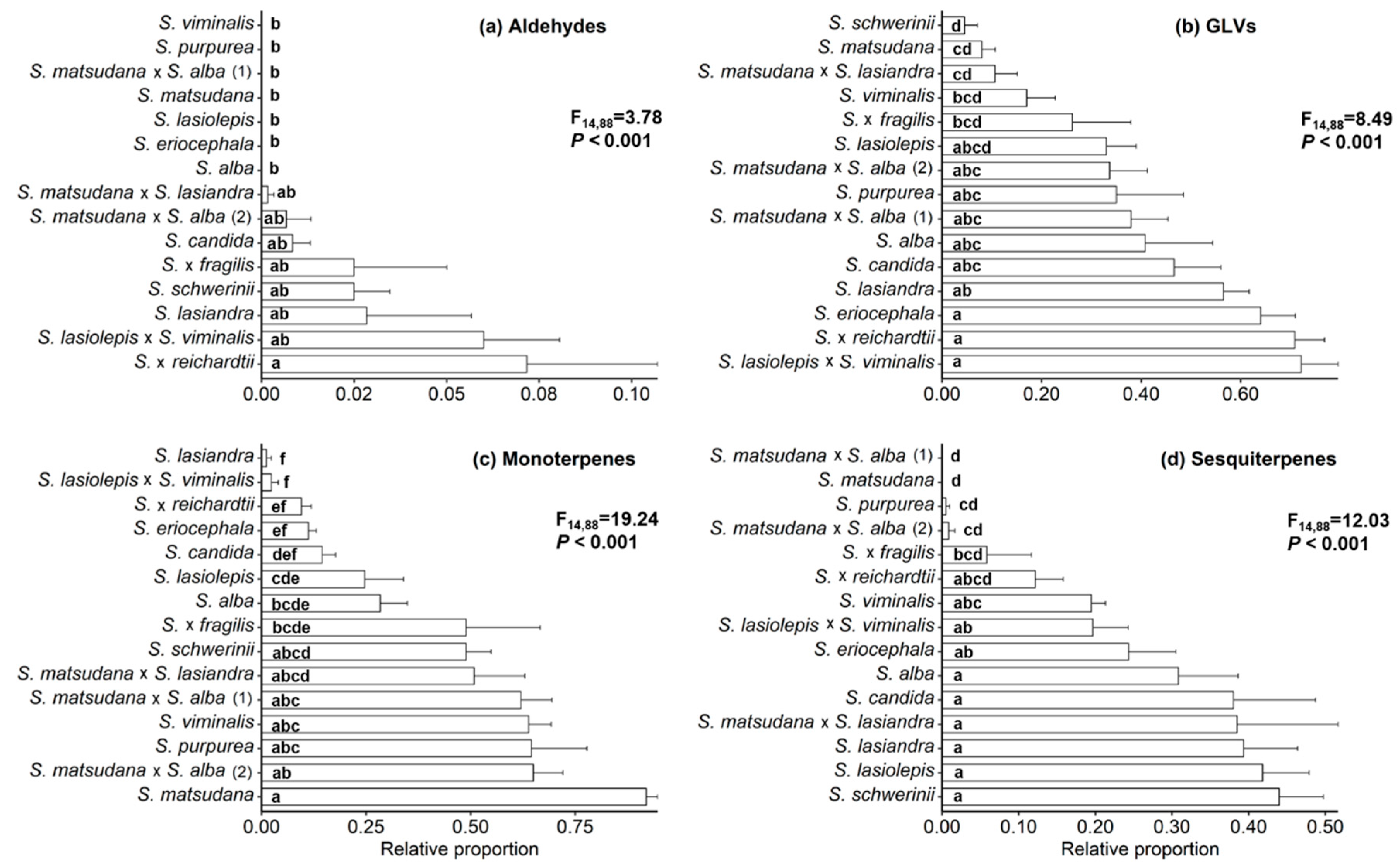
3.1. Characterisation of Willow VOCs
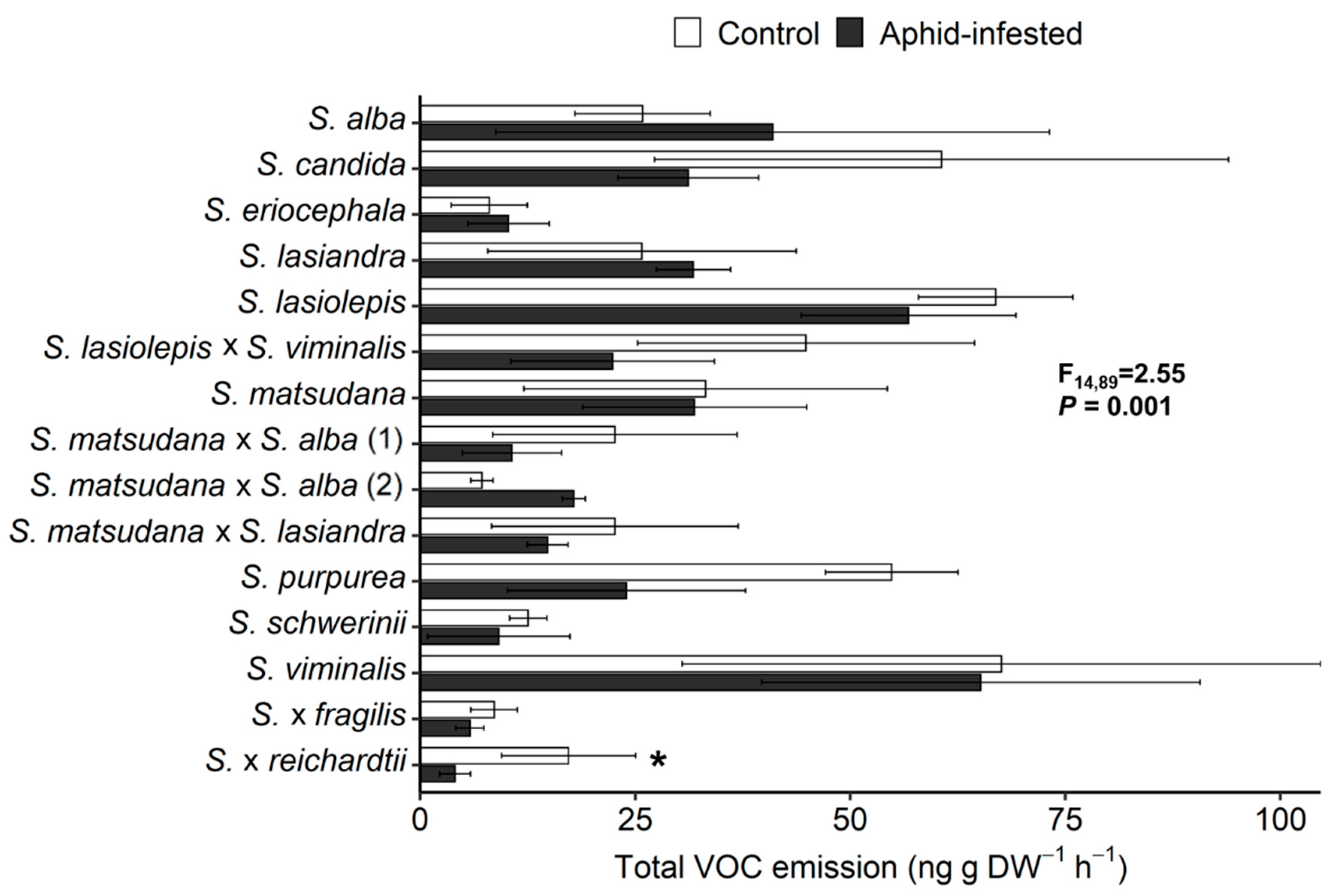
3.2. VOC Response of Willow Species and Hybrids to T. salignus Infestation
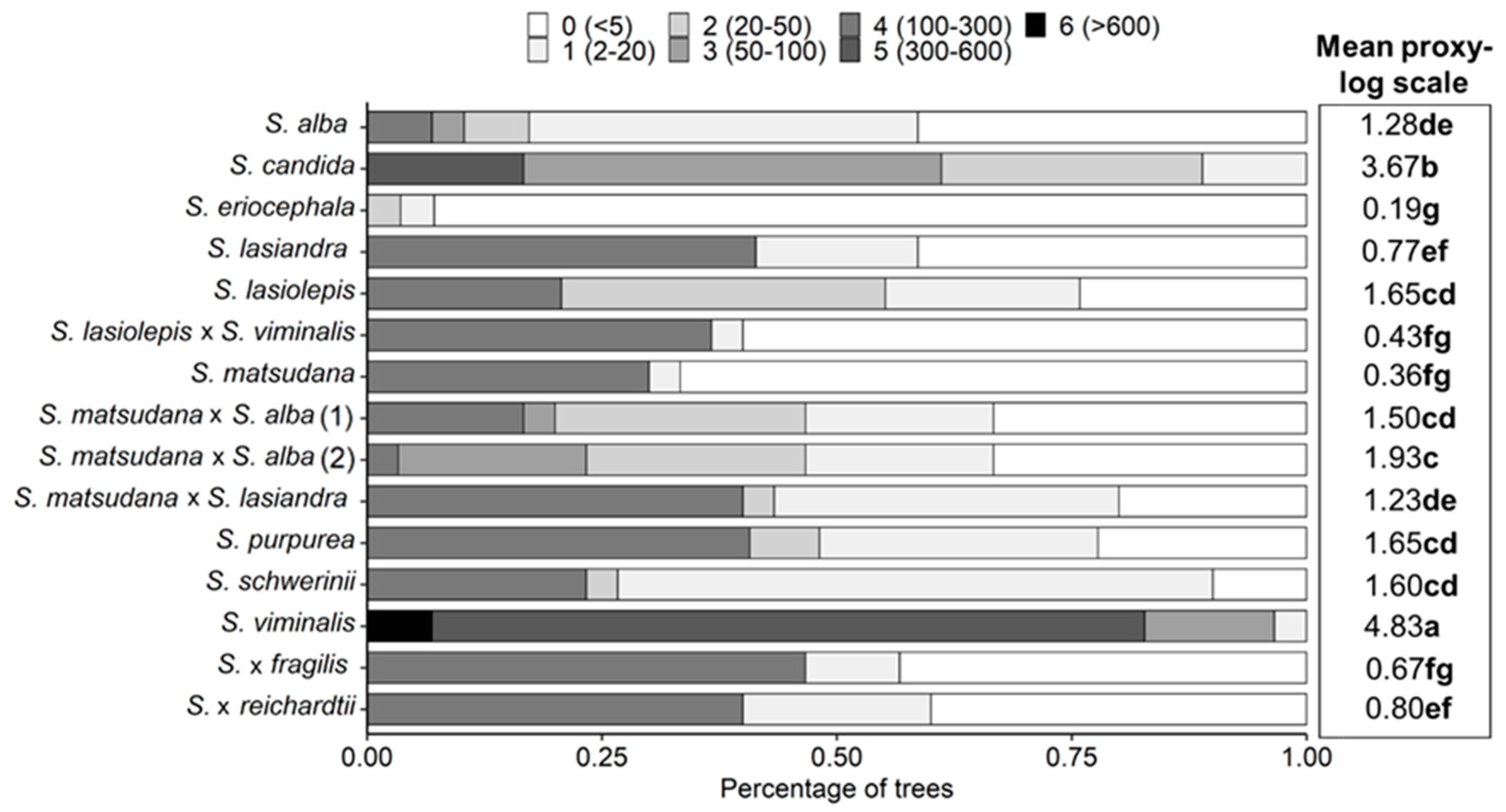
3.3. Correlation between Aphid Infestation and VOC Emissions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jaeger, D.M.; Runyon, J.B.; Richardson, B.A. Signals of speciation: Volatile organic compounds resolve closely related sagebrush taxa, suggesting their importance in evolution. New Phytol. 2016, 211, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Pichersky, E. Metabolic engineering of plant volatiles. Curr. Opin. Biotechnol. 2008, 19, 181–189. [Google Scholar] [CrossRef]
- Clavijo McCormick, A. Can plant–natural enemy communication withstand disruption by biotic and abiotic factors? Ecol. Evol. 2016, 6, 8569–8582. [Google Scholar] [CrossRef] [PubMed]
- Vivaldo, G.; Masi, E.; Taiti, C.; Caldarelli, G.; Mancuso, S. The network of plants volatile organic compounds. Sci. Rep. 2017, 7, 1–18. [Google Scholar] [CrossRef]
- De Moraes, C.M.; Mescher, M.C.; Tumlinson, J.H. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 2001, 410, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Irmisch, S.; Clavijo McCormick, A.; Günther, J.; Schmidt, A.; Boeckler, G.A.; Gershenzon, J.; Unsicker, S.B.; Köllner, T.G. Herbivore-induced poplar cytochrome P450 enzymes of the CYP 71 family convert aldoximes to nitriles which repel a generalist caterpillar. Plant J. 2014, 80, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Amo, L.; Jansen, J.J.; van Dam, N.M.; Dicke, M.; Visser, M.E. Birds exploit herbivore-induced plant volatiles to locate herbivorous prey. Ecol. Lett. 2013, 16, 1348–1355. [Google Scholar] [CrossRef]
- Dudareva, N.; Pichersky, E. Biochemical and molecular genetic aspects of floral scents. Plant Physiol. 2000, 122, 627–634. [Google Scholar] [CrossRef]
- Clavijo McCormick, A.; Irmisch, S.; Reinecke, A.; Boeckler, G.A.; Veit, D.; Reichelt, M.; Hansson, B.S.; Gershenzon, J.; Köllner, T.G.; Unsicker, S.B. Herbivore-induced volatile emission in black poplar: Regulation and role in attracting herbivore enemies. Plant Cell Environ. 2014, 37, 1909–1923. [Google Scholar] [CrossRef]
- Baldwin, I.T.; Kessler, A.; Halitschke, R. Volatile signaling in plant–plant–herbivore interactions: What is real? Curr. Opin. Plant Biol. 2002, 5, 351–354. [Google Scholar] [CrossRef]
- Owen, S.M.; Peñuelas, J. Opportunistic emissions of volatile isoprenoids. Trends Plant Sci. 2005, 10, 420–426. [Google Scholar] [PubMed]
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [PubMed]
- Clavijo McCormick, A.; Unsicker, S.B.; Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [PubMed]
- Arneth, A.; Schurgers, G.; Hickler, T.; Miller, P. Effects of species composition, land surface cover, CO2 concentration and climate on isoprene emissions from European forests. Plant Biol. 2007, 9, 150–162. [Google Scholar]
- Winters, A.J.; Adams, M.A.; Bleby, T.M.; Rennenberg, H.; Steigner, D.; Steinbrecher, R.; Kreuzwieser, J. Emissions of isoprene, monoterpene and short-chained carbonyl compounds from Eucalyptus spp. in southern Australia. Atmos. Environ. 2009, 43, 3035–3043. [Google Scholar]
- Kesselmeier, J.; Staudt, M. Biogenic volatile organic compounds (VOC): An overview on emission, physiology and ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar]
- Ashman, T.-L.; Bradburn, M.; Cole, D.H.; Blaney, B.H.; Raguso, R.A. The scent of a male: The role of floral volatiles in pollination of a gender dimorphic plant. Ecology 2005, 86, 2099–2105. [Google Scholar]
- Masante-Roca, I.; Anton, S.; Delbac, L.; Dufour, M.C.; Gadenne, C. Attraction of the grapevine moth to host and non-host plant parts in the wind tunnel: Effects of plant phenology, sex, and mating status. Entomol. Exp. Appl. 2007, 122, 239–245. [Google Scholar]
- Michereff, M.F.F.; Laumann, R.A.; Borges, M.; Michereff-Filho, M.; Diniz, I.R.; Neto, A.L.F.; Moraes, M.C.B. Volatiles mediating a plant-herbivore-natural enemy interaction in resistant and susceptible soybean cultivars. J. Chem. Ecol. 2011, 37, 273–285. [Google Scholar]
- Shiojiri, K.; Karban, R. Plant age, communication, and resistance to herbivores: Young sagebrush plants are better emitters and receivers. Oecologia 2006, 149, 214–220. [Google Scholar]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Giacomuzzi, V.; Cappellin, L.; Khomenko, I.; Biasioli, F.; Schütz, S.; Tasin, M.; Knight, A.L.; Angeli, S. Emission of volatile compounds from apple plants infested with Pandemis heparana larvae, antennal response of conspecific adults, and preliminary field trial. J. Chem. Ecol. 2016, 42, 1265–1280. [Google Scholar] [CrossRef] [PubMed]
- McClung, C.R. Plant circadian rhythms. Plant Cell 2006, 18, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Clavijo McCormick, A.; Boeckler, G.A.; Köllner, T.G.; Gershenzon, J.; Unsicker, S.B. The timing of herbivore-induced volatile emission in black poplar (Populus nigra) and the influence of herbivore age and identity affect the value of individual volatiles as cues for herbivore enemies. BMC Plant Biol. 2014, 14, 304. [Google Scholar] [CrossRef]
- Walling, L.L. The myriad plant responses to herbivores. J. Plant Growth Regul. 2000, 19, 195–216. [Google Scholar] [CrossRef]
- Joó, É.; Van Langenhove, H.; Šimpraga, M.; Steppe, K.; Amelynck, C.; Schoon, N.; Müller, J.-F.; Dewulf, J. Variation in biogenic volatile organic compound emission pattern of Fagus sylvatica L. due to aphid infection. Atmos. Environ. 2010, 44, 227–234. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Crafts-Brandner, S.J.; Cañas, L.A. Volatile emissions triggered by multiple herbivore damage: Beet armyworm and whitefly feeding on cotton plants. J. Chem. Ecol. 2003, 29, 2539–2550. [Google Scholar] [CrossRef]
- Copeland, N.; Cape, J.N.; Heal, M.R. Volatile organic compound emissions from Miscanthus and short rotation coppice willow bioenergy crops. Atmos. Environ. 2012, 60, 327–335. [Google Scholar] [CrossRef]
- Hakola, H.; Rinne, J.; Laurila, T. The hydrocarbon emission rates of tea-leafed willow (Salix phylicifolia), silver birch (Betula pendula) and European aspen (Populus tremula). Atmos. Environ. 1998, 32, 1825–1833. [Google Scholar] [CrossRef]
- Peacock, L.; Lewis, M.; Powers, S. Volatile compounds from Salix spp. varieties differing in susceptibility to three willow beetle species. J. Chem. Ecol. 2001, 27, 1943–1951. [Google Scholar] [CrossRef]
- Goggin, F.L. Plant–aphid interactions: Molecular and ecological perspectives. Curr. Opin. Plant Biol. 2007, 10, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Darshanee, H.L.C.; Khan, I.A.; Zhang, Z.-F.; Liu, T.-X. Host selection behavior of the green peach aphid, Myzus persicae, in response to volatile organic compounds and nitrogen contents of cabbage cultivars. Front. Plant Sci. 2019, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Inui, Y.; Miyamoto, Y.; Ohgushi, T. Comparison of volatile leaf compounds and herbivorous insect communities on three willow species. Popul. Ecol. 2003, 45, 41–46. [Google Scholar] [CrossRef]
- Argus, G.W. Classification of Salix in the new world. Bot. Electron. News 1999, 227, 1–6. [Google Scholar]
- Argus, G.W. Salix (Salicaceae) distribution maps and a synopsis of their classification in North America, North of Mexico. Harv. Pap. Bot. 2007, 12, 335–368. [Google Scholar] [CrossRef]
- Glenny, D.; Jones, T. Key to Willow Species and Hybrids Present in New Zealand. Available online: https://www.landcareresearch.co.nz/resources/identification/plants/salix-key (accessed on 16 June 2020).
- Isebrands, J.; Aronsson, P.; Carlson, M.; Ceulemans, R.; Coleman, M.; Dickinson, N.; Dimitriou, J.; Doty, S.; Gardiner, E.; Heinsoo, K. Environmental applications of poplars and willows. In Poplars and Willows: Trees for Society and the Environment; Isebrands, J.G., Richardson, J., Eds.; CABI and FAO: Rome, Italy, 2014; pp. 258–336. [Google Scholar]
- Newstrom-Lloyd, L.; McIvor, I.; Jones, T.; Gabarret, M.; Polturat, B. Winning with Willows: Extending the Supply of Nutritious Pollen for Bees in Spring. Available online: https://www.poplarandwillow.org.nz/documents/winning-with-willows.pdf (accessed on 19 September 2019).
- Wilkinson, A.G. Poplars and willows for soil erosion control in New Zealand. Biomass Bioenerg. 1999, 16, 263–274. [Google Scholar] [CrossRef]
- Martin, N.A. Giant Willow Aphid—Tuberolachnus salignus. Available online: http://nzacfactsheets.landcareresearch.co.nz/factsheet/InterestingInsects/Giant-willow-aphid---Tuberolachnus-salignus.html (accessed on 12 December 2019).
- Sopow, S.; Jones, T.; Mclvor, I.; McLean, J.; Pawson, S. Potential impacts of Tuberolachnus salignus (giant willow aphid) in New Zealand and options for control. Agric. Forest Entomol. 2017, 19, 225–234. [Google Scholar] [CrossRef]
- Sopow, S. Giant Willow Aphid in New Zealand. Available online: https://www.scionresearch.com/__data/assets/pdf_file/0006/48183/GiantWillowAphid.pdf (accessed on 7 February 2020).
- Fernandez, P.C.; Meiners, T.; Björkman, C.; Hilker, M. Electrophysiological responses of the blue willow leaf beetle, Phratora vulgatissima, to volatiles of different Salix viminalis genotypes. Entomol. Exp. Appl. 2007, 125, 157–164. [Google Scholar] [CrossRef]
- Braccini, C.L.; Vega, A.S.; Coll Araoz, M.V.; Teal, P.E.; Cerrillo, T.; Zavala, J.A.; Fernandez, P.C. Both volatiles and cuticular plant compounds determine oviposition of the willow sawfly Nematus oligospilus on leaves of Salix spp. (Salicaceae). J. Chem. Ecol. 2015, 11, 985–996. [Google Scholar] [CrossRef]
- Yoneya, K.; Ozawa, R.; Takabayashi, J. Specialist leaf beetle larvae use volatiles from willow leaves infested by conspecifics for reaggregation in a tree. J. Chem. Ecol. 2010, 36, 671–679. [Google Scholar] [CrossRef]
- Aradottir, G.; Karp, A.; Hanley, S.; Shield, I.; Woodcock, C.; Dewhirst, S.; Collins, C.; Leather, S.; Harrington, R. Host selection of the giant willow aphid (Tuberolachnus salignus). Redia 2009, XCII, 223–225. [Google Scholar]
- Collins, C. Aspect of the Ecology of Two Stem-Feeding Willow Aphid Species. Ph.D. Thesis, Imperial College, University of London, London, UK, 2001. [Google Scholar]
- Effah, E.; Barrett, D.P.; Peterson, P.G.; Godfrey, A.J.R.; Potter, M.A.; Holopainen, J.K.; Clavijo McCormick, A. Natural variation in volatile emissions of the invasive weed Calluna vulgaris in New Zealand. Plants 2020, 9, 283. [Google Scholar]
- R Development Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Zhang, B.; Du, N.; Li, Y.; Shi, P.; Wei, G. Distinct biogeographic patterns of rhizobia and non-rhizobial endophytes associated with soybean nodules across China. Sci. Total Environ. 2018, 643, 569–578. [Google Scholar]
- Sheehan, E.V.; Stevens, T.F.; Gall, S.C.; Cousens, S.L.; Attrill, M.J. Recovery of a temperate reef assemblage in a marine protected area following the exclusion of towed demersal fishing. PLoS ONE 2014, 8, e83883. [Google Scholar]
- Arbizu, M. pairwiseAdonis: Pairwise Multilevel Comparison Using Adonis, R Package Version 0.0.1; Available online: https://github.com/pmartinezarbizu/pairwiseAdonis (accessed on 19 December 2019).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package, R package version 1.17-4; Available online: https://CRAN.R-project.org/package=vegan (accessed on 8 January 2020).
- Digilio, M.C.; Corrado, G.; Sasso, R.; Coppola, V.; Iodice, L.; Pasquariello, M.; Bossi, S.; Maffei, M.E.; Coppola, M.; Pennacchio, F. Molecular and chemical mechanisms involved in aphid resistance in cultivated tomato. New Phytol. 2010, 187, 1089–1101. [Google Scholar]
- Ngantcha, A.C. DNA Fingerprinting and Genetic Relationships among Willow (Salix spp.). Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2010. [Google Scholar]
- Danner, H.; Boeckler, G.A.; Irmisch, S.; Yuan, J.S.; Chen, F.; Gershenzon, J.; Unsicker, S.B.; Köllner, T.G. Four terpene synthases produce major compounds of the gypsy moth feeding-induced volatile blend of Populus trichocarpa. Phytochemistry 2011, 72, 897–908. [Google Scholar]
- Frost, C.J.; Mescher, M.C.; Dervinis, C.; Davis, J.M.; Carlson, J.E.; De Moraes, C.M. Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytol. 2008, 180, 722–734. [Google Scholar]
- Frost, C.J.; Appel, H.M.; Carlson, J.E.; De Moraes, C.M.; Mescher, M.C.; Schultz, J.C. Within-plant signalling via volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecol. Lett. 2007, 10, 490–498. [Google Scholar]
- Yoneya, K.; Kugimiya, S.; Takabayashi, J. Can herbivore-induced plant volatiles inform predatory insect about the most suitable stage of its prey? Physio. Entomol. 2009, 34, 379–386. [Google Scholar]
- Giorgi, A.; Panseri, S.; Nanayakkara, N.N.M.C.; Chiesa, L.M. HS-SPME-GC/MS analysis of the volatile compounds of Achillea collina: Evaluation of the emissions fingerprint induced by Myzus persicae infestation. J. Plant Biol. 2012, 55, 251–260. [Google Scholar]
- Schwartzberg, E.G.; Böröczky, K.; Tumlinson, J.H. Pea aphids, Acyrthosiphon pisum, suppress induced plant volatiles in broad bean, Vicia faba. J. Chem. Ecol. 2011, 37, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Blande, J.D.; Korjus, M.; Holopainen, J.K. Foliar methyl salicylate emissions indicate prolonged aphid infestation on silver birch and black alder. Tree Physiol. 2010, 30, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Jiang, Y.; Veromann-Jürgenson, L.-L.; Niinemets, Ü. Petiole gall aphid (Pemphigus spyrothecae) infestation of Populus × petrovskiana leaves alters foliage photosynthetic characteristics and leads to enhanced emissions of both constitutive and stress-induced volatiles. Trees 2019, 33, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.J.; Bernasconi, M.; Bertossa, R.; Bigler, F.; Caloz, G.; Dorn, S. The induction of volatile emissions in maize by three herbivore species with different feeding habits: Possible consequences for their natural enemies. Biol. Control 1998, 11, 122–129. [Google Scholar] [CrossRef]
- Frago, E.; Mala, M.; Weldegergis, B.T.; Yang, C.; McLean, A.; Godfray, H.C.J.; Gols, R.; Dicke, M. Symbionts protect aphids from parasitic wasps by attenuating herbivore-induced plant volatiles. Nat. Commun. 2017, 8, 1860. [Google Scholar] [CrossRef]
- Rudgers, J.A.; Strauss, S.Y.; Wendel, J.F. Trade-offs among anti-herbivore resistance traits: Insights from Gossypieae (Malvaceae). Am. J. Bot. 2004, 91, 871–880. [Google Scholar] [CrossRef]
- Koricheva, J.; Nykänen, H.; Gianoli, E. Meta-analysis of trade-offs among plant antiherbivore defenses: Are plants jacks-of-all-trades, masters of all? Am. Nat. 2004, 163, E64–E75. [Google Scholar] [CrossRef]
- Wei, J.; Wang, L.; Zhao, J.; Li, C.; Ge, F.; Kang, L. Ecological trade-offs between jasmonic acid-dependent direct and indirect plant defences in tritrophic interactions. New Phytol. 2011, 189, 557–567. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Kautz, S.; Lion, U.; Heil, M. Trade-offs between direct and indirect defences of lima bean (Phaseolus lunatus). J. Ecol. 2008, 96, 971–980. [Google Scholar]
- Niinemets, Ü. Costs of production and physiology of emission of volatile leaf isoprenoids. In Advances in Plant Physiology; Hemantaranjan, A., Ed.; Scientific Publishers: Jodhpur, India, 2004; Volume 7, pp. 241–278. [Google Scholar]
- Robert, C.A.M.; Erb, M.; Hiltpold, I.; Hibbard, B.E.; Gaillard, M.D.P.; Bilat, J.; Degenhardt, J.; Cambet-Petit-Jean, X.; Turlings, T.C.J.; Zwahlen, C. Genetically engineered maize plants reveal distinct costs and benefits of constitutive volatile emissions in the field. Plant Biotechnol. J. 2013, 11, 628–639. [Google Scholar] [CrossRef]
- Miresmailli, S.; Gries, R.; Gries, G.; Zamar, R.H.; Isman, M.B. Population density and feeding duration of cabbage looper larvae on tomato plants alter the levels of plant volatile emissions. Pest Manag. Sci. 2012, 68, 101–107. [Google Scholar] [CrossRef] [PubMed]
- De Backer, L.; Megido, R.C.; Fauconnier, M.-L.; Brostaux, Y.; Francis, F.; Verheggen, F. Tuta absoluta-induced plant volatiles: Attractiveness towards the generalist predator Macrolophus pygmaeus. Arthropod Plant Interact. 2015, 9, 465–476. [Google Scholar] [CrossRef]
- Horiuchi, J.-I.; Arimura, G.-I.; Ozawa, R.; Shimoda, T.; Takabayashi, J.; Nishioka, T. A comparison of the responses of Tetranychus urticae (Acari: Tetranychidae) and Phytoseiulus persimilis (Acari: Phytoseiidae) to volatiles emitted from lima bean leaves with different levels of damage made by T. urticae or Spodoptera exigua (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 2003, 38, 109–116. [Google Scholar]
- De Boer, J.G.; Posthumus, M.A.; Dicke, M. Identification of volatiles that are used in discrimination between plants infested with prey or nonprey herbivores by a predatory mite. J. Chem. Ecol. 2004, 30, 2215–2230. [Google Scholar] [CrossRef] [PubMed]
- Rioja, T.; Ceballos, R.; Holuigue, L. Herbivore-induced plant volatiles emitted from avocado shoots infested by Oligonychus yothersi (Acari: Tetranychidae) increases the attraction of micro-coleopterans. Chil. J. Agric. Res. 2018, 78, 447–458. [Google Scholar] [CrossRef]
- Tun, K.M.; Clavijo McCormick, A.; Jones, T.; Minor, M. The potential of harlequin ladybird beetle Harmonia axyridis as a predator of the giant willow aphid Tuberolachnus salignus: Voracity, life history and prey preference. BioControl 2020, 65, 313–321. [Google Scholar]
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green leaf volatiles: A plant’s multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17811. [Google Scholar] [CrossRef]


| Species/Hybrid | Code | Type | Sex | Geographical Origin |
|---|---|---|---|---|
| S. candida | PN 385 | Shrub | Male | North America |
| S. eriocephala | PN 376 | Shrub | Male | North America |
| S. lasiolepis | PN 751 | Shrub | Male | North America |
| S. lasiolepis × S. viminalis | NZ 04-106-073 | Shrub | Male | Hybridized in New Zealand |
| S. purpurea | PN 249 | Shrub | Female | Europe, North Africa |
| S. schwerinii | PN 386 | Shrub | Male | Eastern Asia |
| S. viminalis | PN 220 | Shrub | Male | Europe, Western Asia |
| S. × reichardtii | PN 714 | Shrub | Male | Europe |
| S. alba | PN 357 | Tree | Male | Europe, Western and Central Asia |
| S. lasiandra | PN 747 | Tree | Male | North America |
| S. matsudana | PN 227 | Tree | Female | Eastern Asia |
| S. matsudana × S. alba (1) | NZ 1040 | Tree | Female | Hybridized in New Zealand |
| S. matsudana × S. alba (2) | NZ 1184 | Tree | Male/female | Hybridized in New Zealand |
| S. matsudana × S. lasiandra | NZ 03-003-073 | Tree | Male | Hybridized in New Zealand |
| S. × fragilis | PN 218 | Tree | Female | Europe and Western Asia |
| Major VOC Groups | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldehyde | GLVs | Monoterpenes | Sesquiterpenes | |||||||||||||||||
| Species and Hybrids | Nonanal | (Z)-3-hexenol | (Z)-3-hexenyl acetate | (Z)-3-hexenyl benzoate | (Z)-3-hexenyl-α-methylbutyrate | (Z)-β-ocimene | (E)-β-ocimene | α-ocimene | β-myrcene | α-cubebene | (E,E)-α-farnesene | (Z,E)-α-farnesene | (E)-β-farnesene | Germacrene D | β-caryophyllene | Cedrene | Copaene | δ-cadinene | (E)-α-bergamotene | Number of VOCs Emitted |
| S. candida | + | + | + | + | + | o | + | + | + | + | + | + | o | + | + | + | + | o | o | 15 |
| S. eriocephala | o | + | + | o | + | o | + | o | o | + | + | + | o | o | + | o | + | o | o | 9 |
| S. lasiolepis | o | o | + | o | o | o | + | + | o | + | + | + | o | + | + | + | + | + | o | 11 |
| S. lasiolepis × S. viminalis | + | + | + | o | o | o | + | o | o | o | + | + | o | o | o | o | o | o | o | 6 |
| S. purpurea | o | + | + | o | o | o | + | + | o | o | + | o | o | o | o | o | o | o | o | 5 |
| S. schwerinii | + | o | + | o | + | + | + | + | + | + | + | + | o | o | + | + | + | + | + | 15 |
| S. viminalis | o | + | + | o | o | + | + | + | + | o | + | + | + | o | o | o | o | o | + | 10 |
| S. × reichardtii | + | + | + | o | o | o | + | o | o | o | + | + | o | o | + | o | o | o | o | 7 |
| S. alba | o | + | + | o | + | o | + | o | o | o | + | + | o | o | + | o | o | o | + | 8 |
| S. lasiandra | + | o | + | o | o | o | + | o | o | o | + | + | o | o | o | o | o | o | o | 5 |
| S. matsudana | o | o | + | o | o | + | + | + | o | o | o | o | o | o | o | o | o | o | o | 4 |
| S. matsudana × S. alba (1) | o | + | + | o | o | + | + | + | o | o | o | o | o | o | o | o | o | o | o | 5 |
| S. matsudana × S. alba (2) | + | o | + | o | o | + | + | + | o | o | + | o | o | o | o | o | o | o | o | 6 |
| S. matsudana × S. lasiandra | + | o | + | o | o | + | + | + | o | o | + | + | o | o | o | o | o | o | + | 8 |
| S. × fragilis | + | + | + | o | o | o | + | + | o | o | + | o | + | o | o | o | o | o | o | 7 |
| Number of species and hybrids | 8 | 9 | 15 | 1 | 4 | 6 | 15 | 10 | 3 | 4 | 13 | 10 | 2 | 2 | 6 | 3 | 4 | 2 | 4 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tun, K.M.; Minor, M.; Jones, T.; McCormick, A.C. Volatile Profiling of Fifteen Willow Species and Hybrids and Their Responses to Giant Willow Aphid Infestation. Agronomy 2020, 10, 1404. https://doi.org/10.3390/agronomy10091404
Tun KM, Minor M, Jones T, McCormick AC. Volatile Profiling of Fifteen Willow Species and Hybrids and Their Responses to Giant Willow Aphid Infestation. Agronomy. 2020; 10(9):1404. https://doi.org/10.3390/agronomy10091404
Chicago/Turabian StyleTun, Kyaw Min, Maria Minor, Trevor Jones, and Andrea Clavijo McCormick. 2020. "Volatile Profiling of Fifteen Willow Species and Hybrids and Their Responses to Giant Willow Aphid Infestation" Agronomy 10, no. 9: 1404. https://doi.org/10.3390/agronomy10091404
APA StyleTun, K. M., Minor, M., Jones, T., & McCormick, A. C. (2020). Volatile Profiling of Fifteen Willow Species and Hybrids and Their Responses to Giant Willow Aphid Infestation. Agronomy, 10(9), 1404. https://doi.org/10.3390/agronomy10091404







