The Antifungal Activity of Gallic Acid and Its Derivatives against Alternaria solani, the Causal Agent of Tomato Early Blight
Abstract
1. Introduction
2. Materials and Methods
2.1. Tested Compounds
2.2. Plant Materials and Growth Conditions
2.3. Isolation and Identification of the Pathogen
2.4. Pathogenicity Test
2.5. Antifungal Activity
2.6. The Half-Maximal Inhibitory Concentration (IC50)
2.7. Pot Experiment, Disease Assessments, and Collection of Samples
2.8. Enzymes Activity
2.9. Chlorophyll Content
2.10. Total Soluble Phenolic Compounds
2.11. Total Soluble Flavonoids
2.12. Vegetative Growth and Yield Assessment
2.13. Statistical Analysis
3. Results
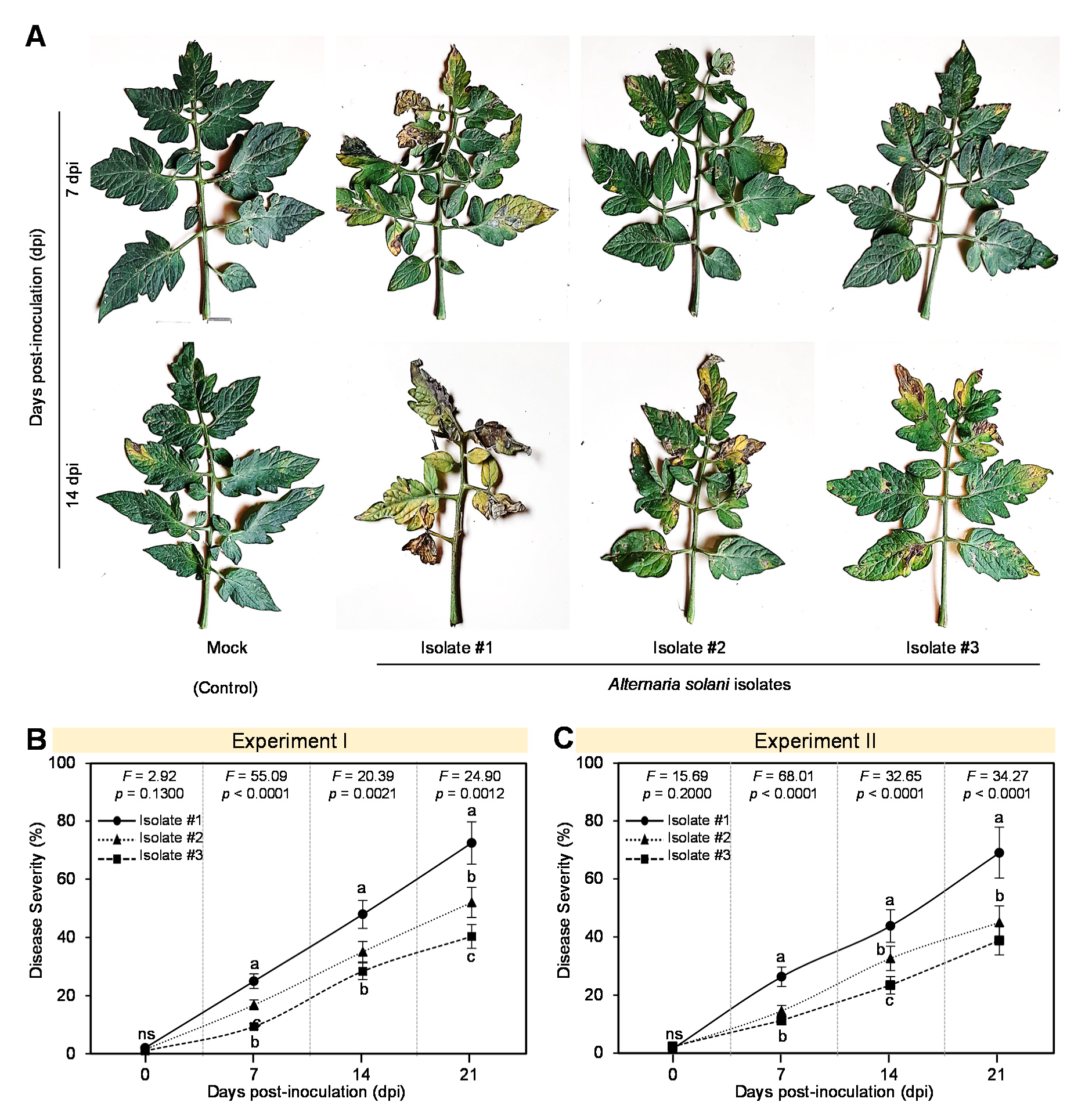
3.1. Pathogenicity Test of Different Isolates of A. solani on Tomato Plants
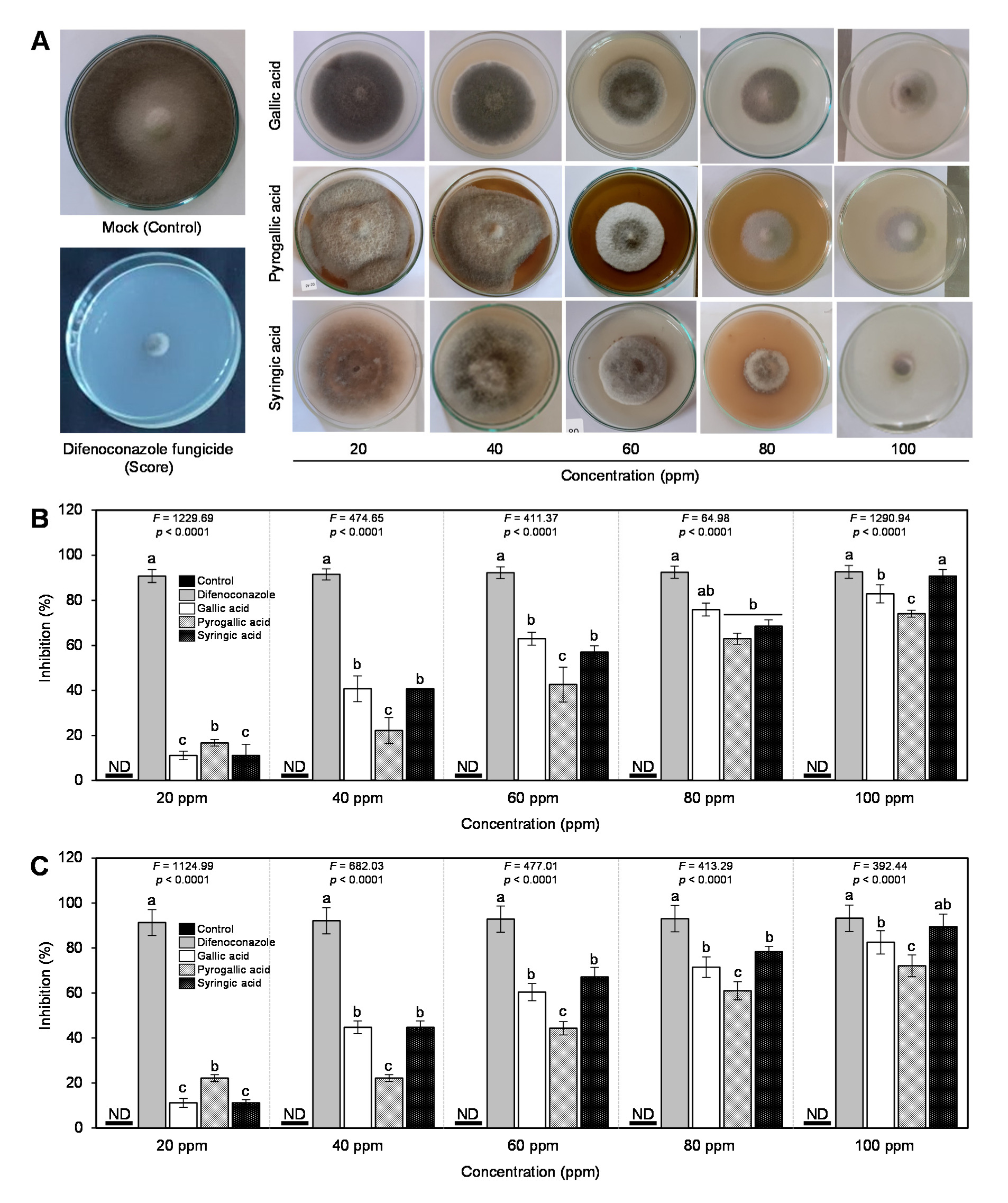
3.2. In Vitro Antifungal Activity of Gallic Acid and Its Derivatives
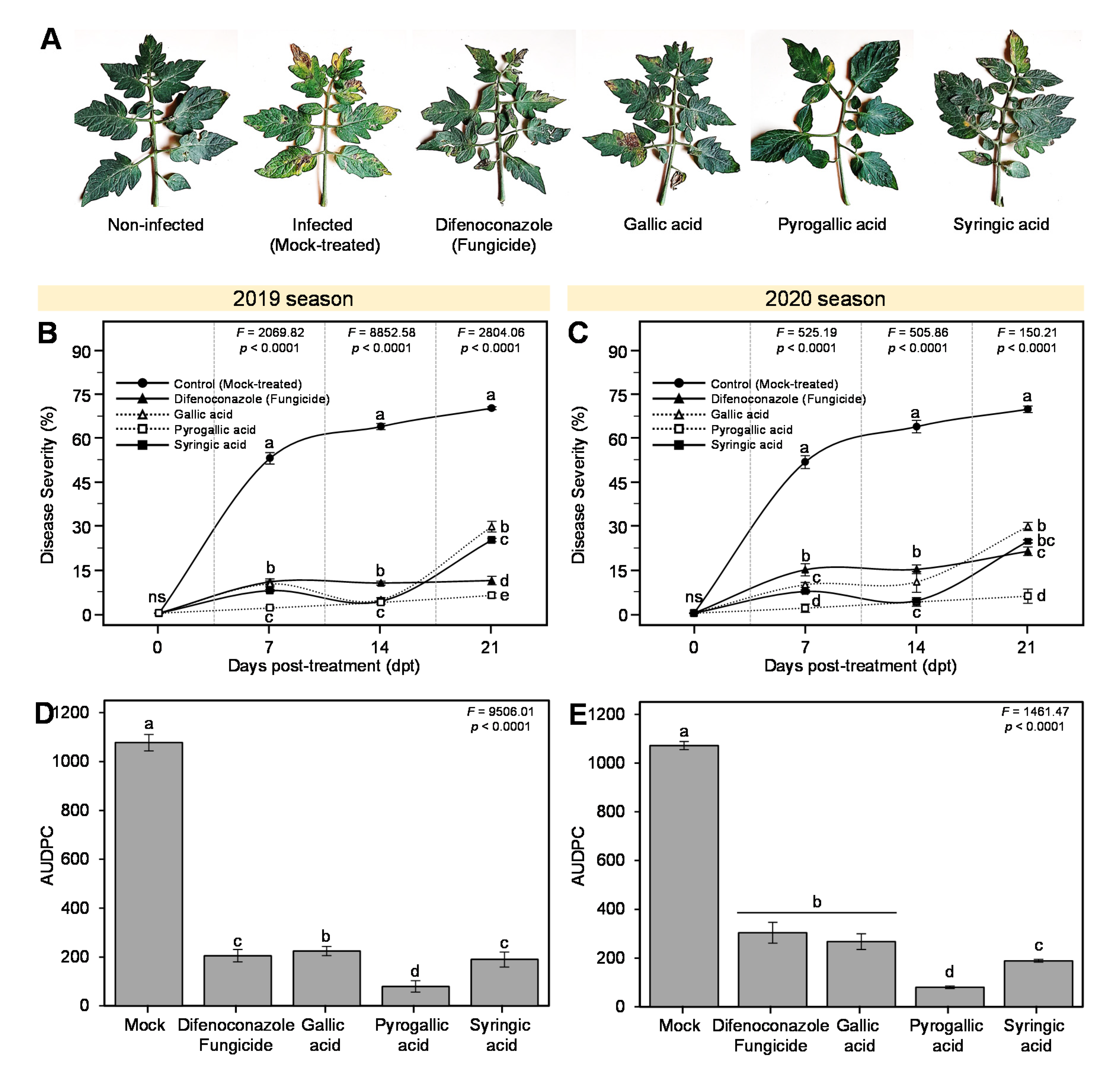
3.3. Effects of Gallic Acid and Its Derivatives on the Development of Early Blight Disease
3.4. Impact of Gallic Acid and Its Derivatives on Growth Parameters of A. solani-Infected Tomato Plants
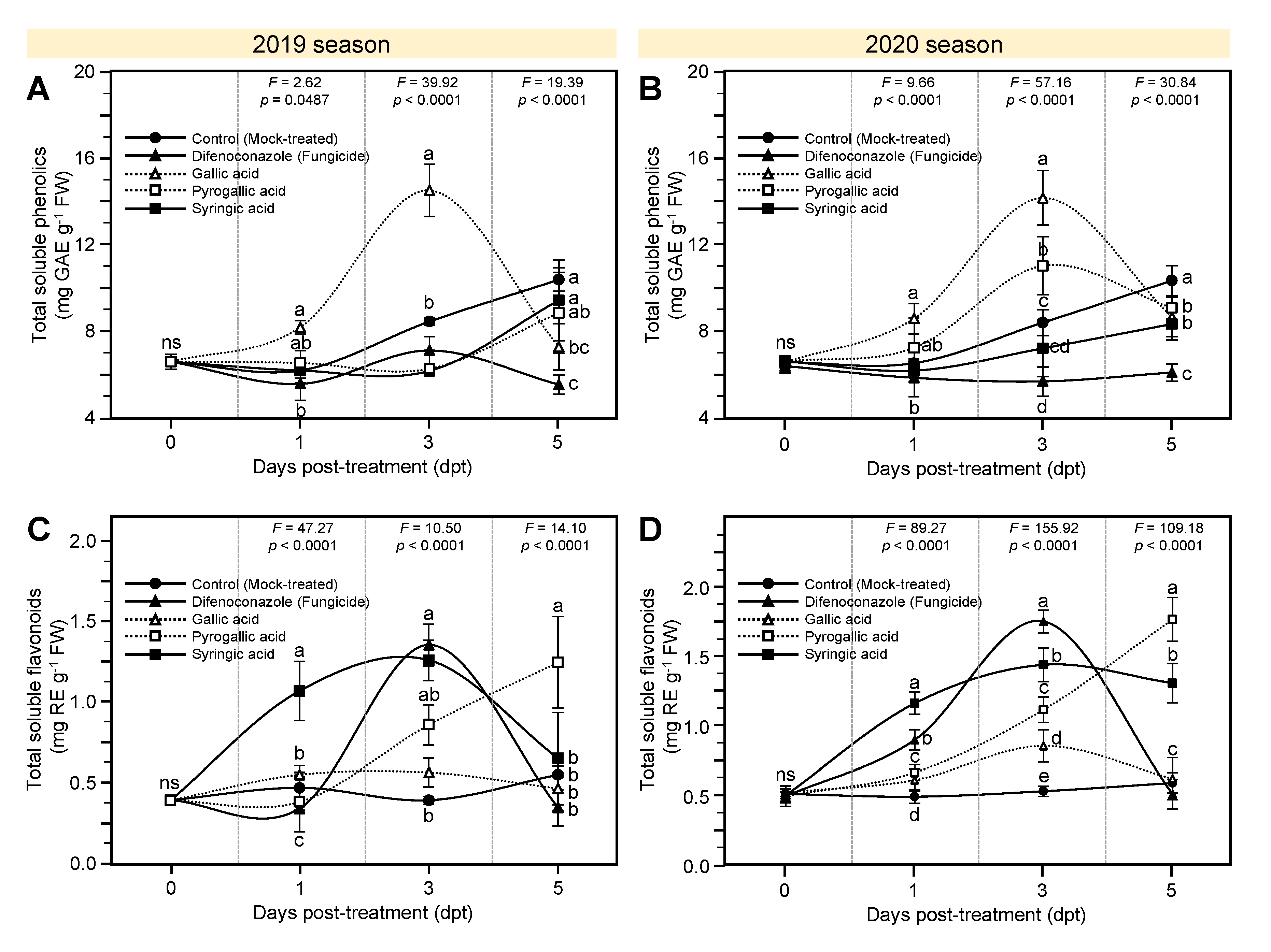
3.5. Effects of Gallic Acid and Its Derivatives on Chlorophyll, Total Phenolics, and Total Flavonoid Content of A. solani-Infected Tomato Plants
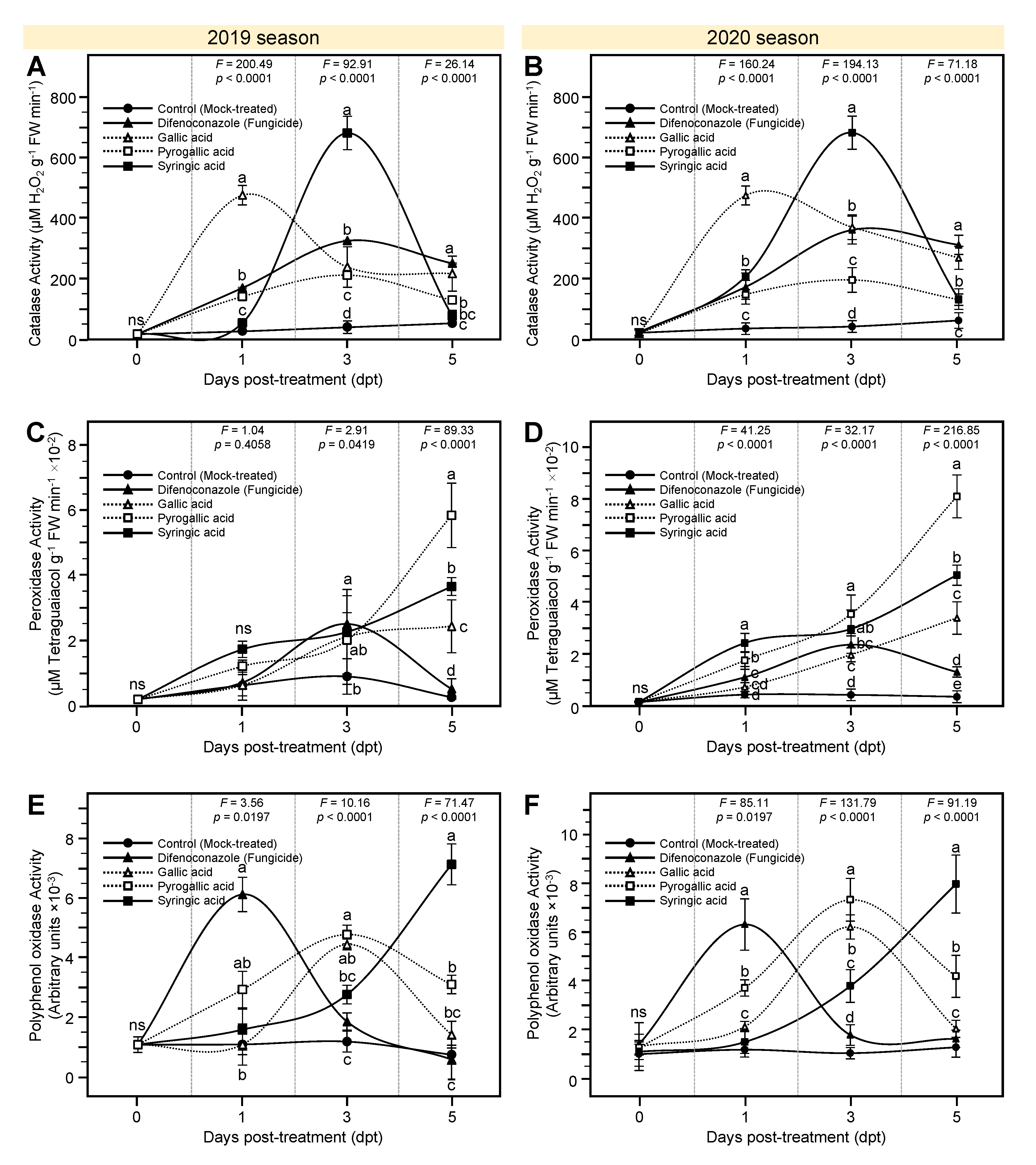
3.6. Effects of Gallic Acid and Its Derivatives on the Activity of Defense-Related Enzymes of A. solani-Infected Tomato Plants
3.7. Impact of Gallic Acid and Its Derivatives on Yield Components of A. solani-Infected Tomato Plants
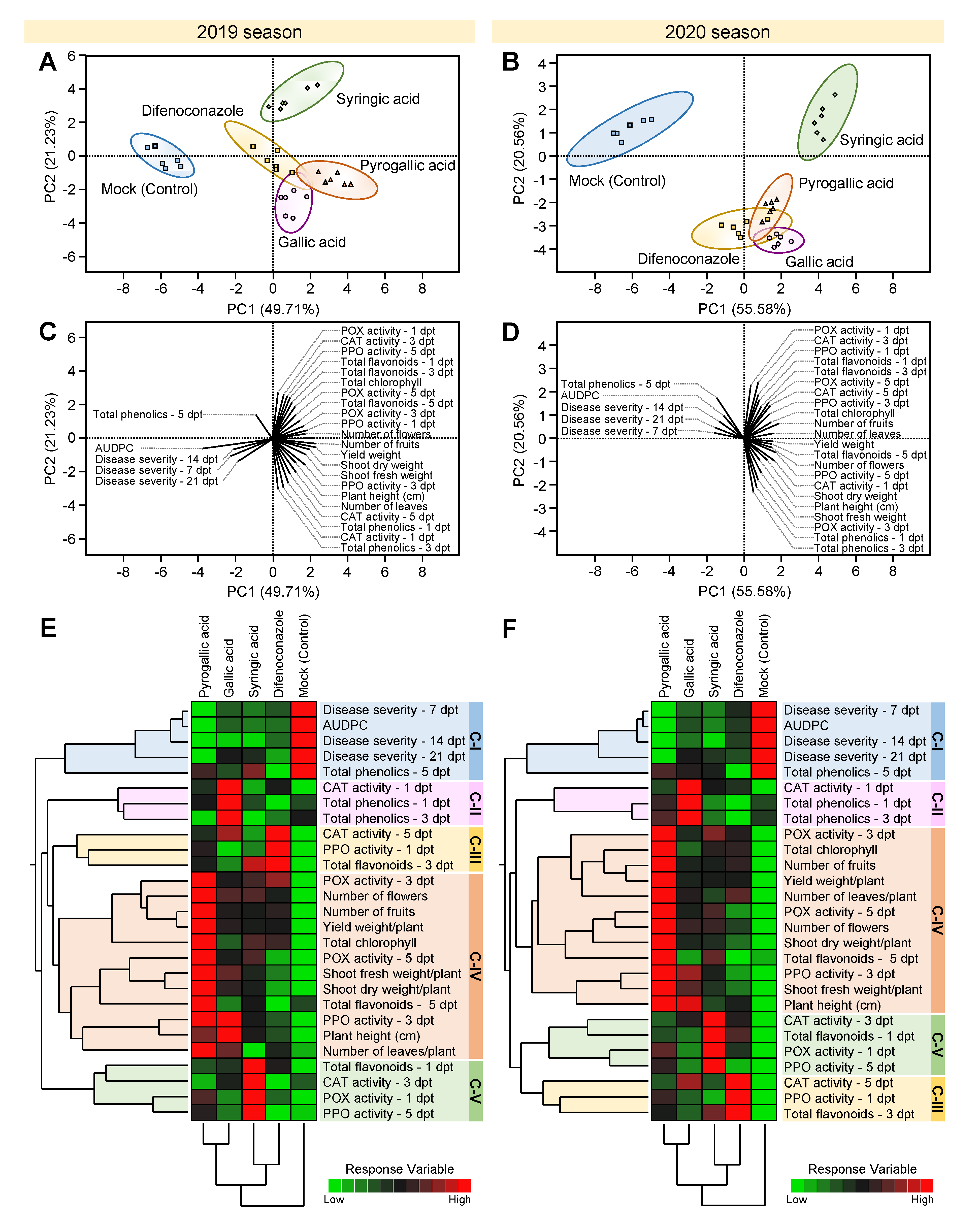
3.8. PCA and HCA Analyses Revealed the Differences between Gallic Acid and Its Derivatives
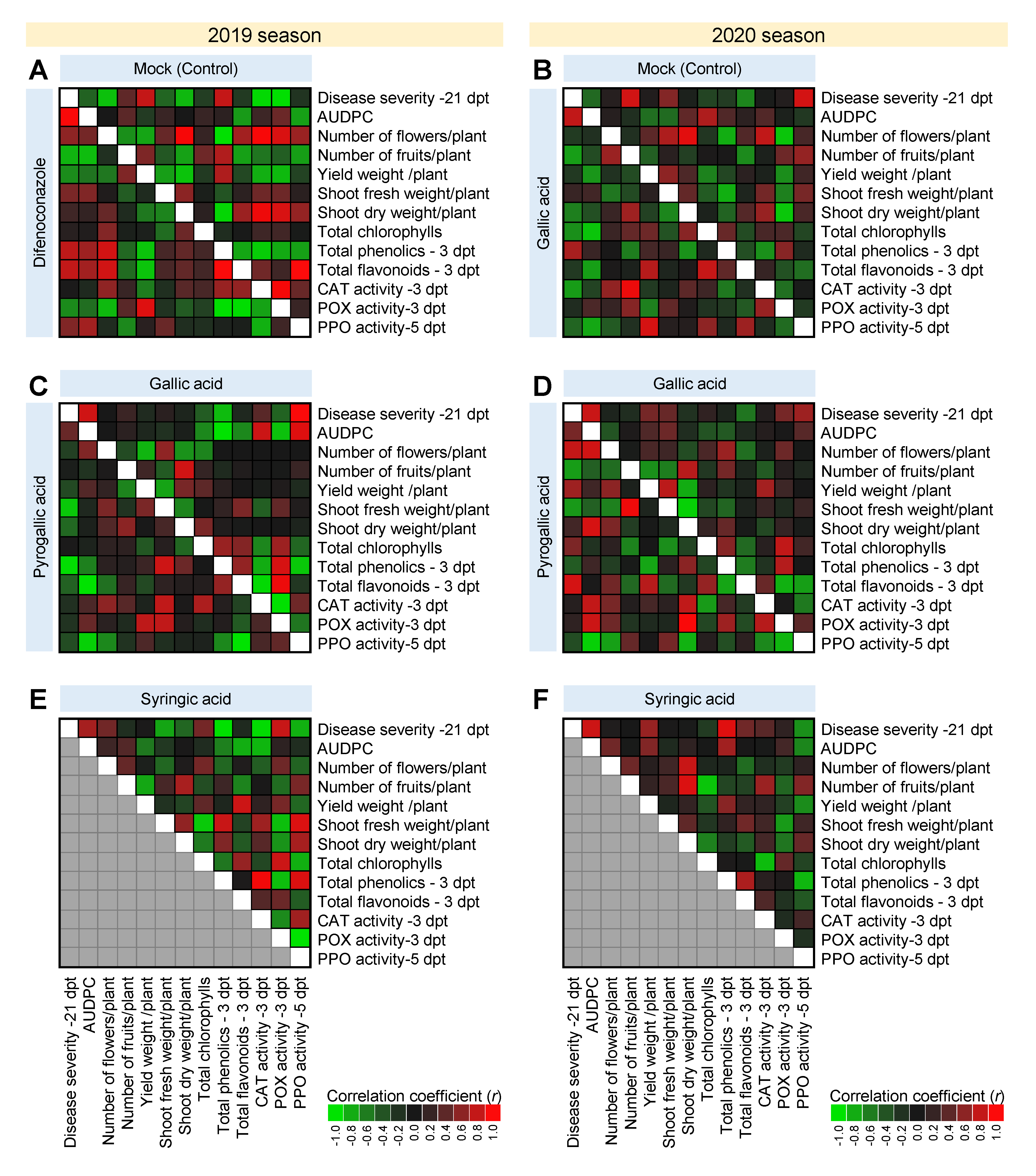
3.9. Correlation Analysis between Disease Parameters, Growth Traits, Yield Components, and Other Phytochemical Responses of A. solani-Infected Tomato Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT Food and Agriculture Organization of the United Nations. 2018. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 13 February 2020).
- Castagna, A.; Chiavaro, E.; Dall’Asta, C.; Rinaldi, M.; Galaverna, G.; Ranieri, A. Effect of postharvest UV-B irradiation on nutraceutical quality and physical properties of tomato fruits. Food Chem. 2013, 137, 151–158. [Google Scholar] [CrossRef]
- Htun, A.A.; Sein, L.L.A.; He, L.; Liu, H.F.; Deng, J.X. First report of Alternaria blumeae causing leaf blight on tomato in Myanmar. Plant Dis. 2020. [Google Scholar] [CrossRef]
- Jones, J.B.; Zitter, T.A.; Momol, T.M.; Miller, S.A. PART I: Infectious Diseases. In Compendium of Tomato Diseases and Pests, 2nd ed.; Jones, J.B., Zitter, T.A., Momol, T.M., Miller, S.A., Eds.; The American Phytopathological Society: Saint Paul, MN, USA, 2016; pp. 15–119. [Google Scholar]
- Neergaard, P. Danish Species of Alternaria and Stemphylium: Taxonomy, Parasitism, Economic Significance; Oxford University Press: London, UK, 1945. [Google Scholar]
- Chaerani, R.; Voorrips, R.E. Tomato early blight (Alternaria solani): The pathogen, genetics, and breeding for resistance. J. Gen. Plant Pathol. 2006, 72, 335–347. [Google Scholar] [CrossRef]
- Attia, M.S.; El-Sayyad, G.S.; Abd Elkodous, M.; El-Batal, A.I. The effective antagonistic potential of plant growth-promoting rhizobacteria against Alternaria solani-causing early blight disease in tomato plant. Sci. Hortic. (Amsterdam) 2020, 266, 109289. [Google Scholar]
- Louis, B.; Waikhom, S.D.; Singh, W.M.; Talukdar, N.C.; Roy, P. Diversity of Ascomycetes at the Potato Interface: New Devastating Fungal Pathogens Posing Threat to Potato Farming. Plant Pathol. J. 2014, 13, 18–27. [Google Scholar]
- Rogerson, C.T.; Sherf, A.E.; MacNab, A.A. Vegetable Diseases and Their Control. Brittonia 1987, 39, 378. [Google Scholar] [CrossRef]
- Palm, M.E.; Rotem, J. The Genus Alternaria: Biology, Epidemiology, and Pathogenicity. Mycologia 1997, 89, 347. [Google Scholar] [CrossRef]
- Van Der Waals, J.E.; Korsten, L.; Denner, F.D.N. Early blight in South Africa: Knowledge, attitudes and control practices of potato growers. Potato Res. 2005, 46, 27–37. [Google Scholar] [CrossRef]
- Aslam, M.; Habib, A.; Sahi, S.T.; Khan, R.R. Effect of bion and salicylic acid on peroxidase activity and total phenolics in tomato against alternaria solani. Pak. J. Agric. Sci. 2020, 57, 53–62. [Google Scholar]
- Sarfraz, M.; Khan, S.A.; Moosa, A.; Farzand, A.; Ishaq, U.; Naeem, I.; Khan, W.A. Promising Antifungal Potential of Selective Botanical Extracts, Fungicides and Trichoderma Isolates Against Alternaria solani. Cercet. Agron. Mold. 2018, 51, 65–74. [Google Scholar] [CrossRef]
- He, M.-H.; Wang, Y.-P.; Wu, E.-J.; Shen, L.-L.; Yang, L.-N.; Wang, T.; Shang, L.-P.; Zhu, W.; Zhan, J. Constraining Evolution of Alternaria alternata Resistance to a Demethylation Inhibitor (DMI) Fungicide Difenoconazole. Front. Microbiol. 2019, 10, 1609. [Google Scholar]
- Nottensteiner, M.; Absmeier, C.; Zellner, M. QoI Fungicide Resistance Mutations in Alternaria solani and Alternaria alternata are Fully Established in Potato Growing Areas in Bavaria and Dual Resistance against SDHI Fungicides is Upcoming. Gesunde Pflanz. 2019, 71, 155–164. [Google Scholar]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic Compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E., Carrillo-Lopez, A., Eds.; Woodhead Publishing-Elsevier: Cambridge, UK, 2019; pp. 253–271. ISBN 9780128132784. [Google Scholar]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signpost: Cerala, India, 2006; pp. 23–67. ISBN 813-080-034-9. [Google Scholar]
- Singh, S.; Singh, A.; Jaiswal, J.; Singh, T.D.; Singh, V.P.; Pandey, V.B.; Tiwari, A.; Singh, U.P. Antifungal activity of the mixture of quaternary alkaloids isolated from Argemone mexicana against some phytopathogenic fungi. Arch. Phytopathol. Plant Prot. 2010, 43, 769–774. [Google Scholar] [CrossRef]
- Pane, C.; Fratianni, F.; Parisi, M.; Nazzaro, F.; Zaccardelli, M. Control of Alternaria post-harvest infections on cherry tomato fruits by wild pepper phenolic-rich extracts. Crop Prot. 2016, 84, 81–87. [Google Scholar]
- Pane, C.; Fratianni, F.; Raimo, F.; Nazzaro, F.; Zaccardelli, M. Efficacy of phenolic-rich extracts from leaves of pepper landraces against Alternaria leaf blight of tomato. J. Plant Pathol. 2017, 99, 239–244. [Google Scholar]
- Gupta, M.; Sharma, S.; Bhadauria, R. Phytotoxicity of Momordica charantia extracts against Alternaria alternata. J. Pharm. Sci. Res. 2017, 9, 28–34. [Google Scholar]
- Papoutsis, K.; Vuong, Q.V.; Tesoriero, L.; Pristijono, P.; Stathopoulos, C.E.; Gkountina, S.; Lidbetter, F.; Bowyer, M.C.; Scarlett, C.J.; Golding, J.B. Microwave irradiation enhances the in vitro antifungal activity of citrus by-product aqueous extracts against Alternaria alternata. Int. J. Food Sci. Technol. 2018, 53, 1510–1517. [Google Scholar]
- Li, Z.-J.; Liu, M.; Dawuti, G.; Dou, Q.; Ma, Y.; Liu, H.-G.; Aibai, S. Antifungal Activity of Gallic Acid In Vitro and In Vivo. Phyther. Res. 2017, 31, 1039–1045. [Google Scholar]
- Binutu, O.A.; Cordell, G.A. Gallic Acid Derivatives from Mezoneuron benthamianum Leaves. Pharm. Biol. 2000, 38, 284–286. [Google Scholar]
- Ahn, Y.-J.; Lee, H.-S.; Oh, H.-S.; Kim, H.-T.; Lee, Y.-H. Antifungal activity and mode of action of Galla rhois-derived phenolics against phytopathogenic fungi. Pestic. Biochem. Physiol. 2005, 81, 105–112. [Google Scholar]
- Benhamou, N.; Bélanger, R.R. Benzothiadiazole-mediated induced resistance to Fusarium oxysporum f. sp. radicis-lycopersici in tomato. Plant Physiol. 1998, 118, 1203–1212. [Google Scholar] [CrossRef]
- Ellis, M.B.; Ellis, P. Microfungi on Land Plants; Croom Helm Australia: Sydney, New South Wales, Australia, 1987. [Google Scholar]
- Joly, P. Le genre alternaria, recherches physiologiques, biologiques et systematiques. Encycl. Mycol. 1964, 33, 1–250. [Google Scholar]
- Simmons, E.G. Alternaria taxonomy: Current status, viewpoint, challenge. In Alternaria: Biology, Plant Diseases, and Metabolites; Chelkowski, J., Visconti, A., Eds.; Elsevier: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Siciliano, I.; Gilardi, G.; Ortu, G.; Gisi, U.; Gullino, M.L.; Garibaldi, A. Identification and characterization of Alternaria species causing leaf spot on cabbage, cauliflower, wild and cultivated rocket by using molecular and morphological features and mycotoxin production. Eur. J. Plant Pathol. 2017, 149, 401–413. [Google Scholar] [CrossRef]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 3rd ed.; Burgess Publishing Company: Minneapolis, MN, USA, 1972. [Google Scholar]
- Pandey, K.K.; Pandey, P.K.; Satpathy, S. Integrated Management of Diseases and Insects of Tomato, Chilli and Cole Crops; Indian Institute of Vegetable Research: Kelabela, Uttar Pradesh, India, 2002. [Google Scholar]
- Grover, R.; Moore, J.D. Toximetric studies of fungicides against the brown root organisms, Sclerotinia fructicola and S. laxa. Phytopathology 1962, 52, 876–880. [Google Scholar]
- Pandey, K.K.; Pandey, P.K.; Kalloo, G.; Banerjee, M.K. Resistance to early blight of tomato with respect to various parameters of disease epidemics. J. Gen. plant Pathol. 2003, 69, 364–371. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Rathmell, W.G.; Sequeira, L. Soluble Peroxidase in Fluid from the Intercellular Spaces of Tobacco Leaves. Plant Physiol. 1974, 53, 317–318. [Google Scholar] [CrossRef]
- Malik, C.P.; Singh, M.B. Plant Enzymology and Histo-Enzymology; Kalyani Publishers: Darya Ganj, New Delhi, India, 1980. [Google Scholar]
- Yadawa, U.L. A rapid and nondestructive method to determine chlorophyll in intact leaves. HortScience 1986, 21, 1449–1450. [Google Scholar]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- SAS Institute. JMP Data Analysis Software—Version 14. Available online: https://www.jmp.com (accessed on 15 December 2019).
- Natural History Museum-University of Oslo. PAleontological STatistics (PAST)—Version 3. Available online: https://folk.uio.no/ohammer/past/ (accessed on 18 May 2019).
- MedCalc Software Ltd. MedCalc Statistical Software—Version 19.3.1. Available online: https://www.medcalc.org (accessed on 22 March 2020).
- Gan, R.-Y.; Chan, C.-L.; Yang, Q.-Q.; Li, H.-B.; Zhang, D.; Ge, Y.-Y.; Gunaratne, A.; Ge, J.; Corke, H. Bioactive compounds and beneficial functions of sprouted grains. In Sprouted Grains: Nutritional Value, Production, and Applications; Feng, H., Nemzer, B., De Vries, J.W., Eds.; Woodhead Publishing and AACC International Press: Cambridge, UK, 2019; pp. 191–246. ISBN 9780128115251. [Google Scholar]
- Vondráková, Z.; Malbeck, J.; Trávníčková, A.; Černý, R.; Cvikrová, M. Phenolic acids in selected scab-resistant and mildew-tolerant apple cultivars. Acta Physiol. Plant. 2020, 42, 1–10. [Google Scholar] [CrossRef]
- Vagiri, M.; Johansson, E.; Rumpunen, K. Phenolic compounds in black currant leaves—An interaction between the plant and foliar diseases? J. Plant Interact. 2017, 12, 193–199. [Google Scholar] [CrossRef]
- Zheng, W.; Zheng, Q.; Xue, Y.; Hu, J.; Gao, M.T. Influence of rice straw polyphenols on cellulase production by Trichoderma reesei. J. Biosci. Bioeng. 2017, 123, 731–738. [Google Scholar] [CrossRef]
- Guleria, S.; Kumar, A. Azadirachta indica leaf extract induces resistance in sesame against Alternaria leaf spot disease. J. Cell Mol. Biol. 2006, 5, 81–86. [Google Scholar]
- Mvumi, C.; Ngadze, E.; Marais, D.; du Toit, E.S.; Mvumi, B.M. Moringa (Moringa oleifera) leaf extracts inhibit spore germination of Alternaria solani, causal agent of early blight disease of tomato (Solanum lycopersicum). S. Afr. J. Plant Soil 2017, 34, 161–165. [Google Scholar] [CrossRef]
- Dethoup, T.; Songkumarn, P.; Rueangrit, S.; Suesa-ard, S.; Kaewkrajay, C. Fungicidal activity of Thai medicinal plant extracts against Alternaria brassicicola causing black spot of Chinese kale. Eur. J. Plant Pathol. 2018, 152, 157–167. [Google Scholar] [CrossRef]
- Yuan, S.; Li, W.; Li, Q.; Wang, L.; Cao, J.; Jiang, W. Defense Responses, Induced by p-Coumaric Acid and Methyl p-Coumarate, of Jujube (Ziziphus jujuba Mill.) Fruit against Black Spot Rot Caused by Alternaria alternata. J. Agric. Food Chem. 2019, 67, 2801–2810. [Google Scholar] [CrossRef]
- Mandal, D.; Pal, R.; Mohanty, A.K. Management of early blight of tomato. Indian J. Hortic. 2019, 76, 548–551. [Google Scholar] [CrossRef]
- Gopi, K.; Renu, K.; Sannanaik Vishwanath, B.; Jayaraman, G. Protective effect of Euphorbia hirta and its components against snake venom induced lethality. J. Ethnopharmacol. 2015, 165, 180–190. [Google Scholar] [CrossRef]
- Baharuddin, N.; Abdullah, H.; Abdul Wahab, W.N. Anti-Candida activity of Quercus infectoria gall extracts against Candida species. J. Pharm. Bioallied Sci. 2015, 7, 15–20. [Google Scholar] [CrossRef]
- Hirasawa, M.; Takada, K. Multiple effects of green tea catechin on the antifungal activity of antimycotics against Candida albicans. J. Antimicrob. Chemother. 2004, 53, 225–229. [Google Scholar]
- Nguyen, D.M.C.; Seo, D.J.; Lee, H.B.; Kim, I.S.; Kim, K.Y.; Park, R.D.; Jung, W.J. Antifungal activity of gallic acid purified from Terminalia nigrovenulosa bark against Fusarium solani. Microb. Pathog. 2013, 56, 8–15. [Google Scholar]
- Sahu, R.; Sharaff, M.; Pradhan, M.; Sethi, A.; Bandyopadhyay, T.; Mishra, V.K.; Chand, R.; Chowdhury, A.K.; Joshi, A.K.; Pandey, S.P. Elucidation of defense-related signaling responses to spot blotch infection in bread wheat (Triticum aestivum L.). Plant J. 2016, 86, 35–49. [Google Scholar] [CrossRef]
- Sharma, S.; Sahu, R.; Navathe, S.; Mishra, V.K.; Chand, R.; Singh, P.K.; Joshi, A.K.; Pandey, S.P. Natural Variation in Elicitation of Defense-Signaling Associates to Field Resistance Against the Spot Blotch Disease in Bread Wheat (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 636. [Google Scholar] [CrossRef]
- Chong, K.P.; Rossall, S.; Atong, M. In Vitro Antimicrobial Activity and Fungitoxicity of Syringic Acid, Caffeic Acid and 4-hydroxybenzoic Acid against Ganoderma Boninense. J. Agric. Sci. 2009, 1, 15. [Google Scholar]
- Chong, K.P.; Atong, M.; Rossall, S. The role of syringic acid in the interaction between oil palm and Ganoderma boninense, the causal agent of basal stem rot. Plant Pathol. 2012, 61, 953–963. [Google Scholar]
- Sánchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar]
- Alves, M.J.; Ferreira, I.C.F.R.; Froufe, H.J.C.; Abreu, R.M.V.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar]
- Shi, C.; Sun, Y.; Zheng, Z.; Zhang, X.; Song, K.; Jia, Z.; Chen, Y.; Yang, M.; Liu, X.; Dong, R.; et al. Antimicrobial activity of syringic acid against Cronobacter sakazakii and its effect on cell membrane. Food Chem. 2016, 197, 100–106. [Google Scholar] [CrossRef]
- Kozlowska, M.; Krzywanski, Z. The possible role of phenolic compounds in red raspberry resistance to Didymella applanata (Niessl) Sacc. In Symposium on Natural Phenols in Plant Resistance; Geibel, M., Treutter, D., Feucht, W., Eds.; International Society for Horticultural Science: Weihenstephan, Germany, 1993; pp. 671–674. [Google Scholar]
- Fu, Y.H.; Quan, W.X.; Li, C.C.; Qian, C.Y.; Tang, F.H.; Chen, X.J. Allelopathic effects of phenolic acids on seedling growth and photosynthesis in rhododendron delavayi franch. Photosynthetica 2019, 57, 377–387. [Google Scholar] [CrossRef]
- Dangl, J.L.; Jones, J.D.G. Plant pathogens and integrated defence responses to infection. Nature 2001, 411, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 2009, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Shi, J.; Ma, X.; Wei, H.; Hu, J.; Tsang, Y.F.; Gao, M.T. Effect of Phenolic Acids Derived from Rice Straw on Botrytis cinerea and Infection on Tomato. Waste Biomass Valorization 2020, 1–9. [Google Scholar] [CrossRef]
- Jia, L.G.; Sheng, Z.W.; Xu, W.F.; Li, Y.X.; Liu, Y.G.; Xia, Y.J.; Zhang, J.H. Modulation of anti-oxidation ability by proanthocyanidins during germination of arabidopsis thaliana seeds. Mol. Plant 2012, 5, 472–481. [Google Scholar] [CrossRef]
- Abdul-Maksoud, R.S.; Fouad, R.A.; Elsayed, T.G.; Ibrahem, R.A.; Badawi, A.E. The impact of catalase and glutathione peroxidase-1 genetic polymorphisms on their enzyme activities among Egyptian patients with keratoconus. J. Gene Med. 2020, 22, e3192. [Google Scholar] [CrossRef]
- Heller, J.; Tudzynski, P. Reactive Oxygen Species in Phytopathogenic Fungi: Signaling, Development, and Disease. Annu. Rev. Phytopathol. 2011, 49, 369–390. [Google Scholar] [CrossRef]
- Mahmoudi, N. Effect of beta-aminobutyric acid on activity of peroxidase and polyphenol oxidase enzymes in cucumber infected with nematode Meloidogyne javanica. Rudn J. Agron. Anim. Ind. 2019, 14, 7–17. [Google Scholar] [CrossRef]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V. Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
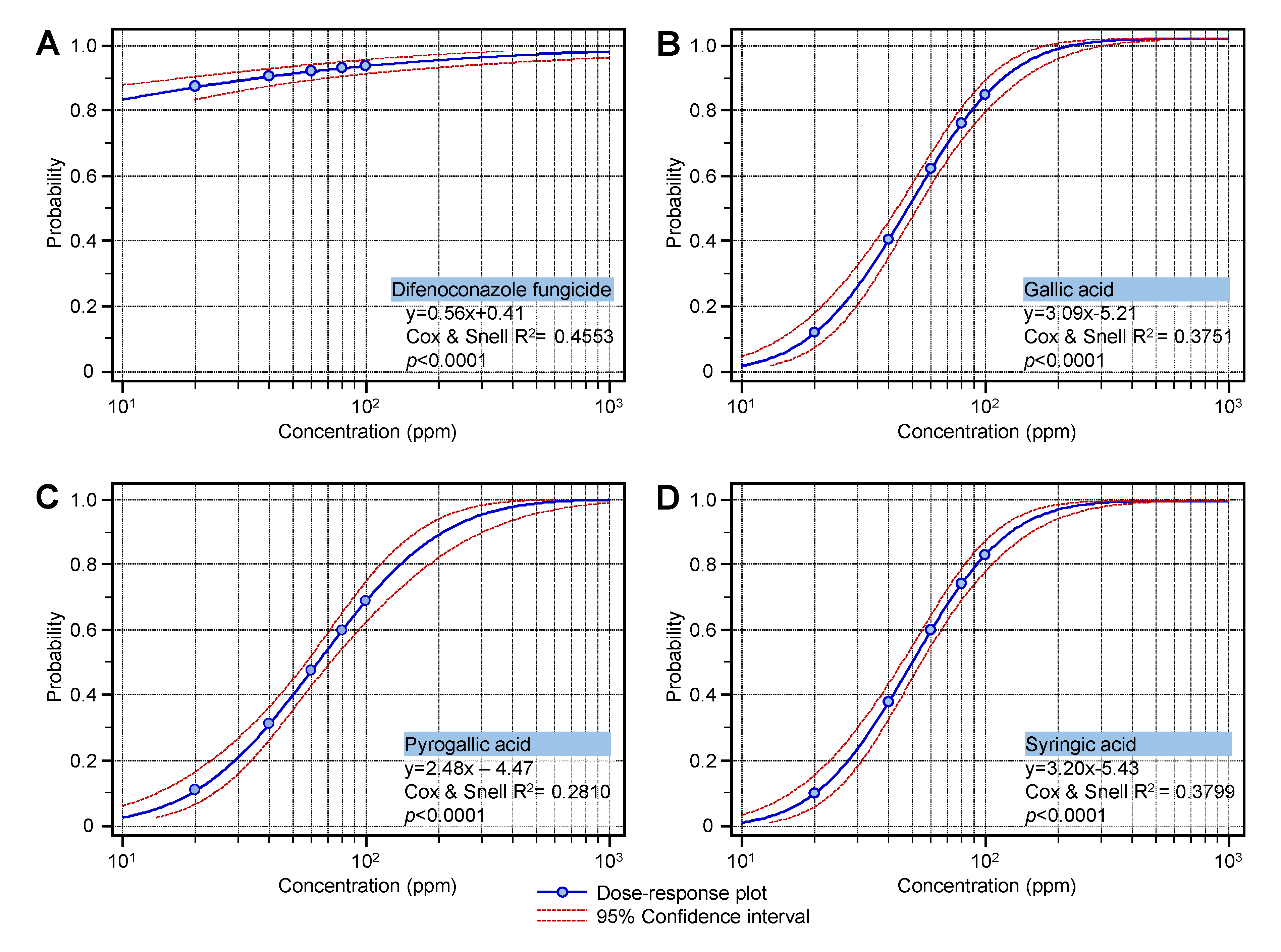
| Compounds | IC50 (ppm) | 95% Confidence Interval | IC99 (ppm) | 95% Confidence Interval | Overall Model Fit | ||||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | χ2 | p-Value | Cox and Snell R2 | |||
| Difenoconazole fungicide (Score) | 0.19 | 0.01 | 0.84 | 2572.24 | 770.93 | 26582.69 | 364.52 | <0.0001 | 0.4553 |
| Gallic acid | 48.81 | 44.29 | 53.44 | 276.85 | 213.61 | 398.68 | 282.10 | <0.0001 | 0.3751 |
| Syringic acid | 49.75 | 45.30 | 54.31 | 264.94 | 206.83 | 374.43 | 286.73 | <0.0001 | 0.3799 |
| Pyrogallic acid | 63.75 | 57.16 | 71.89 | 552.61 | 365.36 | 1041.11 | 197.93 | <0.0001 | 0.2810 |
| Treatment | Plant Height (cm) | Number of Leaves per Plant | Shoot Fresh Weight (g plant−1) | Shoot Dry Weight (g plant−1) | Chlorophyll Content (SPAD) |
|---|---|---|---|---|---|
| 2019 season | |||||
| Mock (Control) | 24.83 ± 5.08 ns | 7.67 ± 1.37 ns | 20.20 ± 1.27 e | 4.86 ± 0.07 e | 10.55 ± 6.89 c |
| Difenoconazole fungicide (Score) | 26.83 ± 3.76 ns | 8.33 ± 2.25 ns | 23.53 ± 1.89 d | 5.05 ± 0.07 d | 27.15 ± 4.67 ab |
| Gallic acid | 30.83 ± 3.31 ns | 9.00 ± 1.79 ns | 32.83 ± 1.97 b | 6.11 ± 0.09 b | 18.00 ± 5.44 bc |
| Pyrogallic acid | 29.50 ± 1.52 ns | 9.67 ± 0.82 ns | 38.63 ± 1.38 a | 7.45 ± 0.16 a | 36.68 ± 8.63 a |
| Syringic acid | 28.17 ± 5.95 ns | 7.50 ± 0.55 ns | 27.61 ± 1.43 c | 5.78 ± 0.08 c | 28.37 ± 9.84 ab |
| 2020 season | |||||
| Mock (Control) | 23.67 ± 4.68 b | 8.50 ± 2.26 ns | 21.52 ± 1.31 d | 4.90 ± 0.66 b | 12.20 ± 6.06 c |
| Difenoconazole fungicide (Score) | 27.67 ± 2.73 ab | 10.17 ± 2.40 ns | 24.99 ± 2.65 cd | 5.41 ± 0.51 b | 27.55 ± 4.51 b |
| Gallic acid | 30.83 ± 2.64 a | 9.83 ± 1.33 ns | 33.82 ± 1.51 b | 5.88 ± 0.71 b | 18.65 ± 5.43 bc |
| Pyrogallic acid | 31.17 ± 2.48 a | 10.83 ± 1.47 ns | 40.59 ± 2.99 a | 8.07 ± 0.84 a | 39.93 ± 6.52 a |
| Syringic acid | 26.50 ± 6.32 ab | 9.33 ± 1.03 ns | 27.96 ± 2.21 c | 5.83 ± 0.11 b | 26.90 ± 5.43 b |
| Treatment | Number of Flowers per Plant | Number of Fruits per Plant | Fruit Yield (Kg plant−1) | Fruit Yield Increase over Control (%) |
|---|---|---|---|---|
| 2019 season | ||||
| Mock (Control) | 5.00 ± 0.89 c | 10.33 ± 1.63 c | 0.85 ± 0.07 d | - |
| Difenoconazole fungicide (Score) | 7.67 ± 1.21 b | 16.00 ± 0.89 b | 1.50 ± 0.04 bc | 176.32 ± 5.27 |
| Gallic acid | 8.50 ± 0.55 ab | 15.67 ± 1.03 b | 1.55 ± 0.04 b | 182.39 ± 5.13 |
| Pyrogallic acid | 9.67 ± 1.37 a | 19.67 ± 1.21 a | 2.07 ± 0.06 a | 243.05 ± 7.01 |
| Syringic acid | 8.50 ± 1.52 ab | 15.17 ± 0.75 b | 1.46 ± 0.04 c | 171.04 ± 4.24 |
| 2020 season | ||||
| Mock (Control) | 6.50 ± 1.97 b | 10.50 ± 1.87 c | 0.92 ± 0.10 c | - |
| Difenoconazole fungicide (Score) | 8.17 ± 1.60 ab | 16.83 ± 1.94 b | 1.63 ± 0.19 b | 176.63 ± 21.07 |
| Gallic acid | 9.33 ± 1.51 ab | 15.33 ± 2.34 b | 1.59 ± 0.21 b | 172.64 ± 23.14 |
| Pyrogallic acid | 11.00 ± 1.41 a | 21.33 ± 4.03 a | 2.50 ± 0.17 a | 272.10 ± 18.63 |
| Syringic acid | 9.33 ± 2.42 ab | 15.5 ± 1.05 b | 1.62 ± 0.12 b | 176.45 ± 13.45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Nagar, A.; Elzaawely, A.A.; Taha, N.A.; Nehela, Y. The Antifungal Activity of Gallic Acid and Its Derivatives against Alternaria solani, the Causal Agent of Tomato Early Blight. Agronomy 2020, 10, 1402. https://doi.org/10.3390/agronomy10091402
El-Nagar A, Elzaawely AA, Taha NA, Nehela Y. The Antifungal Activity of Gallic Acid and Its Derivatives against Alternaria solani, the Causal Agent of Tomato Early Blight. Agronomy. 2020; 10(9):1402. https://doi.org/10.3390/agronomy10091402
Chicago/Turabian StyleEl-Nagar, Asmaa, Abdelnaser A. Elzaawely, Naglaa A. Taha, and Yasser Nehela. 2020. "The Antifungal Activity of Gallic Acid and Its Derivatives against Alternaria solani, the Causal Agent of Tomato Early Blight" Agronomy 10, no. 9: 1402. https://doi.org/10.3390/agronomy10091402
APA StyleEl-Nagar, A., Elzaawely, A. A., Taha, N. A., & Nehela, Y. (2020). The Antifungal Activity of Gallic Acid and Its Derivatives against Alternaria solani, the Causal Agent of Tomato Early Blight. Agronomy, 10(9), 1402. https://doi.org/10.3390/agronomy10091402







