Occurrence and Management of PSII-Inhibitor-Resistant Chenopodium album L. in Atlantic Canadian Potato Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Common Lambsquarters Populations
2.2. Identifying Target-Site Mutation
2.3. Single-Dose Assays
2.4. Herbicide Dose–Response
2.5. Evaluation of PRE-Emergence Herbicide Options
2.6. Statistical Analysis
3. Results and Discussion
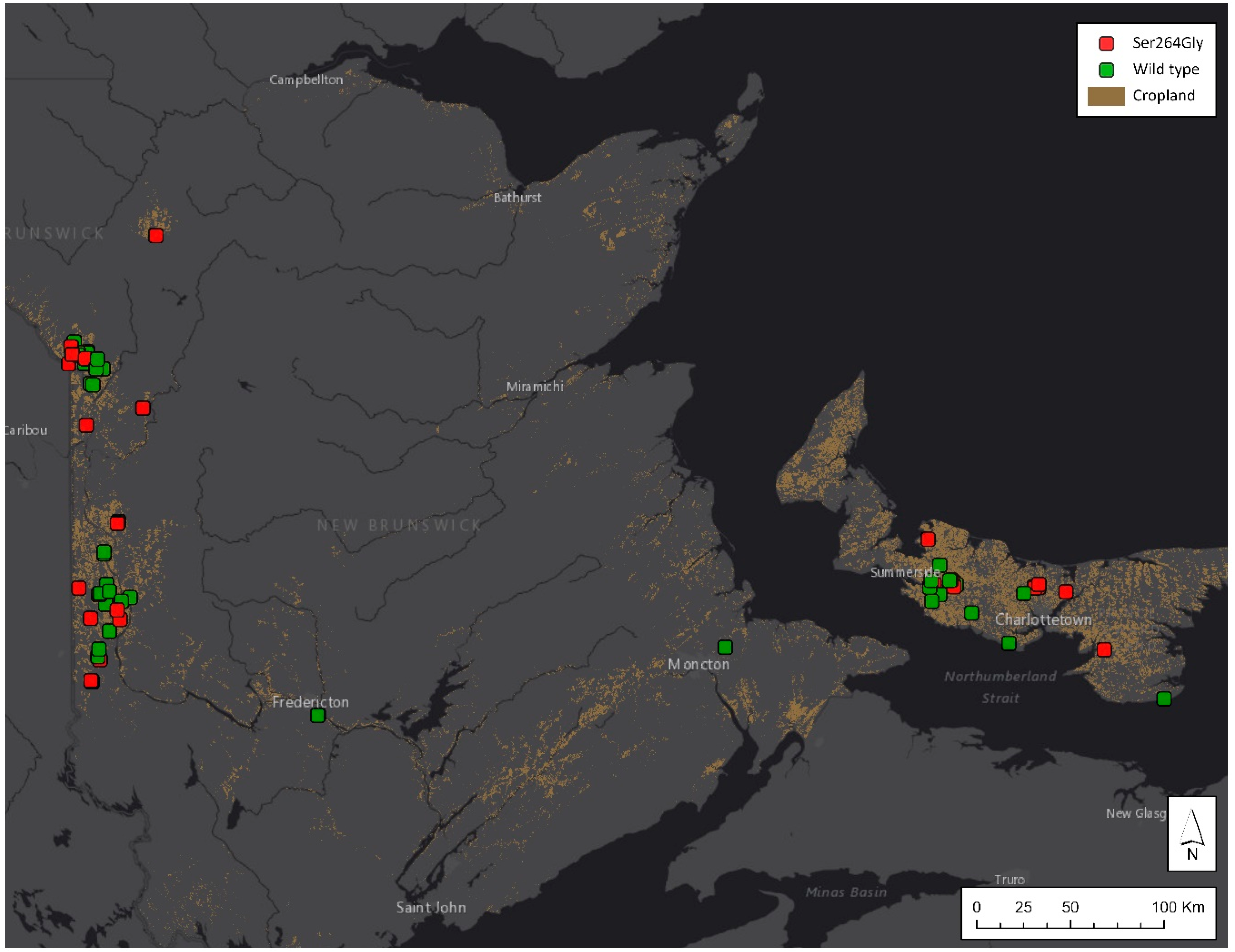
3.1. PSII-Inhibitor-Resistant Common Lambsquarters Is Widespread across Atlantic Canada
3.2. PSII-Inhibitor-Resistant Common Lambsquarters Is Controlled by Other Modes of Action
3.3. Potato Yield Was Not Impacted by Preemergent Herbicides
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Statistics Canada Table 32-10-0358-01 Area, Production and Farm Value of Potatoes. Available online: https://doi.org/10.25318/3210035801-eng (accessed on 26 May 2020).
- USDA-NASS Agricultural Chemical Use Program—Potatoes. Available online: www.nass.usda.gov/Surveys/Guide_to_NASS_Surveys/Chemical_Use/ (accessed on 26 May 2020).
- Shaner, D.L.; Jachetta, J.J.; Senseman, S.; Burke, I.; Hanson, B.; Jugulam, M.; Tan, S.; Reynolds, J.; Strek, H.; McAllister, R.; et al. Metribuzin. In Herbicide Handbook, 10th ed.; Shaner, D.L., Ed.; Allen Press: Lawrence, KS, USA, 2014; pp. 308–310. [Google Scholar]
- Heap, I. The International Herbicide-Resistant Weed Database. 2020. Available online: www.weedscience.org (accessed on 25 May 2020).
- Gronwald, J.W. Resistance to Photosystem II Inhibiting Herbicides. In Herbicide Resistance in Plants: Biology and Biochemistry; Powles, S.B., Holtum, J.A.M., Eds.; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Beckie, H.J.; Tardif, F.J. Herbicide cross resistance in weeds. Crop. Prot. 2012, 35, 15–28. [Google Scholar] [CrossRef]
- Anderson, M.P.; Gronwald, J.W. Atrazine resistance in a velvetleaf (Abutilon theophrasti) biotype due to enhanced glutathione S-transferase activity. Plant Physiol. 1991, 96, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Boydston, R.A.; Felix, J.; Al-Khatib, K. Preemergence herbicides for potential use in potato production. Weed Technol. 2012, 26, 731–739. [Google Scholar] [CrossRef]
- Eberlein, C.V.; Patterson, P.E.; Guttieri, M.J.; Stark, J.C. Efficacy and economics of cultivation for weed control in potato (Solanum tuberosum). Weed Technol. 1997, 11, 257–264. [Google Scholar] [CrossRef]
- Bandeen, J.D.; McLaren, R.D. Resistance of Chenopodium album to triazine herbicides. Can. J. Plant Sci. 1976, 56, 411–412. [Google Scholar] [CrossRef][Green Version]
- Ellis, A.T.; Steckel, L.E.; Main, C.L.; De Melo, M.S.; West, D.R.; Mueller, T.C. A survey for diclofop-methyl resistance in Italian ryegrass from Tennessee and how to manage resistance in wheat. Weed Technol. 2010, 24, 303–309. [Google Scholar] [CrossRef]
- Vink, J.P.; Soltani, N.; Robinson, D.E.; Tardif, F.J.; Lawton, M.B.; Sikkema, P.H. Occurrence and distribution of glyphosate-resistant giant ragweed (Ambrosia trifida L.) in southwestern Ontario. Can. J. Plant Sci. 2012, 92, 533–539. [Google Scholar] [CrossRef]
- Bettini, P.; McNally, S.; Sevignac, M.; Darmency, H.; Gasquez, J.; Dron, M. Atrazine resistance in Chenopodium album: Low and high levels of resistance to the herbicide are related to the same chloroplast psbA gene mutation. Plant Physiol. 1987, 84, 1442–1446. [Google Scholar] [CrossRef]
- Beckie, H.J.; Heap, I.M.; Smeda, R.J.; Hall, L.M. Screening for herbicide resistance in weeds. Weed Technol. 2000, 14, 428–445. [Google Scholar] [CrossRef]
- Burgos, N.R.; Tranel, P.J.; Streibig, J.C.; Davis, V.M.; Shaner, D.; Norsworthy, J.K.; Ritz, C. Confirmation of resistance to herbicides and evaluation of resistance levels. Weed Sci. 2013, 61, 4–20. [Google Scholar] [CrossRef]
- Davis, G.; Letarte, J.; Grainger, C.M.; Rajcan, I.; Tardif, F.J. Widespread herbicide resistance in pigweed species in Ontario carrot production is due to multiple photosystem II mutations. Can. J. Plant Sci. 2020, 100, 56–67. [Google Scholar] [CrossRef]
- Mechant, E.; De Marez, T.; Hermann, O.; Olsson, R.; Bulcke, R. Target site resistance to metarnitron in Chenopodium album L. J. Plant Dis. Prot. 2008, XXI, 37–40. [Google Scholar]
- Chomas, A.J.; Kells, J.J. Triazine-resistant common lambsquarters (Chenopodium album) control in corn with preemergence herbicides. Weed Technol. 2004, 18, 551–554. [Google Scholar] [CrossRef]
- Bailey, W.A.; Wilson, H.P.; Hines, T.E. Response of potato (Solanum tuberosum) and selected weeds to sulfentrazone. Weed Technol. 2002, 16, 651–658. [Google Scholar] [CrossRef]
- Hutchinson, P.J.; Hancock, D.M.; Beutler, B.R. Efficacy of reduced sulfentrazone rates applied preemergence with metribuzin in potato (Solanum tuberosum). Weed Technol. 2005, 19, 954–958. [Google Scholar] [CrossRef]
- Hutchinson, P.J. Common lambsquarters and hairy nightshade control in potato with dimethenamid-P alone and in tank mixtures and comparison of control by dimethenamid-P with S-metolachlor and metolachlor. Weed Technol. 2012, 26, 279–283. [Google Scholar] [CrossRef]
- Moran, M.; Sikkema, P.H.; Swanton, C.J. Efficacy of saflufenacil plus dimethenamid-P for weed control in corn. Weed Technol. 2011, 25, 330–334. [Google Scholar] [CrossRef]
- Mohseni-Moghadam, M.; Doohan, D. Fomesafen crop tolerance and weed control in processing tomato. Weed Technol. 2017, 31, 441–446. [Google Scholar] [CrossRef]
- Peachey, E.; Doohan, D.; Koch, T. Selectivity of fomesafen based systems for preemergence weed control in cucurbit crops. Crop. Prot. 2012, 40, 91–97. [Google Scholar] [CrossRef]
- Eleftherohorinos, I.G.; Vasilakoglou, I.B.; Dhima, K.V. Metribuzin resistance in Amaranthus retroflexus and Chenopodium album in Greece. Weed Sci. 2000, 48, 69–74. [Google Scholar] [CrossRef]
- Hutchinson, P.J.; Ransom, C.V.; Tonks, D.J.; Eberlein, C.V. ‘Russet Burbank’ potato tolerance to dimethenamid-p. Weed Technol. 2004, 18, 850–852. [Google Scholar] [CrossRef]
- Wilson, D.E.; Nissen, S.J.; Thompson, A. Potato (Solanum tuberosum) variety and weed response to sulfentrazone and flumioxazin. Weed Technol. 2002, 16, 567–574. [Google Scholar] [CrossRef]
- Hutchinson, P.J.; Beutler, B.R.; Hancock, D.M. Weed control and potato (Solanum tuberosum) crop response with low rates of sulfentrazone applied postemergence with metribuzin. Weed Technol. 2006, 20, 1023–1029. [Google Scholar] [CrossRef]
- Health Canada Pest Management Regulatory Agency Consumer Product Safety Label Search. Available online: https://pr-rp.hc-sc.gc.ca/ls-re/lbl_detail-eng.php?p_disp_regn=%2732621%27&p_regnum=32621 (accessed on 18 August 2020).
- Norsworthy, J.K.; Ward, S.M.; Shaw, D.R.; Llewellyn, R.S.; Nichols, R.L.; Webster, T.M.; Bradley, K.W.; Frisvold, G.; Powles, S.B.; Burgos, N.R.; et al. Reducing the risks of herbicide resistance: Best management practices and recommendations. Weed Sci. 2012, 60, 31–62. [Google Scholar] [CrossRef]
| Herbicide Active Ingredient | Herbicide Rate (g a.i. ha−1) | Trade Name | WSSA Herbicide Group | Chemical Family |
|---|---|---|---|---|
| Metribuzin | 1100 | Sencor 75DF | 5 | Triazinone |
| Linuron | 2208 | Lorox L | 7 | Substituted urea |
| S-metolachlor | 1600 | Dual II Magnum | 15 | Acetamide |
| Saflufenacil | 25.2 | Eragon | 14 | Uracil amide |
| Dimethenamid-P | 693.36 | Frontier Max | 15 | Acetamide |
| Sulfentrazone | 105.12 | Authority | 14 | Aryl triazinone |
| Fomesafen | 240 + 0.1% NIS 1 | Reflex + Agral 90 | 14 | Amide |
| Metribuzin + linuron | 825 + 1800 | Sencor 75DF + Lorox L | 5 + 7 | See above |
| S-metolachlor + metribuzin | 1570 + 372.5 | Boundary LQD | 15 + 5 | See above |
| Metribuzin + sulfentrazone | 600 + 105.12 | Sencor STZ | 5 + 14 | See above |
| Saflufenacil + dimethenamid-P | 74.8 + 660 | Integrity | 14 + 15 | See above |
| Fomesafen + S-metolachlor + metribuzin | 240 + 1570 + 372.5 + 0.1% NIS | Reflex + Boundary LQD + Agral 90 | 14 + 15 + 5 | See above |
| Location | Populations Screened | Single-Dose Assay (% Resistant) | % with Ser264Gly Mutation (Populations Screened) | ||
|---|---|---|---|---|---|
| Metribuzin 1 | Atrazine 2 | Linuron 3 | |||
| NB | 35 | 29% | 37% | 6% | 42% (121) |
| PE | 13 | 54% | 20%4 | 8% | 53% (70) |
| Herbicide | Population | d | b | LD50 kg ha−1 | LD90 kg ha−1 | Resistance Index |
|---|---|---|---|---|---|---|
| Metribuzin | S | 0.72 ± 0.05 | 0.53 ± 0.00 | 0.003 ± 0.00 | 0.18 | – |
| R1 | 1.57 ± 0.20 | 2.21 ± 1.04 | 0.38 ± 0.10 | 1.02 | 127 | |
| R2 | 4.90 ± 0.29 | 1.33 ± 0.24 | 0.79 ± 0.14 | 4.08 | 263 | |
| R3 | 6.07 ± 0.28 | 1.48 ± 0.22 | 0.76 ± 0.10 | 3.36 | 253 | |
| R4 | 2.29 ± 0.19 | 1.90 ± 0.59 | 0.61 ± 0.12 | 1.92 | 203 | |
| Linuron | S | 1.26 ± 0.09 | 0.57 ± 0.28 | 0.005 ± 0.01 | 0.13 | – |
| R1 | 1.81 ± 0.05 | 5.29 ± 0.54 | 0.04 ± 0.00 | 0.05 | – | |
| R2 | 1.31 ± 0.07 | 0.65 ± 0.17 | 0.01 ± 0.01 | 0.22 | – | |
| R3 | 1.02 ± 0.06 | 2.22 ± 0.62 | 0.03 ± 0.00 | 0.06 | – | |
| R4 | 1.74 ± 0.06 | 7.86 ± 1.17 | 0.04 ± 0.00 | 0.05 | – |
| Cross Resistance Pattern | |||
|---|---|---|---|
| Population | Metribuzin | Atrazine | Linuron |
| NB5 | H | H | H |
| NB6 | L | H | L |
| NB7 | L | H | L |
| PE16 | S | L | L |
| Treatment | Common Lambsquarters | ||
|---|---|---|---|
| 4 WAA 1 (% Control) | 8 WAA (% Control) | Biomass (g m−2) | |
| Weed-free control | 100 | 100 | 0.0 |
| Weedy control | 0 | 0 | 63.2 a |
| Metribuzin | 81 ab | 88 ab | 3.2 b |
| Linuron | 89 ab | 77 ab | 1.9 b |
| S-metolachlor | 65 b | 65 ab | 26.8 ab |
| Saflufenacil | 84 ab | 69 ab | 9.0 b |
| Dimethenamid-P | 68 ab | 66 ab | 14.8 b |
| Sulfentrazone | 79 ab | 86 ab | 12.2 b |
| Fomesafen | 82 ab | 62 b | 19.1 b |
| Metribuzin + linuron | 91 ab | 84 ab | 16.3 b |
| S-metolachlor + metribuzin | 90 ab | 92 ab | 2.7 b |
| Metribuzin + sulfentrazone | 95 ab | 93 ab | 1.6 b |
| Saflufenacil + dimethenamid-P | 97 a | 92 ab | 2.8 b |
| Fomesafen + S-metolachlor + metribuzin | 99 a | 95 a | 0.3 b |
| Treatment | Total Tuber Yield (T ha−1) | Marketable Yield (T ha−1) | Can#1 Yield (T ha−1) |
|---|---|---|---|
| Weed-free control | 34.94 | 30.75 | 20.99 |
| Weedy control | 29.39 | 27.11 | 16.19 |
| Metribuzin | 34.95 | 31.87 | 19.74 |
| Linuron | 32.67 | 31.10 | 19.43 |
| S-metolachlor | 34.79 | 32.18 | 21.67 |
| Saflufenacil | 29.39 | 27.73 | 18.16 |
| Dimethenamid-P | 36.24 | 33.98 | 22.73 |
| Sulfentrazone | 32.76 | 30.62 | 17.89 |
| Fomesafen | 33.83 | 31.92 | 21.15 |
| Metribuzin + linuron | 34.12 | 31.75 | 19.10 |
| S-metolachlor + metribuzin | 33.70 | 31.08 | 19.36 |
| Metribuzin + sulfentrazone | 37.47 | 33.72 | 22.46 |
| Saflufenacil + dimethenamid-P | 34.77 | 31.26 | 20.52 |
| Fomesafen + S-metolachlor + metribuzin | 33.73 | 30.81 | 20.12 |
| Contrasts | p value | ||
| Weed-free vs. weedy | 0.067 | 0.172 | 0.064 |
| Weedy vs. metribuzin | 0.066 | 0.076 | 0.167 |
| Weedy vs. linuron | 0.272 | 0.135 | 0.206 |
| Weedy vs. S-metolachlor | 0.074 | 0.060 | 0.036 |
| Weedy vs. saflufenacil | 0.999 | 0.815 | 0.439 |
| Weedy vs. dimethenamid-P | 0.025 | 0.012 | 0.013 |
| Weedy vs. sulfentrazone | 0.259 | 0.187 | 0.503 |
| Weedy vs. fomesafen | 0.139 | 0.073 | 0.056 |
| Weedy vs. metribuzin + linuron | 0.116 | 0.084 | 0.255 |
| Weedy vs. S-metolachlor + metribuzin | 0.151 | 0.137 | 0.215 |
| Weedy vs. metribuzin + sulfentrazone | 0.009 | 0.016 | 0.017 |
| Weedy vs. saflufenacil + dimethenamid-P | 0.075 | 0.120 | 0.094 |
| Weedy vs. fomesafen + S-metolachlor + metribuzin | 0.148 | 0.670 | 0.127 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McKenzie-Gopsill, A.; Graham, G.; Laforest, M.; Ibarra, S.; Hann, S.; Wagg, C. Occurrence and Management of PSII-Inhibitor-Resistant Chenopodium album L. in Atlantic Canadian Potato Production. Agronomy 2020, 10, 1369. https://doi.org/10.3390/agronomy10091369
McKenzie-Gopsill A, Graham G, Laforest M, Ibarra S, Hann S, Wagg C. Occurrence and Management of PSII-Inhibitor-Resistant Chenopodium album L. in Atlantic Canadian Potato Production. Agronomy. 2020; 10(9):1369. https://doi.org/10.3390/agronomy10091369
Chicago/Turabian StyleMcKenzie-Gopsill, Andrew, Gavin Graham, Martin Laforest, Sebastian Ibarra, Sheldon Hann, and Cameron Wagg. 2020. "Occurrence and Management of PSII-Inhibitor-Resistant Chenopodium album L. in Atlantic Canadian Potato Production" Agronomy 10, no. 9: 1369. https://doi.org/10.3390/agronomy10091369
APA StyleMcKenzie-Gopsill, A., Graham, G., Laforest, M., Ibarra, S., Hann, S., & Wagg, C. (2020). Occurrence and Management of PSII-Inhibitor-Resistant Chenopodium album L. in Atlantic Canadian Potato Production. Agronomy, 10(9), 1369. https://doi.org/10.3390/agronomy10091369





