Chlorophyll Fluorescence Kinetics May Be Useful to Identify Early Drought and Irrigation Effects on Photosynthetic Apparatus in Field-Grown Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Climate Conditions
2.2. Chlorophyll Fluorescence Measurements
2.3. Measurements of Chlorophyll Content
2.4. Assessment of Reflectance-Based Vegetation Index (NDVI)
2.5. Assessment of the Crop Water Status
2.6. Analysis of the Canopy Absorbance
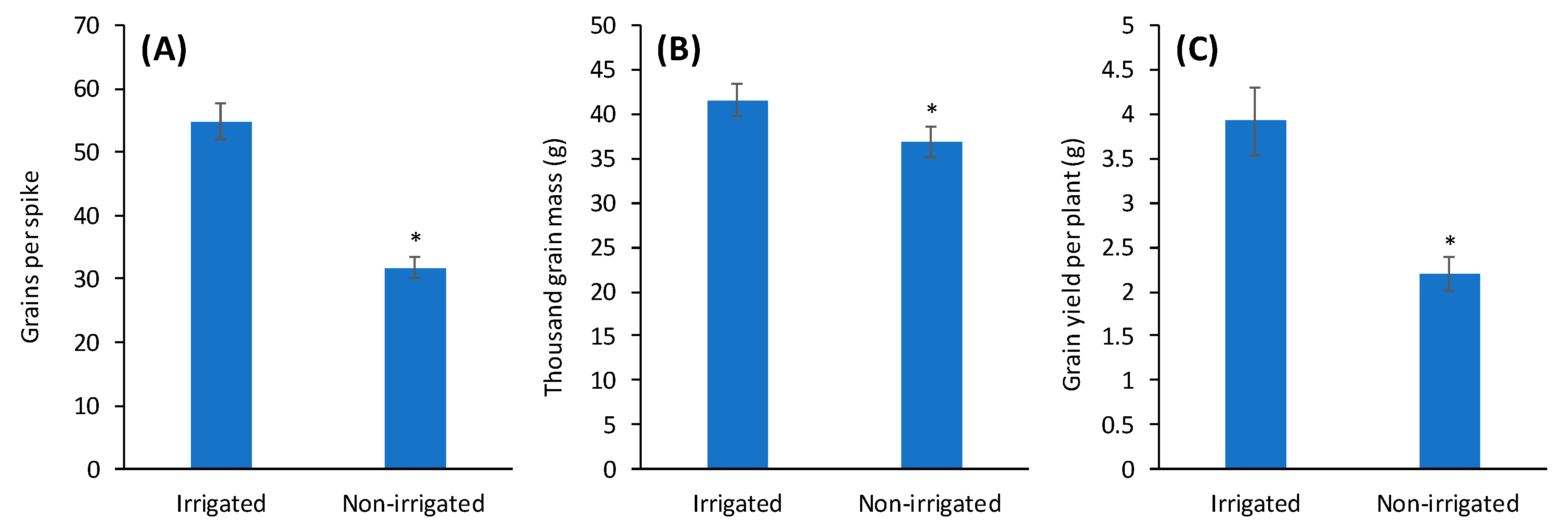
2.7. Analysis of the Yield Components
2.8. Statistical Analyses
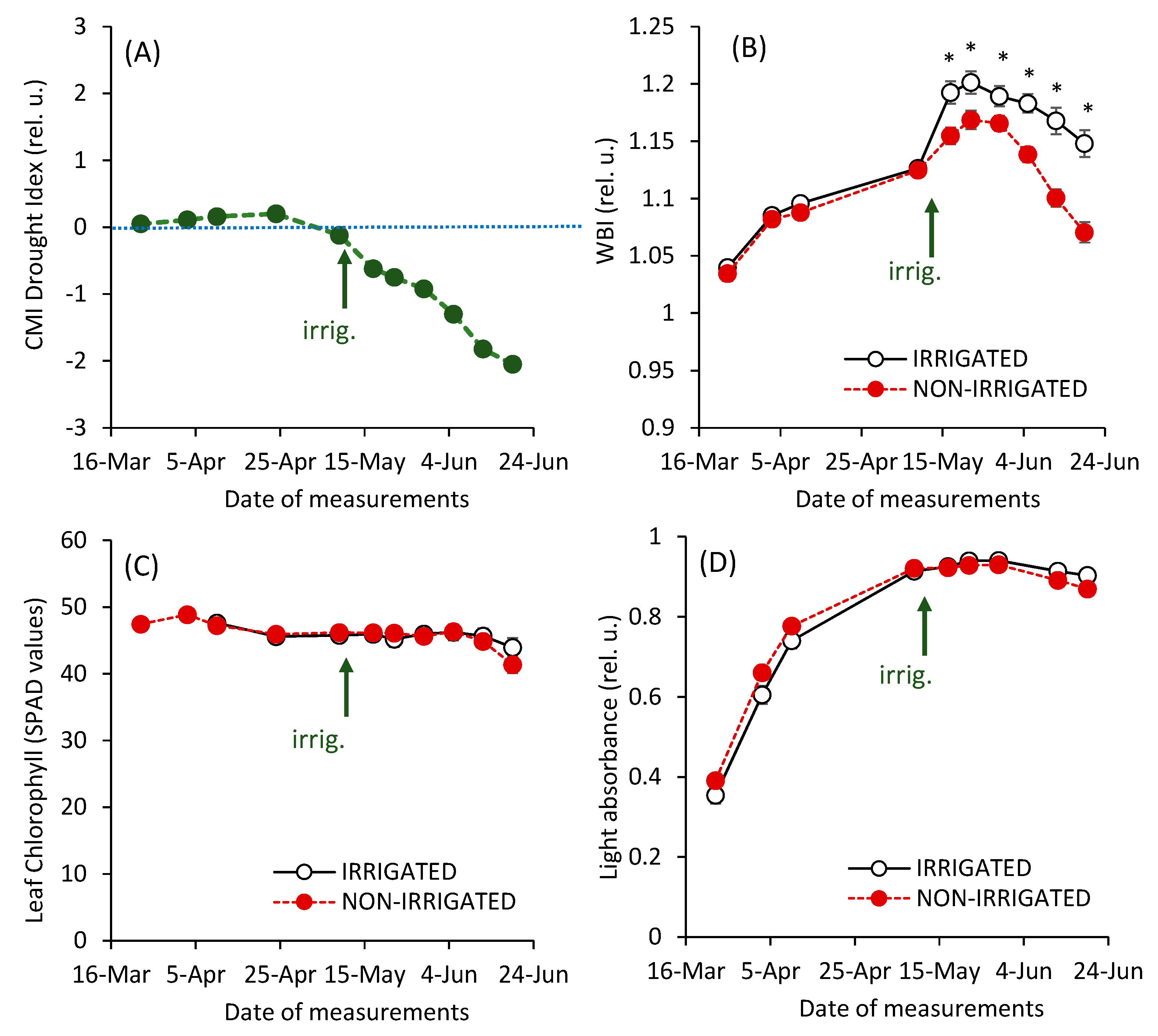
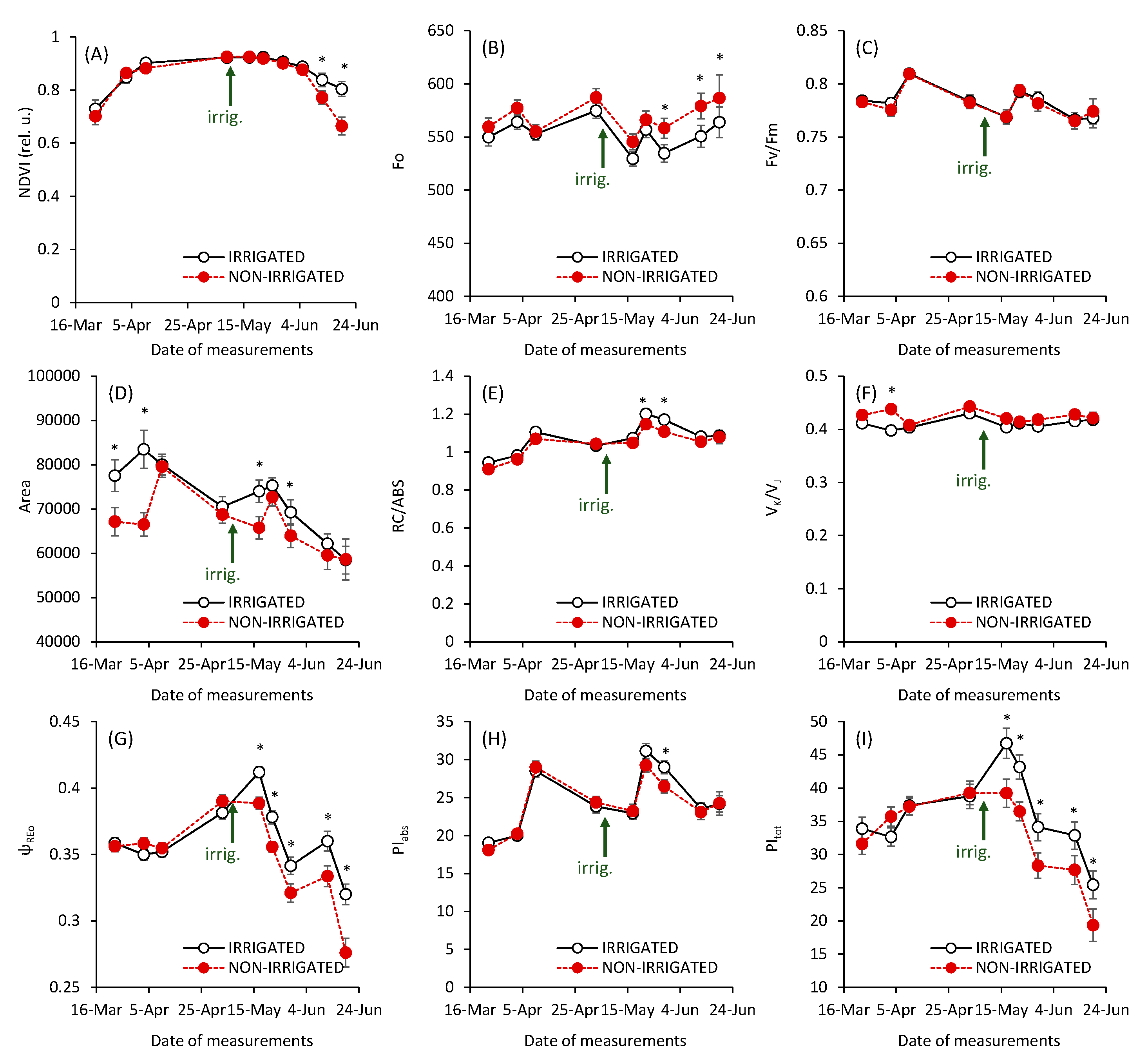
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fujimori, S.; Iizumi, T.; Hasegawa, T.; Takakura, J.Y.; Takahashi, K.; Hijioka, Y. Macroeconomic Impacts of Climate Change Driven by Changes in Crop Yields. Sustainability 2018, 10, 3673. [Google Scholar] [CrossRef]
- Nelson, G.C.; Valin, H.; Sands, R.D.; Havlik, P.; Ahammad, H.; Deryng, D.; Elliott, J.; Fujimori, S.; Hasegawa, T.; Heyhoe, E.; et al. Climate change effects on agriculture: Economic responses to biophysical shocks. Proc. Natl. Acad. Sci. USA 2014, 111, 3274–3279. [Google Scholar] [CrossRef]
- Carvalho, F.P. Agriculture, pesticides, food security and food safety. Environ. Sci. Policy 2006, 9, 685–692. [Google Scholar] [CrossRef]
- Tester, M.; Langridge, P. Breeding Technologies to Increase Crop Production in a Changing World. Science 2010, 327, 818–822. [Google Scholar] [CrossRef]
- Förch, W.; Kristjanson, P.; Cramer, L.; Barahona, C.; Thornton, P.K. Back to baselines: Measuring change and sharing data. Agric. Food Secur. 2014, 3, 13. [Google Scholar] [CrossRef][Green Version]
- Eckardt, N.A.; Cominelli, E.; Galbiati, M.; Tonelli, C. The Future of Science: Food and Water for Life. Plant Cell 2009, 21, 368–372. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Nisbett, N.; Pretty, J.; Robinson, S.; Toulmin, C.; Whiteley, R. The future of the global food system. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2769–2777. [Google Scholar] [CrossRef]
- Podlich, D.W.; Winkler, C.R.; Cooper, M. Mapping As You Go. Crop. Sci. 2004, 44, 1560–1571. [Google Scholar] [CrossRef]
- Govindaraj, M.; Vetriventhan, M.; Srinivasan, M. Importance of Genetic Diversity Assessment in Crop Plants and Its Recent Advances: An Overview of Its Analytical Perspectives. Genet. Res. Int. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Araus, J.L.; Kefauver, S.C.; Zaman-Allah, M.; Olsen, M.S.; Cairns, J.E. Translating High-Throughput Phenotyping into Genetic Gain. Trends Plant Sci. 2018, 23, 451–466. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Araus, J.L.; Crossa, J.; Cairns, J.E.; Palacios, N.; Mahuku, G.; Das, B.; Magorokosho, C. High-throughput and precision phenotyping in cereal breeding programs. In Cereal Genomics-II; Gupta, P.K., Varshney, R.K., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 341–374. [Google Scholar]
- Xu, Y. Envirotyping for deciphering environmental impacts on crop plants. Theor. Appl. Genet. 2016, 129, 653–673. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ganapathysubramanian, B.; Singh, A.K.; Sarkar, S. Machine Learning for High-Throughput Stress Phenotyping in Plants. Trends Plant Sci. 2016, 21, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Cen, H.; Weng, H.; Yao, J.; He, M.; Lv, J.; Hua, S.; Li, H.; He, Y. Chlorophyll Fluorescence Imaging Uncovers Photosynthetic Fingerprint of Citrus Huanglongbing. Front. Plant Sci. 2017, 8, 8. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Rastogi, A.; Zivcak, M.; Brestic, M.; Daszkowska-Golec, A.; Sitko, K.; Alsharafa, K.Y.; Lotfi, R.; Stypinski, P.; Samborska, I.A.; et al. Prompt chlorophyll fluorescence as a tool for crop phenotyping: An example of barley landraces exposed to various abiotic stress factors. Photosynthetica 2018, 56, 953–961. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Hauptvogel, P.; Misheva, S.; Kocheva, K.; Yang, X.; Li, X.; Allakhverdiev, S.I. Wheat plant selection for high yields entailed improvement of leaf anatomical and biochemical traits including tolerance to non-optimal temperature conditions. Photosynth. Res. 2018, 136, 245–255. [Google Scholar] [CrossRef]
- Zivcak, M.; Kalaji, H.M.; Shao, H.-B.; Olsovska, K.; Brestic, M. Photosynthetic proton and electron transport in wheat leaves under prolonged moderate drought stress. J. Photochem. Photobiol. B Biol. 2014, 137, 107–115. [Google Scholar] [CrossRef]
- Zivcak, M.; Olsovska, K.; Slamka, P.; Galambošová, J.; Rataj, V.; Shao, H.B.; Brestic, M. Application of chlorophyll fluorescence performance indices to assess the wheat photosynthetic functions influenced by nitrogen deficiency. Plant Soil Environ. 2014, 60, 210–215. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Kalaji, H.M.; Carpentier, R.; Allakhverdiev, S.I. Photosystem II thermostability in situ: Environmentally induced acclimation and genotype-specific reactions in Triticum aestivum L. Plant Physiol. Biochem. 2012, 57, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Zivcak, M.; Brestic, M.; Olsovska, K.; Slamka, P. Performance index as a sensitive indicator of water stress in Triticum aestivum L. Plant Soil Environ. 2008, 54, 133–139. [Google Scholar] [CrossRef]
- Rastogi, A.; Kovar, M.; He, X.; Zivcak, M.; Kataria, S.; Kalaji, H.M.; Skalicky, M.; Ibrahimova, U.F.; Hussain, S.; Mbarki, S.; et al. Special issue in honour of Prof. Reto J. Strasser—JIP-test as a tool to identify salinity tolerance in sweet sorghum genotypes. Photosynthetica 2020, 58, 518–528. [Google Scholar] [CrossRef]
- Faseela, P.; Sinisha, A.K.; Brestic, M.; Puthur, J. Special issue in honour of Prof. Reto J. Strasser—Chlorophyll a fluorescence parameters as indicators of a particular abiotic stress in rice. Photosynthetica 2020, 58, 293–300. [Google Scholar] [CrossRef]
- Badr, A.; Bruggemann, W. Special issue in honour of Prof. Reto J. Strasser—Comparative analysis of drought stress response of maize genotypes using chlorophyll fluorescence measurements and leaf relative water content. Photosynthetica 2020, 58, 638–645. [Google Scholar] [CrossRef]
- Roostaei, M.; Mohammadi, S.A.; Amri, A.; Majidi, E.; Nachit, M.; Haghparast, R. Chlorophyll fluorescence parameters and drought tolerance in a mapping population of winter bread wheat in the highlands of Iran. Russ. J. Plant Physiol. 2011, 58, 351–358. [Google Scholar] [CrossRef]
- Chaichi, M.; Sanjarian, F.; Razavi, K.; Gonzalez-Hernandez, J.L. Phenotypic diversity among Iranian bread wheat landraces, as a screening tool for drought tolerance. Acta Physiol. Plant. 2019, 41, 90. [Google Scholar] [CrossRef]
- Palmer, W.C. Meteorologic Drought; Weather Bureau, Research Paper No. 45; US Department of Commerce, Weather Bureau: Washington, DC, USA, 1965.
- Strasser, R.J.; Srivastava, A.; Govindjee, G. Polyphasic chlorophyll a fluorescence transient inplants and cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation & Adaptation; Yunus, M., Pathre, U., Eds.; Taylor & Francis: London, UK, 2000; pp. 443–480. [Google Scholar]
- Rouse, J.W.; Haas, R.H.; Shell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS; NASA Scientific and Technical Information Office: Washington, DC, USA, 1974.
- Peñuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Save, R. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Hlavinka, P.; Trnka, M.; Semerádová, D.; Dubrovský, M.; Zalud, Z.; Možný, M. Effect of drought on yield variability of key crops in Czech Republic. Agric. For. Meteorol. 2009, 149, 431–442. [Google Scholar] [CrossRef]
- Hänsel, S.; Ustrnul, Z.; Łupikasza, E.; Skalák, P. Assessing seasonal drought variations and trends over Central Europe. Adv. Water Resour. 2019, 127, 53–75. [Google Scholar] [CrossRef]
- Pu, R.; Ge, S.; Kelly, N.M.; Gong, P. Spectral absorption features as indicators of water status in coast live oak (Quercus agrifolia) leaves. Int. J. Remote Sens. 2003, 24, 1799–1810. [Google Scholar] [CrossRef]
- Claudio, H.C.; Cheng, Y.; Fuentes, D.A.; Gamon, J.A.; Luo, H.; Oechel, W.; Qiu, H.L.; Rahman, A.F.; Sims, D. Monitoring drought effects on vegetation water content and fluxes in chaparral with the 970 nm water band index. Remote Sens. Environ. 2006, 103, 304–311. [Google Scholar] [CrossRef]
- Ranjan, R.; Sahoo, R.N.; Chopra, U.K.; Pramanik, M.; Singh, A.K.; Pradhan, S. Assessment of Water Status in Wheat (Triticum aestivum L.) Using Ground Based Hyperspectral Reflectance. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 377–388. [Google Scholar] [CrossRef]
- Hailemichael, G.; Catalina, A.; González, M.R.; Martin, P. Relationships between Water Status, Leaf Chlorophyll Content and Photosynthetic Performance in Tempranillo Vineyards. S. Afr. J. Enol. Vitic. 2016, 37, 149–156. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Balatova, Z.; Drevenakova, P.; Olsovska, K.; Kalaji, H.M.; Yang, X.; Allakhverdiev, S.I. Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynth. Res. 2013, 117, 529–546. [Google Scholar] [CrossRef]
- Samarah, N.H. Effects of drought stress on growth and yield of barley. Agron. Sustain. Dev. 2005, 25, 145–149. [Google Scholar] [CrossRef]
- Kaur, V.; Behl, R.K. Grain yield in wheat as affected by short periods of high temperature, drought and their interaction during pre- and post-anthesis stages. Cereal Res. Commun. 2010, 38, 514–520. [Google Scholar] [CrossRef]
- Ahmed, N.; Chowdhry, M.A.; Khaliq, I.; Maekawa, M. The inheritance of yield and yield components of five wheat hybrid populations under drought conditions. Indones. J. Agric. Sci. 2016, 8, 53–59. [Google Scholar] [CrossRef]
- Hassan, M.A.; Yang, M.; Rasheed, A.; Yang, G.; Reynolds, M.; Xia, X.; Xiao, Y.; He, Z. A rapid monitoring of NDVI across the wheat growth cycle for grain yield prediction using a multi-spectral UAV platform. Plant Sci. 2018, 282, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Jamali, S.; Seaquist, J.; Ardö, J.; Eklundh, L. Investigating temporal relationships between rainfall, soil moisture and MODIS-derived NDVI and EVI for six sites in Africa. Savanna 2011, 21, 38. [Google Scholar]
- Ahmed, M.; Else, B.; Eklundh, L.; Ardö, J.; Seaquist, J. Dynamic response of NDVI to soil moisture variations during different hydrological regimes in the Sahel region. Int. J. Remote Sens. 2017, 38, 5408–5429. [Google Scholar] [CrossRef]
- Liu, S.; Roberts, D.A.; Chadwick, O.A.; Still, C.J. Spectral responses to plant available soil moisture in a Californian grassland. Int. J. Appl. Earth Obs. Geoinf. 2012, 19, 31–44. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Low-Oxygen Stress in Plants; Springer Science and Business Media LLC: Basel, Switzerland, 2004; Volume 19, pp. 321–362. [Google Scholar]
- Stirbet, A. Govindjee on the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Zlatev, Z.S. Drought-Induced Changes in Chlorophyll Fluorescence of Young Wheat Plants. Biotechnol. Biotechnol. Equip. 2009, 23, 438–441. [Google Scholar] [CrossRef]
- Wang, G.P.; Li, F.; Zhang, J.; Zhao, M.R.; Hui, Z.; Wang, W. Overaccumulation of glycine betaine enhances tolerance of the photosynthetic apparatus to drought and heat stress in wheat. Photosynthetica 2010, 48, 30–41. [Google Scholar] [CrossRef]
- Li, G.L.; Wu, H.X.; Sun, Y.Q.; Zhang, S.Y. Response of chlorophyll fluorescence parameters to drought stress in sugar beet seedlings. Russ. J. Plant Physiol. 2013, 60, 337–342. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Kunderlikova, K.; Sytar, O.; Shao, H.; Kalaji, H.M.; Allakhverdiev, S.I. Low PSI content limits the photoprotection of PSI and PSII in early growth stages of chlorophyll b-deficient wheat mutant lines. Photosynth. Res. 2015, 125, 151–166. [Google Scholar] [CrossRef]
- Srivastava, A.; Guissé, B.; Greppin, H.; Strasser, R.J. Regulation of antenna structure and electron transport in PSII of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP. Biochim. Biophys. 1997, 1320, 95–106. [Google Scholar] [CrossRef]
- Cascio, C.; Schaub, M.; Novak, K.; Desotgiu, R.; Bussotti, F.; Strasser, R.J. Foliar responses to ozone of Fagus sylvatica L. seedlings grown in shaded and in full sunlight conditions. Environ. Exp. Bot. 2010, 68, 188–197. [Google Scholar] [CrossRef]
- Bussotti, F.; Nali, C.; Lorenzini, G. Chlorophyll fluorescence: From theory to (good) practice. An introduction. Environ. Exp. Bot. 2011, 73, 1–2. [Google Scholar] [CrossRef]
- Nikiforou, C.; Manetas, Y. Inherent nitrogen deficiency in Pistacia lentiscus preferentially affects photosystem I: A seasonal field study. Funct. Plant Biol. 2011, 38, 848–855. [Google Scholar] [CrossRef]
- Pollastrini, M.; Desotgiu, R.; Cascio, C.; Bussotti, F.; Cherubini, P.; Saurer, M.; Gerosa, G.; Marzuoli, R. Growth and physiological responses to ozone and mild drought stress of tree species with different ecological requirements. Trees 2010, 24, 695–704. [Google Scholar] [CrossRef]
- Pšidová, E.; Zivcak, M.; Stojnić, S.; Orlović, S.; Gömöry, D.; Kučerová, J.; Ditmarová, Ľ.; Střelcová, K.; Brestic, M.; Kalaji, H.M. Altitude of origin influences the responses of PSII photochemistry to heat waves in European beech (Fagus sylvatica L.). Environ. Exp. Bot. 2018, 152, 97–106. [Google Scholar] [CrossRef]
- Pollastrini, M.; Salvatori, E.; Fusaro, L.; Manes, F.; Marzuoli, R.; Gerosa, G.; Brüggemann, W.; Strasser, R.J.; Bussotti, F. Selection of tree species for forests under climate change: Is PSI functioning a better predictor for net photosynthesis and growth than PSII? Tree Physiol. 2020, tpaa084. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Kunderlikova, K.; Olsovska, K.; Allakhverdiev, S.I. Effect of photosystem I inactivation on chlorophyll a fluorescence induction in wheat leaves: Does activity of photosystem I play any role in OJIP rise? J. Photochem. Photobiol. B Biol. 2015, 152, 318–324. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazár, D.; Kromdijk, J.; Govindjee, G. Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M.; Strasser, R.J. In vivo Assessment of Stress Impact on Plant’s Vitality: Applications in Detecting and Evaluating the Beneficial Role of Mycorrhization on Host Plants. In Mycorrhiza; Springer: Berlin/Heidelberg, Germany, 2008; pp. 679–703. [Google Scholar]
- Zhu, Y.; Yu, L.; Fan, J.; Ruan, Y.; Cui, Z.; Hu, K.; Zhang, F. Characteristics of chlorophyll fluorescence parameters of leaves during leaf elongation of maize seedling. J. Henan Agric. Sci. 2011, 40, 36–42. [Google Scholar]
- Begovic, L.; Galic, V.; Abicic, I.; Loncaric, Z.; Lalic, A.; Mlinaric, S. Implications of intra-seasonal climate variations on chlorophyll a fluorescence and biomass in winter barley breeding program. Photosynthetica 2020, 58, 995–1008. [Google Scholar] [CrossRef]
- Wang, Y.W.; Xu, C.; Lv, C.F.; Wu, M.; Cai, X.J.; Liu, Z.T.; Song, X.M.; Chen, G.; Lv, C.G. Chlorophyll a fluorescence analysis of high-yield rice (Oryza sativa L.) LYPJ during leaf senescence. Photosynthetica 2016, 54, 422–429. [Google Scholar] [CrossRef]
| IX | X | XI | XII | I | II | III | IV | V | VI | III-VI | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rainfall (mm) | 1951–2000 | 45 | 42 | 48 | 46 | 30 | 31 | 31 | 41 | 59 | 76 | 207 |
| Season | 39 | 50 | 34 | 15 | 25 | 49 | 19 | 48 | 27 | 20 | 114 | |
| Difference | −6 | +8 | −14 | −31 | −5 | +18 | −12 | +7 | −32 | −56 | −93 (−45%) | |
| Temperature (°C) | 1951–2000 | 14.7 | 9.7 | 4.4 | 0.2 | −1.6 | 0.2 | 4.4 | 9.6 | 14.5 | 17.7 | 11.55 |
| Season | 17.4 | 9.2 | 4.8 | −0.5 | −6.7 | 2 | 7.8 | 9.3 | 15.6 | 20.7 | 13.35 | |
| Difference | +2.7 | −0.5 | +0.4 | −0.7 | −5.1 | +1.8 | +3.4 | −0.3 | +1.1 | +3 | +1.8 |
| Parameter | Definition | Formula |
|---|---|---|
| Fo, Fm | Minimum and maximum fluorescence intensity | |
| Ft | Fluorescence intensity in time t | |
| Mo | Initial slope of the fluorescence curve | |
| Area | Area between fluorescence curve and Fm | |
| Fv/Fm = φPo | Maximum quantum yield of PS II photochemistry | Fv/Fm = (Fm – Fo)/Fm |
| VK, VJ, VI | Variable fluorescence at time 0.3 ms (VK), 2 ms (VJ), and 30 ms (VI) | Vt = (Ft – F0)/(Fm – F0) |
| VK/VJ | Relative variable fluorescence of K-step; an indicator of the damage of oxygen evolving complex in PSII | |
| ψo | Probability of electron transport from PSII RC to PSII acceptor side | ψo = 1 – VJ |
| ψREo = ΔVI-P | Amplitude of variable fluorescence in I-P phase; probability of electron transport from PSII acceptor side to the PSI electron acceptors | ψREo = 1 – VI |
| RC/ABS | Number of active PSII RCs per absorbed light | RC/ABS = VJ × φPo × 1/Mo |
| PIabs | Performance Index on absorption base | PIabs = (RC/ABS) × φPo/(1 – φPo) × (ψo/(1 – ψo) |
| PItot | Total performance index | PItot = PIabs × (1 – VI)/(VI – VJ) |
| Parameter | Significance of Differences between the Variants (Irrigated vs. Non-irrigated) | F Value of the Factor * | |||
|---|---|---|---|---|---|
| 3 Weeks beforethe Start of Irrigation | 3 weeks after the Start of Irrigation | Irrigation | Genotype | Interaction (Irrigation x Genotype) | |
| PItot | ns | *** | ● 53.48 | ● 21.59 | ● 8.77 |
| ψREo | ns | *** | ◆ 13.1 | ◆ 11.1 | ◆ 3.4 |
| Fo | ns | *** | ● 62.4 | ● 21.4 | ◆ 2.5 |
| AREA | *** | *** | ● 65.3 | ▲ 18.2 | ● 10.2 |
| RC/ABS | *** | *** | ◆ 12.7 | ● 24.5 | ▲ 7.5 |
| PIabs | ns | ns | - | - | - |
| Fv/Fm | ns | ns | - | - | - |
| NDVI | ns | ns | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botyanszka, L.; Zivcak, M.; Chovancek, E.; Sytar, O.; Barek, V.; Hauptvogel, P.; Halabuk, A.; Brestic, M. Chlorophyll Fluorescence Kinetics May Be Useful to Identify Early Drought and Irrigation Effects on Photosynthetic Apparatus in Field-Grown Wheat. Agronomy 2020, 10, 1275. https://doi.org/10.3390/agronomy10091275
Botyanszka L, Zivcak M, Chovancek E, Sytar O, Barek V, Hauptvogel P, Halabuk A, Brestic M. Chlorophyll Fluorescence Kinetics May Be Useful to Identify Early Drought and Irrigation Effects on Photosynthetic Apparatus in Field-Grown Wheat. Agronomy. 2020; 10(9):1275. https://doi.org/10.3390/agronomy10091275
Chicago/Turabian StyleBotyanszka, Lenka, Marek Zivcak, Erik Chovancek, Oksana Sytar, Viliam Barek, Pavol Hauptvogel, Andrej Halabuk, and Marian Brestic. 2020. "Chlorophyll Fluorescence Kinetics May Be Useful to Identify Early Drought and Irrigation Effects on Photosynthetic Apparatus in Field-Grown Wheat" Agronomy 10, no. 9: 1275. https://doi.org/10.3390/agronomy10091275
APA StyleBotyanszka, L., Zivcak, M., Chovancek, E., Sytar, O., Barek, V., Hauptvogel, P., Halabuk, A., & Brestic, M. (2020). Chlorophyll Fluorescence Kinetics May Be Useful to Identify Early Drought and Irrigation Effects on Photosynthetic Apparatus in Field-Grown Wheat. Agronomy, 10(9), 1275. https://doi.org/10.3390/agronomy10091275








