Growth, Nutritional Quality and Health-Promoting Compounds in Chinese Kale Grown under Different Ratios of Red:Blue LED Lights
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Conditions and Light Treatments
2.2. Growth Characteristics Measurement
2.3. Sample Extraction
2.4. Pigments Content
2.5. Nutritional Quality
2.6. Amino Acids Composition and Content
2.7. Health-Promoting Compounds Content
2.8. Antioxidant Activity
2.9. Statistical Analysis
3. Results
3.1. Plant Growth and Biomass Production as Affected by Light Treatment
3.2. Effects of Light Treatments on Pigment Content in Chinese Kale
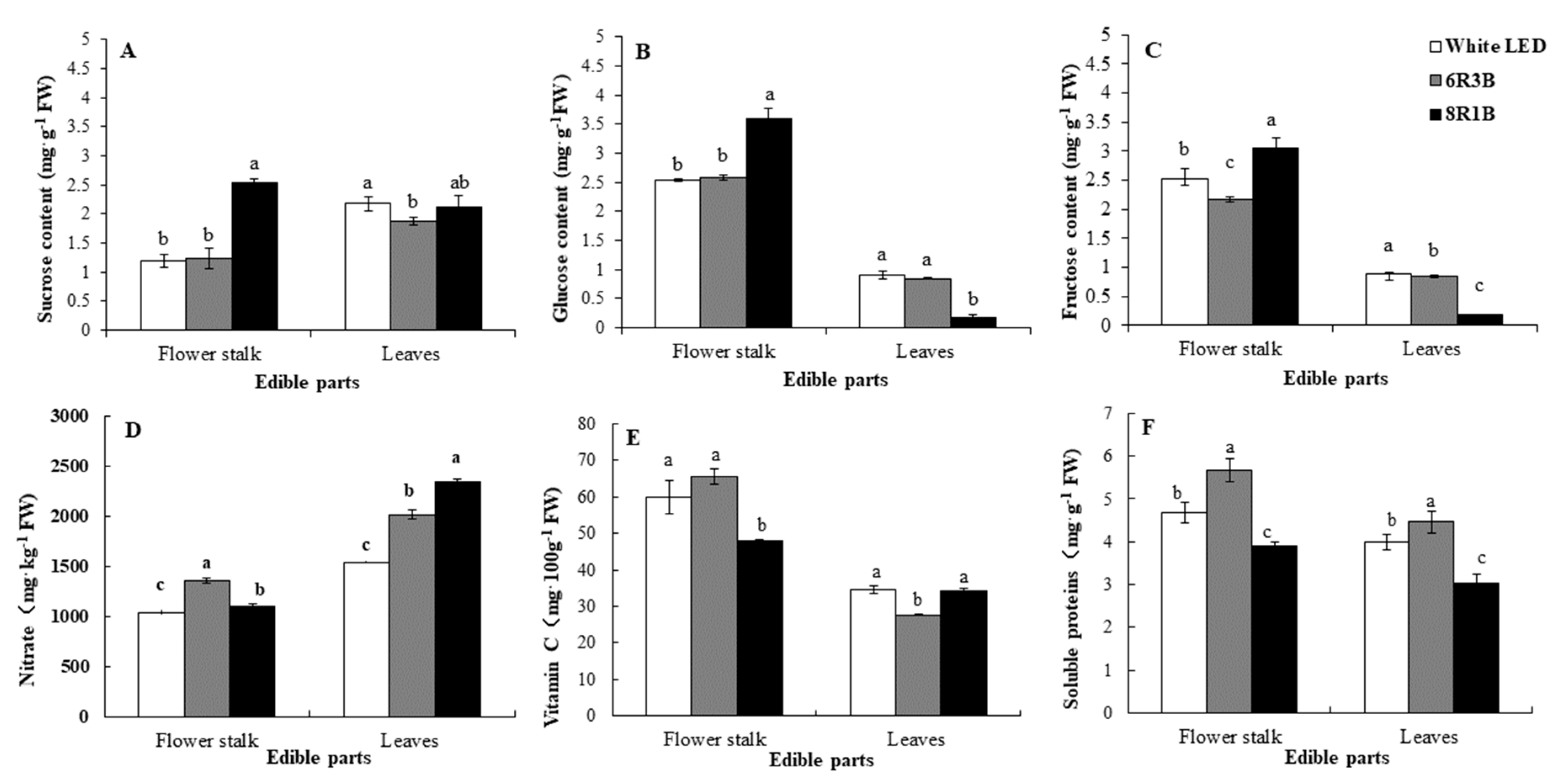
3.3. Effects of Light Treatments on Nutritional Quality in Chinese Kale
3.4. Effects of Light Treatments on Amino Acid Content in Chinese Kale
3.5. Effects of Light Treatments on Health-Promoting Compounds in Chinese Kale
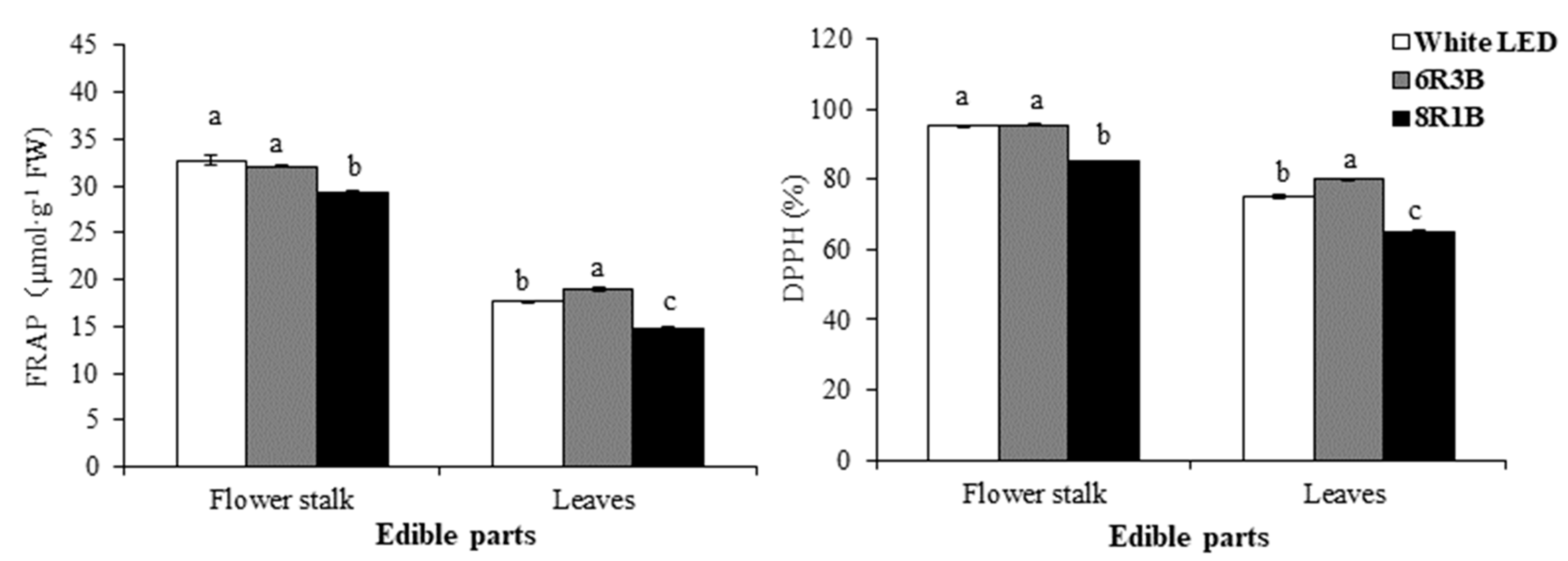
3.6. Effects of Light Treatments on Antioxidant Activity in Chinese Kale
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sun, B.; Liu, N.; Zhao, Y.T.; Yan, H.Z.; Wang, Q.M. Variation of glucosinolates in three edible parts of Chinese kale (Brassica alboglabra Bailey) varieties. Food Chem. 2011, 124, 941–947. [Google Scholar] [CrossRef]
- He, H.J.; Chen, H.; Schnitzler, W.H. Glucosinolate composition and contents in Brassica vegetables. Sci. Agric. Sin. 2002, 35, 192–197. (In Chinese) [Google Scholar]
- Sun, B.; Yan, H.Z.; Liu, N.; Wei, J.; Wang, Q.M. Effect of 1-MCP treatment on postharvest quality characters, antioxidants and glucosinolates of Chinese kale. Food Chem. 2012, 131, 519–526. [Google Scholar] [CrossRef]
- Deng, M.D.; Qian, H.M.; Chen, L.L.; Sun, B.; Chang, J.Q.; Miao, H.Y.; Cai, C.X.; Wang, Q.M. Influence of pre-harvest red light irradiation on main phytochemicals and antioxidant activity of Chinese kale sprouts. Food Chem. 2017, 222, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.I.; Kim, J.S.; Kim, D.; Kim, C.Y.; Son, J.E. Harvest strategies to maximize the annual production of bioactive compounds, glucosinolates, and total antioxidant activities of kale in plant factories. Hortic. Environ. Biotechnol. 2019, 60, 883–894. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E. Genetic and environmental factors affecting plant lutein/zeaxanthin. Agro Food Ind. Hi-Tech 2008, 19, 44–46. [Google Scholar]
- Kopsell, D.A.; Pantaniaopoulos, N.I.; Sams, C.E.; Kopsell, D.E. Shoot tissue pigment levels increase in ‘Florida Broadleaf’ mustard (Brassica juncea L.) microgreens following high light treatment. Sci. Hortic. 2012, 140, 96–99. [Google Scholar] [CrossRef]
- Goto, E. Plant production in a closed plant factory with artificial lighting. Acta Hortic. 2012, 956, 37–49. [Google Scholar] [CrossRef]
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. Jpn. Acad. Ser. B 2013, 89, 447–461. [Google Scholar] [CrossRef]
- Kozai, T. Sustainable plant factory: Closed plant production systems with artificial light for high resource use efficiencies and quality produce. Acta Hortic. 2013, 1004, 27–40. [Google Scholar] [CrossRef]
- Kozai, T. PFAL business and R&D in the world: Current status and perspectives. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Academic Press: Cambridge, UK, 2015; pp. 35–68. [Google Scholar]
- Senger, H. The effect of blue light on plants and microorganisms. Photochem. Photobiol. 1982, 35, 911–920. [Google Scholar] [CrossRef]
- Saebo, A.; Krekling, T.; Appelgren, M. Light quality affects photosynthesis and leaf anatomy of birch plantlets in vitro. Plant Cell Tissue Organ Cult. 1995, 41, 177–185. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Cho, J.Y.; Son, D.M.; Kim, J.M.; Seo, B.S.; Yang, S.Y.; Kim, B.W.; Heo, B.G. Effects of LEDs on the germination, growth and physiological activities of amaranth sprouts. Korean J. Hortic. Sci. Technol. 2008, 26, 106–112. [Google Scholar]
- Thwe, A.A.; Kim, Y.B.; Li, X.H.; Seo, J.M.; Kim, S.J.; Suzuki, T.; Chung, S.O.; Park, S.U. Effects of light-emitting diodes on expression of phenylpropanoid biosynthetic genes and accumulation of phenylpropanoids in Fagopyrum tataricum sprouts. J. Agric. Food Chem. 2014, 62, 4839–4845. [Google Scholar] [CrossRef] [PubMed]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Son, K.H.; Oh, M.M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Lee, M.J.; Son, K.H.; Oh, M.M. Increase in biomass and bioactive compounds in Lettuce under various ratios of red to far-red LED light supplemented with blue LED Light. Hortic. Environ. Biotechnol. 2016, 57, 139–147. [Google Scholar] [CrossRef]
- Piovene, C.; Orsini, F.; Bosi, S.; Sanoubar, R.; Bregola, V.; Dinelli, G.; Gianquinto, G. Optimal red:blue ratio in led lighting for nutraceutical indoor horticulture. Sci. Hortic. 2015, 193, 202–208. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red:blue ratio provided by LED lighting. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the role of red:blue LED lights on resource use efficiency and nutritional properties of indoor grown sweet basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Liu, T.; Deng, M.; Miao, H.; Cai, C.; Shen, W.; Wang, Q. Effects of light quality on main health-promoting compounds and antioxidant capacity of Chinese kale sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Gratani, L. A non-destructive method to determine chlorophyll content of leaves. Photosynthetica 1992, 26, 469–473. [Google Scholar]
- Song, J.; Huang, H.; Hao, Y.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Nutritional quality, mineral and antioxidant content in lettuce affected by interaction of light intensity and nutrient solution concentration. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Brazaitytė, A.; Sakalauskienė, S.; Samuolienė, G.; Jankauskienė, J.; Viršilė, A.; Novičkovas, A.; Sirtautas, R.; Miliauskienė, J.; Vaštakaitė, V.; Dabašinskas, L.; et al. The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef]
- Song, S.W.; Li, L.Y.; Liu, H.C.; Sun, G.W.; Chen, R.Y. Effect of ammonium and nitrate ratio on nutritional quality of flowering Chinese Cabbage. Appl. Mech. Mater. 2011, 142, 188–192. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Hu, L.P.; Liu, G.M.; Zhang, D.S.; He, H.J. Evaluation of the nutritional quality of Chinese Kale (Brassica alboglabra Bailey) Using UHPLC-Quadrupole-Orbitrap MS/MS-Based Metabolomics. Molecules 2017, 22, 1262. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Y.; Liu, H.; Li, Y.; Song, S.; Chen, R.Y.; Liu, Y.; Sun, G.W.; Hao, Y.W.; Su, W.; et al. Supplemental blue light increases growth and quality of greenhouse Pak-choi depending on cultivar and supplemental light intensity. J. Integr. Agric. 2018, 17, 2245–2256. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Heaney, R.K.; Spinks, E.A.; Fenwick, G.R. Improved method for the determination of the total glucosinolate content of rapeseed by determination of enzymically released glucose. Analyst 1988, 113, 1515–1518. [Google Scholar] [CrossRef]
- Rapisarda, P.; Fanella, F.; Maccarone, E. Reliability of analytical methods for determining anthocyanins in blood orange juices. J. Agric. Food Chem. 2000, 48, 2249–2252. [Google Scholar] [CrossRef] [PubMed]
- Javanmardi, J.; Stushnoff, C.; Locke, E.; Vivanco, J. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003, 83, 547–550. [Google Scholar] [CrossRef]
- Jia, Z.S.; Tang, M.C.; Wu, J.M. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Hao, X. Different ratios of red and blue LED light effects on coriander productivity and antioxidant properties. Acta Hortic. 2016, 1134, 223–229. [Google Scholar] [CrossRef]
- Huché-Thélier, L.; Crespel, L.; Gourrierec, J.L.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations—Perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Heo, J.; Lee, C.; Chakrabarty, D.; Paek, K. Growth responses of marigold and salvia bedding plants as affected by monochromic or mixture radiation provided by a light-emitting diode (LED). Plant Growth Regul. 2002, 38, 225–230. [Google Scholar] [CrossRef]
- Hoenecke, M.E.; Bula, R.J.; Tibbitts, T.W. Importance of “blue” photon levels for lettuce seedlings grown under red light-emitting diodes. HortScience 1992, 27, 427–430. [Google Scholar] [CrossRef]
- Brown, C.S.; Schuerger, A.C.; Sager, J.C. Growth and photomorphogenesis of pepper plants under red light-emitting diodes with supplemental blue or far-red lighting. J. Am. Soc. Hort. Sci. 1995, 120, 808–813. [Google Scholar] [CrossRef]
- Kim, H.H.; Goins, G.; Wheeler, R.M.; Sager, J.C. Green-light supplementation for enhanced lettuce growth under red- and blue-light-emitting diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef]
- Li, Y.M.; Zheng, Y.J.; Liu, H.C.; Zhang, Y.T.; Hao, Y.W.; Song, S.W.; Lei, B.F. Effect of supplemental blue light intensity on the growth and quality of Chinese kale. Hortic. Environ. Biotechnol. 2019, 60, 49–57. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbate biosynthesis and function in photo protection. Philos. Trans. R. Soc. B Biol. Sci. 2000, 355, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Kato, H.; Rhue, M.R.; Nishimura, T. Role of free amino acids and peptides in food taste. Flavor Chem. 1989, 13, 158–174. [Google Scholar]
- Nishimura, T.; Kato, H. Taste of free amino acids and peptides. Food Rev. Int. 1988, 4, 175–194. [Google Scholar] [CrossRef]
- Xiao, H.; He, D.; Xu, Y.J. Analysis and comparison of the amino acids in six varieties of purple—Caitai. Amino Acids Biot. Resour. 2008, 30, 59–62. (In Chinese) [Google Scholar]
- Kumar, V.; Sharma, A.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Ahmad, P. Differential distribution of amino acids in plants. Amino Acid. 2017, 49, 821–869. [Google Scholar] [CrossRef]
- Brown, P.D.; Tokuhisa, J.G.; Reichelt, M.; Gershenzon, J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 2003, 62, 471–481. [Google Scholar] [CrossRef]
- Bellostas, N.; Sorensen, J.C.; Sorensen, H. Profiling glucosinolates in vegetative and reproductive tissues of four Brassica species of the U-triangle for their biofumigation potential. J. Sci. Food Agric. 2007, 87, 1586–1594. [Google Scholar] [CrossRef]
- Yan, X.F.; Chen, S.X. Regulation of plant glucosinolate metabolism. Planta 2007, 226, 1343–1352. [Google Scholar] [CrossRef]
- Liu, W.; He, H.J.; Song, M. Influence of amino acids on bioactive compounds of Chinese kale. Acta Hortic. 2012, 944, 153–158. [Google Scholar] [CrossRef]
- Ninu, L.; Ahmad, M.; Miarelli, C.; Cashmore, A.R.; Giuliano, G. Cryptovhrome 1 controls tomato development in response to blue light. Plant J. 2002, 18, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Giliberto, L.; Perrotta, G.; Pallara, P.; Weller, J.L.; Fraser, P.D.; Bramley, P.M.; Giuliano, G. Manipulation of the blue light photoreceptor cryptochrome 2 in tomato affects vegetative development, flowering time, and fruit antioxidant content. Plant Physiol. 2005, 137, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xing, T.; Wang, X.J. The role of light in the regulation of anthocyanin accumulation in Gerbera hybrid. Plant Growth Regul. 2004, 44, 243–250. [Google Scholar] [CrossRef]
- Shoji, K.; Goto, E.; Hashida, S.; Goto, F.; Yoshihara, T. Effect of light quality on the polyphenol content and antioxidant activity of sweet basil (Ocimum basilicum L.). Acta Hortic. 2011, 907, 95–99. [Google Scholar] [CrossRef]
- Stutte, G.; Edney, S.; Skerritt, T. Photoregulation of bioprotectant content of red leaf lettuce with light-emitting diodes. HortScience 2009, 44, 79–82. [Google Scholar] [CrossRef]
- Yorio, N.C.; Goins, G.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. Hortscience 2001, 36, 380–383. [Google Scholar] [CrossRef]
- Goins, G.; Ruffe, L.M.; Cranston, N.A.; Yorio, N.C.; Wheeler, R.; Sager, J.C. Salad Crop Production under Different Wavelengths of Red Light-Emitting Diodes (LED); SAE Technical Papers; SAE International: Warrendale, PA, USA, 2001. [Google Scholar]
- Goins, G.; Yorio, N.C.; Vivenzio, H. Performance of salad-type plants using lighting and nutrient delivery concepts intended for spaceflight. J. Aerosp. 1998, 107, 284–289. [Google Scholar]
- Smith, H.L.; McAusland, L.; Murchie, E.H. Don’t ignore the green light: Exploring diverse roles in plant processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
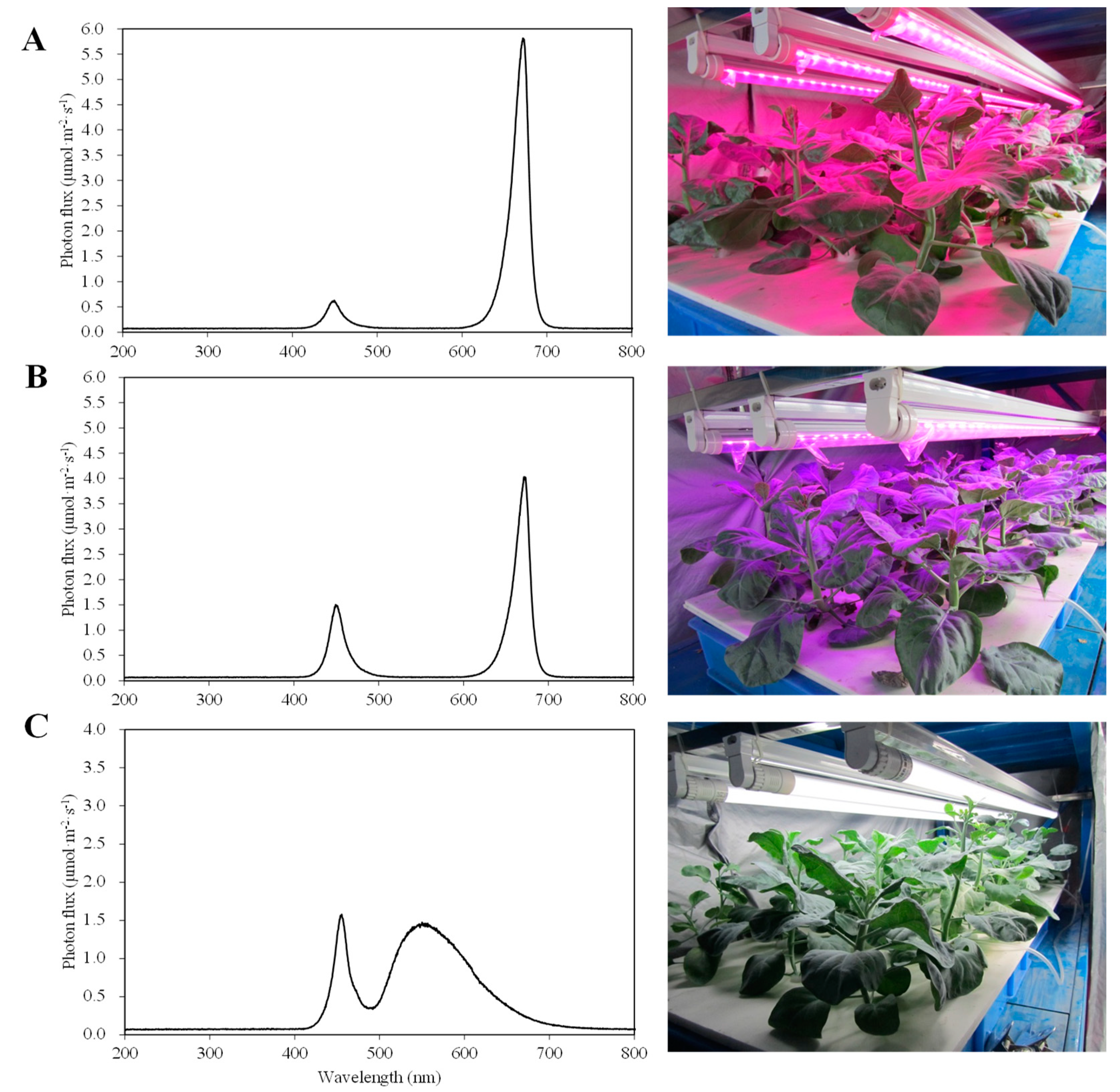

| Treatment | Flower Stalk Height (cm) | Flower Stalk Diameter (mm) | Leaf Traits | Weight (g per Plant) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Number | Length (cm) | Width (cm) | Shoot FW | Plant FW | Shoot DW | Plant DW | |||
| White LED | 22.8 ± 0.5 c | 10.3 ± 0.2 a | 13.2 ± 0.8 b | 10.3 ± 0.4 b | 9.5 ± 0.4 a | 36.1 ± 3.5 b | 39.1 ± 3.2 b | 3.1 ± 0.2 b | 3.4 ± 0.3 a |
| 6R3B | 23.8 ± 0.6 b | 9.8 ± 0.4 b | 12.8 ± 0.5 b | 9.7 ± 0.2 c | 8.9 ± 0.3 b | 33.6 ± 2.3 b | 37.0 ± 2.1 b | 2.3 ± 0.2 c | 2.6 ± 0.2 b |
| 8R1B | 25.5 ± 0.8 a | 10.6 ± 0.3 a | 15.0 ± 1.0 a | 11.3 ± 0.2 a | 9.8 ± 0.3 a | 41.8 ± 2.3 a | 44.9 ± 3.3 a | 3.4 ± 0.3 a | 3.6 ± 0.2 a |
| Light | Photosynthetic Pigment Content (mg/g FW) | |||||
|---|---|---|---|---|---|---|
| Chl a | Chl b | Total Chl | Carotenoids | β-carotene (μg/g DW) | Chl a/chl b | |
| White LED | 1.46 ± 0.04 a | 0.57 ± 0.04 a | 2.06 ± 0.07 a | 0.16 ± 0.01 b | 28.63 ± 0.08b | 2.56 ± 0.04 c |
| 6R3B | 1.41 ± 0.01 a | 0.50 ± 0.03 b | 1.93 ± 0.02 b | 0.17 ± 0.01 b | 36.20 ± 0.71a | 2.82 ± 0.03 b |
| 8R1B | 1.32 ± 0.01 b | 0.42 ± 0.02 c | 1.76 ± 0.02 c | 0.21 ± 0.01 a | 36.78 ± 0.47a | 3.14 ± 0.03 a |
| Amino Acids Composition and Content (mg/100 g FW) | Abbreviations | Light Combination | ||
|---|---|---|---|---|
| White LED | 6R3B | 8R1B | ||
| Essential amino acids (EAAs) | ||||
| Threonine A | Thr | 5.01 ± 0.41 b | 6.30 ± 0.08 a | 6.57 ± 0.13 a |
| Valine BD | Val | 2.32 ± 0.03 b | 3.10 ± 0.05 a | 3.01 ± 0.16 a |
| Mmethionine BD | Met | 0.28 ± 0.02 b | 0.82 ± 0.08 a | 0.21 ± 0.01 a |
| Isoleucine BD | Ile | 1.85 ± 0.04 c | 2.91 ± 0.06 a | 2.67 ± 0.03 b |
| Leucine BD | Leu | 1.25 ± 0.01 c | 2.86 ± 0.08 a | 2.20 ± 0.01 b |
| Phenylalanine BD | Phe | 2.22 ± 0.17 c | 3.47 ± 0.29 a | 2.73 ± 0.08 b |
| Lysine A | Lys | 2.63 ± 0.13 c | 4.35 ± 0.07 a | 3.57 ± 0.08 b |
| Non-essential amino acids (NEAAs) | ||||
| Histidine B | His | 4.07 ± 0.05 c | 5.62 ± 0.07 a | 5.29 ± 0.18 b |
| Arginine B | Arg | 30.37 ± 0.44 c | 43.38 ± 0.50 b | 45.37 ± 0.70 a |
| Phosphatidylserine | P-Ser | 6.00 ± 0.22 a | 3.81 ± 0.05 b | 3.93 ± 0.12 b |
| Aspartic acid C | Asp | 20.75 ± 0.97 c | 28.78 ± 0.58 a | 26.65 ± 0.70 b |
| Serine A | Ser | 9.56 ± 0.25 b | 8.08 ± 0.25 c | 10.89 ± 0.14 a |
| L-Asparagine | ASPNH2 | 8.23 ± 0.40 c | 9.22 ± 0.50 b | 10.40 ± 0.56 a |
| Glutamic acid C | Glu | 27.36 ± 1.50 c | 36.50 ± 1.31 a | 33.13 ± 0.34 b |
| Glutamine A | GluNH2 | 11.45 ± 0.49 b | 9.98 ± 0.55 c | 15.51 ± 0.22 a |
| Glycine A | Gly | 1.10 ± 0.07 a | 0.83 ± 0.04 b | 1.12 ± 0.03 a |
| Alanine A | Ala | 8.87 ± 0.37 b | 8.93 ± 0.30 b | 11.04 ± 0.19 a |
| Cysteine D | Cys | 0.94 ± 0.09 b | 1.16 ± 0.05 a | 1.00 ± 0.04 b |
| Tyrosine | Tyr | 0.87 ± 0.01 c | 1.02 ± 0.10 b | 1.15 ± 0.02 a |
| β-Alanine | β-Ala | 0.39 ± 0.01 c | 1.09 ± 0.01 a | 0.58 ± 0.04 b |
| γ-Aminobutyric acid | GABA | 2.05 ± 0.02 a | 0.78 ± 0.05 c | 1.55 ± 0.01 b |
| Ethanolamine | EOHNH2 | 1.34 ± 0.03b | 1.47 ± 0.01 ab | 1.54 ± 0.10a |
| Ornithine | Orn | 0.94 ± 0.03 a | 0.94 ± 0.05 a | 0.80 ± 0.07 b |
| Methylhistidine | 1-Mehis | 2.27 ± 0.08 b | 1.90 ± 0.04 c | 3.18 ± 0.09 a |
| Hydroxylysine | Hylys | 0.16 ± 0.01 b | 0.33 ± 0.02 a | 0.18 ± 0.01 b |
| Total EAA | 15.56 ± 0.75 c | 23.59 ± 0.92 a | 20.95 ± 0.20 b | |
| Total EAA+NEAA | 152.36 ± 4.68 b | 188.93 ± 2.56 a | 194.36 ± 2.21 a | |
| Total A | 38.62 ± 0.94 b | 38.47 ± 0.13 b | 48.70 ± 0.38 a | |
| Total B | 42.36 ± 0.34 b | 62.16 ± 0.60 a | 61.48 ± 0.38 a | |
| Total C | 48.11 ± 1.42 c | 65.28 ± 1.08 a | 59.78 ± 0.56 b | |
| Total D | 8.86 ± 0.16 c | 14.32 ± 0.33 a | 11.81 ± 0.10 b | |
| Parameters | 6R3B vs. White LED | 8R1B vs. White LED | 6R3B vs. 8R1B |
|---|---|---|---|
| Growth | |||
| Flower stalk height | + | ++ | − |
| Flower stalk diameter | − | n | − |
| Leaf number | n | + | − |
| Leaf length | − | + | −− |
| Leaf width | − | n | − |
| Shoot FW | n | + | − |
| Plant FW | n | + | − |
| Shoot DW | − | + | −− |
| Plant DW | − | n | − |
| Count | −5 | +7 | −11 |
| Quality | |||
| Total Chl | − | −− | + |
| β-carotene | + | + | n |
| Chl a/Chl b | + | ++ | − |
| Sucrose | n | + | − |
| Glucose | n | + | − |
| Fructose | − | + | −− |
| Nitrate | −− | − | + |
| Vitamin C | n | − | + |
| Soluble protein | + | − | ++ |
| Total EAA | ++ | + | + |
| Total EAA+NEAA | + | + | n |
| Total A | n | + | − |
| Total B | + | + | n |
| Total C | ++ | + | + |
| Total D | ++ | + | + |
| TG | ++ | + | + |
| TA | ++ | + | + |
| TP | n | − | n |
| FK | n | − | + |
| FQ | + | − | ++ |
| GSH | + | ++ | − |
| FRAP | n | − | + |
| DPPH | n | − | + |
| Count | +13 | +6 | +15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ji, J.; Song, S.; Su, W.; Liu, H. Growth, Nutritional Quality and Health-Promoting Compounds in Chinese Kale Grown under Different Ratios of Red:Blue LED Lights. Agronomy 2020, 10, 1248. https://doi.org/10.3390/agronomy10091248
Zhang Y, Ji J, Song S, Su W, Liu H. Growth, Nutritional Quality and Health-Promoting Compounds in Chinese Kale Grown under Different Ratios of Red:Blue LED Lights. Agronomy. 2020; 10(9):1248. https://doi.org/10.3390/agronomy10091248
Chicago/Turabian StyleZhang, Yiting, Jiazeng Ji, Shiwei Song, Wei Su, and Houcheng Liu. 2020. "Growth, Nutritional Quality and Health-Promoting Compounds in Chinese Kale Grown under Different Ratios of Red:Blue LED Lights" Agronomy 10, no. 9: 1248. https://doi.org/10.3390/agronomy10091248
APA StyleZhang, Y., Ji, J., Song, S., Su, W., & Liu, H. (2020). Growth, Nutritional Quality and Health-Promoting Compounds in Chinese Kale Grown under Different Ratios of Red:Blue LED Lights. Agronomy, 10(9), 1248. https://doi.org/10.3390/agronomy10091248






