Effects of Daily Light Integral and LED Spectrum on Growth and Nutritional Quality of Hydroponic Spinach
Abstract
1. Introduction
2. Materials and Methods
2.1. Seedling Materials and Growth Conditions
2.2. LED Lighting Treatments
2.3. Measurement Indexes and Methods
2.3.1. Plant Morphological and Growth Characteristics
2.3.2. Photosynthetic Characteristics and Nutritional Indices
2.3.3. Energy Yield and Light Energy Use Efficiency
2.4. Statistical Analysis
3. Results and Discussion
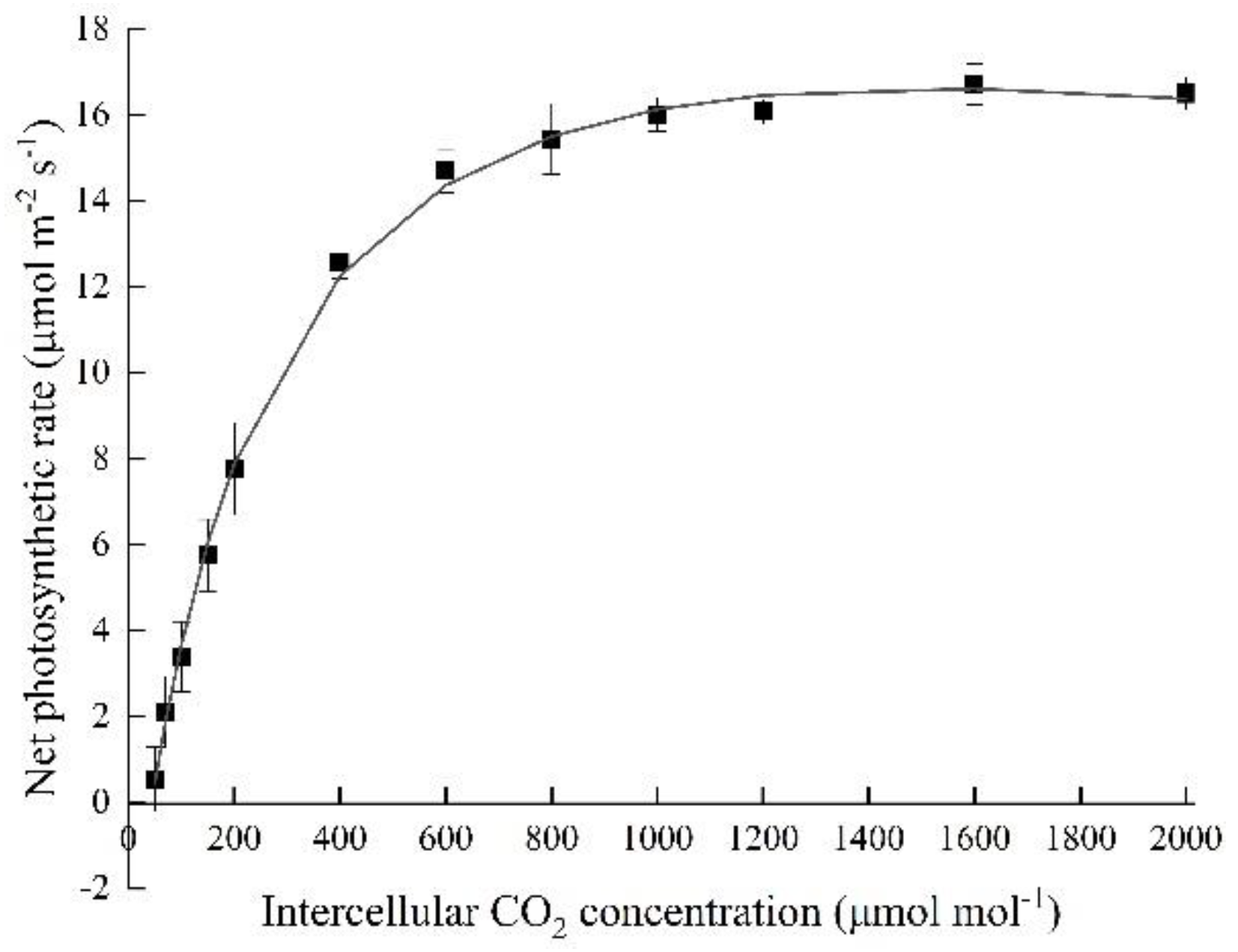
3.1. Photosynthetic and Morphological Characteristics of Hydroponic Spinach
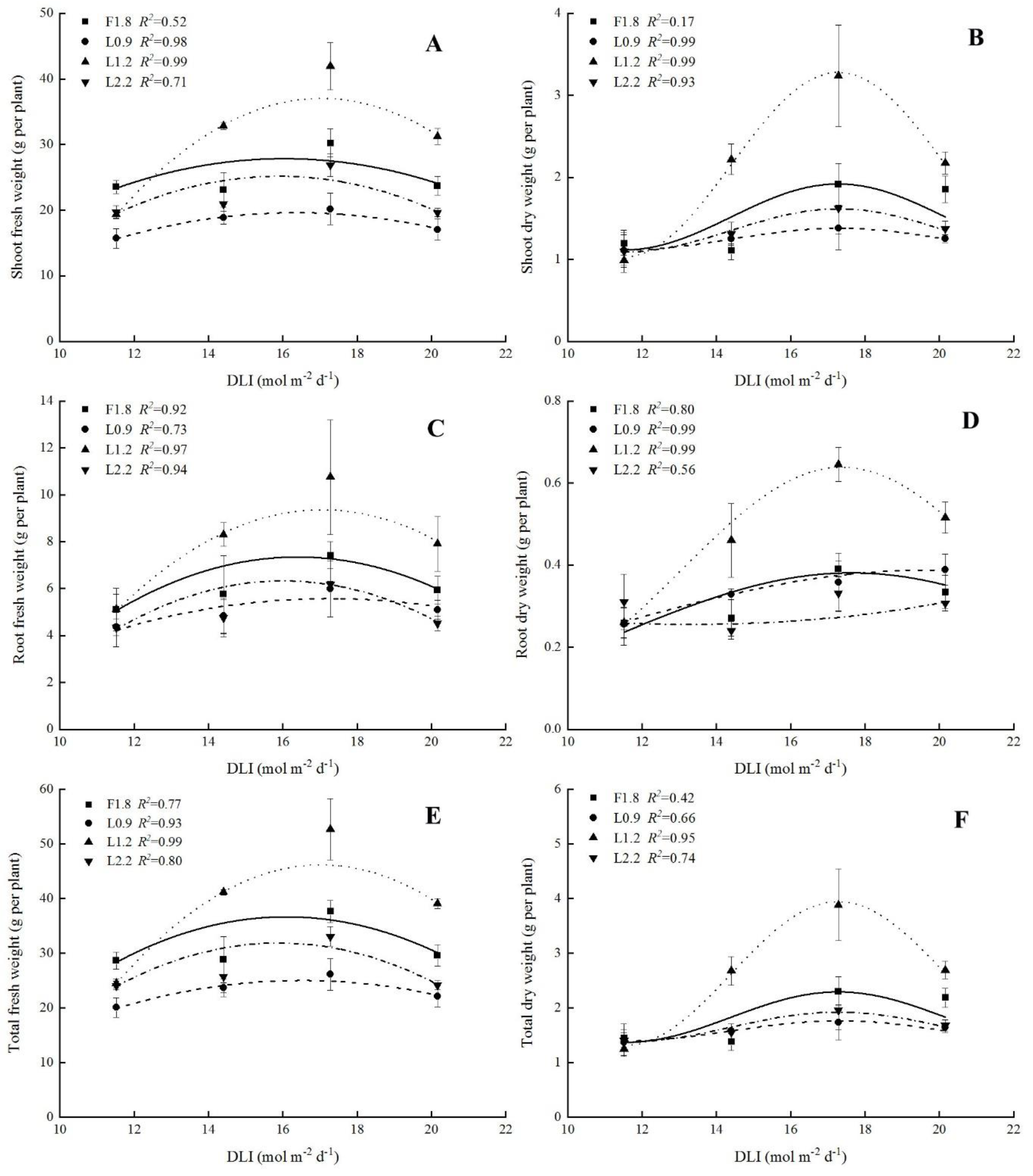
3.2. Biomass Accumulation of Hydroponic Spinach
3.3. Nutritional Quality of Hydroponic Spinach
3.4. Energy Yield and Light Energy Use Efficiency
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.; Hikosaka, S.; Goto, E. Effects of light quality and photosynthetic photon flux on growth and carotenoid pigments in Spinach (Spinacia oleracea L.). Acta Hortic. 2011, 907, 105–110. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.D.; Barman, M.; Mitra, A. Photosynthetic apparatus plays a central role in photosensitive physiological acclimations affecting spinach (Spinacia oleracea L.) growth in response to blue and red photon flux ratios. Environ. Exp. Bot. 2018, 156, 170–182. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G. Overview and concept of closed plant production system (CPPS). In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: London, UK, 2016; pp. 5–78. [Google Scholar]
- He, D.X.; Kozai, T.; Niu, G.; Zhang, X. Light-emitting diodes for horticulture. In Light-Emitting Diodes Materials, Processes, Devices and Applications; Li, J., Zhang, G., Eds.; Springer: Cham, Switzerland, 2019; pp. 513–547. [Google Scholar]
- Zhang, X.; He, D.X.; Niu, G.; Yan, Z.N.; Song, J.X. Effects of environment lighting on the growth, photosynthesis, and quality of hydroponic lettuce in a plant factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Yan, Z.N.; He, D.X.; Niu, G.; Zhou, Q.; Qu, Y.H. Growth, nutritional quality, and energy use efficiency of hydroponic lettuce as influenced by daily light integrals exposed to white versus white plus red light-emitting diodes. HortScience 2019, 54, 1737–1744. [Google Scholar] [CrossRef]
- Dou, H.J.; Niu, G.; Gu, M.M.; Masabni, J.G. Responses of sweet basil to different daily light integrals in photosynthesis, morphology, yield, and nutritional quality. HortScience 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Proietti, S.; Moscatello, S.; Golla, G.; Battistelli, Y. The effect of growing spinach (Spinacia oleracea L.) at two light intensities on the amounts of oxalate, ascorbate and nitrate in their leaves. J. Hort. Sci. Biotechnol. 2004, 79, 606–609. [Google Scholar] [CrossRef]
- Gent, M.P.N. Effect of irradiance and temperature on composition of spinach. HortScience 2016, 51, 133–140. [Google Scholar] [CrossRef]
- Matsuda, R.; Ohashi-kaneko, K.; Fujiwara, K.; Kurata, K. Effects of blue light deficiency on acclimation of light energy partitioning in PSII and CO2 assimilation capacity to high irradiance in Spinach leaves. Plant. Cell Physiol. 2008, 49, 664–670. [Google Scholar] [CrossRef] [PubMed]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1972, 9, 191–216. [Google Scholar] [CrossRef]
- Fukuda, N.; Ikeda, H.; Nara, M. Effects of light quality on the growth of lettuce and spinach cultured by hydroponics under controlled environment. Soc. Agric. Struct. Jpn. 1993, 24, 77–84. [Google Scholar]
- Ohashi-Kaneko, K.; Takase, M.; Kon, N.; Fujiwara, K.; Kurata, K. Effect of light quality on growth and vegetable quality in leaf lettuce, spinach and komatsuna. Environ. Control. Biol. 2007, 45, 189–198. [Google Scholar] [CrossRef]
- Park, S.; Cho, E.; An, J.; Yoon, B.; Choi, K.; Choi, E. Plant growth and ascorbic acid content of Spinacia oleracea grown under different light-emitting diodes and ultraviolet radiation light of plant factory system. Prot. Hort. Plant. Fac. 2019, 28, 1–8. [Google Scholar] [CrossRef]
- Yorio, N.C.; Goins, G.D.; Kagie, H.R.; Wheeler, R.M.; Sager, J.C. Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. HortScience 2001, 36, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Son, K.H.; Jeon, Y.M.; Oh, M.M. Application of supplementary white and pulsed light-emitting diodes to lettuce grown in a plant factory with artificial lighting. Hortic. Environ. Biotechnol. 2016, 57, 560–572. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red:blue ratio provided by LED lighting. Sci. Rep. 2019, 9, 14127:1–14127:11. [Google Scholar] [CrossRef]
- Chung, H.Y.; Chang, M.Y.; Wu, C.C.; Fang, W. Quantitative evaluation of electric light recipes for red leaf lettuce cultivation in plant factories. HortTechnology 2018, 28, 755–763. [Google Scholar] [CrossRef]
- Peixe, A.; Ribeiro, H.; Ribeiro, A.; Soares, M.; Machado, R.; Rato, A.; Coelho, R. Analysis of growth parameters for crop vegetables under broad and narrow LED spectra and fluorescent light tubes at different PPFs. J. Plant. Stud. 2018, 7, 47–60. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant. Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Li, H.S. Experimental Principle and Technology of Plant. Physiology and Biochemistry; Higher Education Press: Beijing, China, 2000; pp. 123–124, 182–184. [Google Scholar]
- Baker, C.J.L. The determination of oxalates in fresh plant material. Analyst 1952, 77, 340–344. [Google Scholar] [CrossRef]
- Song, J.X.; Meng, Q.W.; Du, W.F.; He, D.X. Effects of light quality on growth and development of cucumber seedlings in controlled environment. Int. J. Agric. Biol. Eng. 2017, 10, 312–318. [Google Scholar]
- Hersch, M.; Lorrain, S.; de Wit, M.; Trevisan, M.; Ljung, K.; Bergmann, S.; Fankhauser, C. Light intensity modulates the regulatory network of the shade avoidance response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 6515–6520. [Google Scholar] [CrossRef] [PubMed]
- Cope, K.R.; Snowden, M.C.; Bugbee, B. Photobiological interactions of blue light and photosynthetic photon flux: Effects of monochromatic and broad-spectrum light sources. Photochem. Photobiol. 2014, 90, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Snowden, M.C.; Cope, K.R.; Bugbee, B. Sensitivity of seven diverse species to blue and green light: Interactions with photon flux. PLoS ONE 2016, 11, e0163121. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q.C. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant. Sci. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Cope, K.R.; Bugbee, B. Spectral effects of three types of white light-emitting diodes on plant growth and development: Absolute versus relative amounts of blue light. HortScience 2013, 48, 505–509. [Google Scholar] [CrossRef]
- Loewus, F.A. Biosynthesis and metabolism of ascorbic acid in plants and of analogs of ascorbic acid in fungi. Phytochemistry 1999, 52, 193–210. [Google Scholar] [CrossRef]
- Fu, Y.M.; Li, H.Y.; Yu, J.; Liu, H.; Cao, Z.Y.; Manukovsky, N.S.; Liu, H. Interaction effects of light intensity and nitrogen concentration on growth, photosynthetic characteristics and quality of lettuce (Lactuca sativa L. Var. youmaicai). Sci. Hortic. 2017, 214, 51–57. [Google Scholar] [CrossRef]
- Proietti, S.; Moscatello, S.; Giacomelli, G.A.; Battistelli, A. Influence of the interaction between light intensity and CO2 concentration on productivity and quality of spinach (Spinacia oleracea L.) grown in fully controlled environment. Adv. Space Res. 2013, 52, 1193–1200. [Google Scholar] [CrossRef]
- Smirnoff, N. The Function and metabolism of ascorbic acid in plants. Ann. Bot. 1996, 78, 661–669. [Google Scholar] [CrossRef]
- Chang, C.C.; Beevers, H. Biogenesis of oxalate in plant tissues. Plant. Physiol. 1968, 43, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.D. The fine control of cytosolic pH. Physiol. Plant. 1986, 67, 702–706. [Google Scholar] [CrossRef]
- Rinallo, C.; Modi, G. Content of oxalate in actinidia deliciosa plants grown in nutrient solutions with different nitrogen forms. Biol. Plant. 2002, 45, 137–139. [Google Scholar] [CrossRef]
- Qi, L.D.; Liu, S.Q.; Xu, L.; Yu, W.Y.; Liang, Q.L.; Hao, S.Q. Effects of light qualities on accumulation of oxalate, tannin and nitrate in spinach. Trans. CSAE 2007, 23, 201–205. [Google Scholar]
- Li, K.; Yang, Q.C.; Tong, Y.X.; Cheng, R.F. Using movable light-emitting diodes for electricity savings in a plant factory growing lettuce. HortTechnology 2014, 24, 546–553. [Google Scholar] [CrossRef]
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2013, 89, 447–461. [Google Scholar] [CrossRef]


| Treatment Symbols | DLI x (mol m−2 day−1) | R:B Ratio y | Light Intensity (µmol m−2 s−1) | Photoperiod (h day−1) | Light Source |
|---|---|---|---|---|---|
| D11.5-F1.8 | 11.5 | 1.8 | 200 | 16 | White fluorescent lamp in color temperature of 4200 K |
| D14.4-F1.8 | 14.4 | 250 | |||
| D17.3-F1.8 | 17.3 | 300 | |||
| D20.2-F1.8 | 20.2 | 350 | |||
| D11.5-L0.9 | 11.5 | 0.9 | 200 | 16 | LED lamp with white chip in color temperature of 6500 K |
| D14.4-L0.9 | 14.4 | 250 | |||
| D17.3-L0.9 | 17.3 | 300 | |||
| D20.2-L0.9 | 20.2 | 350 | |||
| D11.5-L1.2 | 11.5 | 1.2 | 200 | 16 | LED lamp with white and red chips in 5:1 ratio, the white chip same to above and red chip in 660 nm |
| D14.4-L1.2 | 14.4 | 250 | |||
| D17.3-L1.2 | 17.3 | 300 | |||
| D20.2-L1.2 | 20.2 | 350 | |||
| D11.5-L2.2 | 11.5 | 2.2 | 200 | 16 | LED lamp with white and red chips in 5:3 ratio, the white and red chips same to above |
| D14.4-L2.2 | 14.4 | 250 | |||
| D17.3-L2.2 | 17.3 | 300 | |||
| D20.2-L2.2 | 20.2 | 350 |
| Parameter | Spectral Fraction of Light Source (%) | |||
|---|---|---|---|---|
| F1.8 | L0.9 | L1.2 | L2.2 | |
| Photon flux (300–800 nm) | 100.0 z | 100.0 | 100.0 | 100.0 |
| Ultraviolet light (300–399 nm) | 1.4 | 0.0 | 0.0 | 0.0 |
| Blue light (400–499 nm) | 20.3 | 27.0 | 25.9 | 20.4 |
| Green light (500–599 nm) | 39.0 | 46.9 | 41.1 | 33.9 |
| Red light (600–699 nm) | 35.8 | 24.2 | 31.4 | 44.1 |
| Far-red light (700–800 nm) | 3.5 | 1.9 | 1.6 | 1.6 |
| R:B ratio y | 1.8 | 0.9 | 1.2 | 2.2 |
| Treatments | Net Photosynthetic Rate (μmol m−2 s−1) | Stomatal Conductance (mmol m−2 s−1) | Intercellular CO2 Concentration (μmol mol−1) | Transpiration Rate (mmol m−2 s−1) |
|---|---|---|---|---|
| D11.5-F1.8 | 13.3 ± 0.6 ab z | 407 ± 21 f | 712 ± 3 d | 4.1 ± 0.1 f |
| D14.4-F1.8 | 13.3 ± 0.6 ab | 583 ± 72 e | 724 ± 8 c | 5.9 ± 0.4 d |
| D17.3-F1.8 | 13.5 ± 0.8 ab | 650 ± 70 cd | 726 ± 6 bc | 6.2 ± 0.2 c |
| D20.2-F1.8 | 11.5 ± 0.9 c | 593 ± 64 e | 731 ± 6 b | 5.6 ± 0.6 de |
| D11.5-L0.9 | 13.2 ± 0.7 ab | 573 ± 23 e | 722 ± 3 c | 6.1 ± 0.1 c |
| D14.4-L0.9 | 14.4 ± 0.5 a | 870 ± 79 ab | 731 ± 8 b | 7.9 ± 0.4 a |
| D17.3-L0.9 | 13.6 ± 0.6 ab | 837 ± 38 b | 729 ± 4 b | 7.8 ± 0.2 a |
| D20.2-L0.9 | 13.8 ± 0.5 ab | 683 ± 81 cd | 730 ± 8 b | 6.6 ± 0.4 bc |
| D11.5-L1.2 | 13.0 ± 0.7 b | 603 ± 35 d | 727 ± 9 c | 6.2 ± 0.2 c |
| D14.4-L1.2 | 12.8 ± 0.6 b | 717 ± 118 c | 729 ± 7 b | 6.4 ± 0.5 bc |
| D17.3-L1.2 | 13.4 ± 0.3 ab | 937 ± 32 a | 738 ± 2 ab | 6.9 ± 0.4 b |
| D20.2-L1.2 | 11.7 ± 0.9 c | 577 ± 46 e | 730 ± 9 b | 4.8 ± 0.1 e |
| D11.5-L2.2 | 13.0 ± 0.4 b | 560 ± 40 e | 727 ± 2 bc | 4.4 ± 0.1 ef |
| D14.4-L2.2 | 12.4 ± 0.6 bc | 700 ± 20 cd | 737 ± 1 ab | 4.9 ± 0.3 e |
| D17.3-L2.2 | 13.3 ± 0.5 ab | 827 ± 75 b | 745 ± 3 a | 5.8 ± 0.2 d |
| D20.2-L2.2 | 12.6 ± 0.8 bc | 633 ± 15 cd | 728 ± 5 b | 4.8 ± 0.3 e |
| DLI | * | * | * | * |
| LQ | * | * | * | * |
| DLI × LQ | * | * | NS | * |
| Treatments | Leaf Number | Leaf Length (cm) | Leaf Width (cm) | Petiole Length (cm) | Petiole Diameter (mm) | Leaf Area (cm2) | Specific Leaf Area (m2 kg−1) |
|---|---|---|---|---|---|---|---|
| D11.5-F1.8 | 10.0 ± 0.8 b z | 12.6 ± 0.2 bc | 5.7 ± 0.6 c | 7.7 ± 1.1 ab | 4.4 ± 0.4 ab | 53.6 ± 5.0 d | 4.40 ± 0.91 c |
| D14.4-F1.8 | 10.3 ± 0.6 b | 11.9 ± 1.9 c | 5.8 ± 0.4 c | 5.6 ± 1.5 cd | 3.9 ± 0.5 bc | 51.2 ± 7.2 d | 5.16 ± 1.31 b |
| D17.3-F1.8 | 11.3 ± 1.0 a | 12.0 ± 0.7 c | 5.9 ± 0.3 c | 5.1 ± 1.6 cd | 4.3 ± 0.5 ab | 51.5 ± 2.5 d | 2.73 ± 0.37 d |
| D20.2-F1.8 | 10.6 ± 0.9 ab | 10.5 ± 0.9 d | 5.6 ± 0.4 c | 4.4 ± 1.8 d | 4.1 ± 0.7 b | 42.1 ± 2.2 e | 2.28 ± 0.15 d |
| D11.5-L0.9 | 9.0 ± 0.8 c | 10.3 ± 0.6 d | 6.1 ± 0.5 c | 4.5 ± 1.5 d | 4.1 ± 0.5 b | 47.5 ± 5.1 de | 4.34 ± 0.54 c |
| D14.4-L0.9 | 8.3 ± 0.6 cd | 11.1 ± 0.2 cd | 6.6 ± 1.0 bc | 6.0 ± 1.1 c | 3.5 ± 0.3 c | 55.1 ± 7.4 cd | 4.92 ± 0.92 bc |
| D17.3-L0.9 | 8.3 ± 0.6 cd | 12.8 ± 1.5 bc | 5.6 ± 0.3 c | 6.4 ± 0.6 c | 3.2 ± 0.5 c | 52.9 ± 12.4 d | 4.80 ± 1.14 bc |
| D20.2-L0.9 | 8.8 ± 0.5 cd | 11.2 ± 0.8 cd | 6.0 ± 0.6 c | 5.8 ± 0.6 cd | 3.7 ± 0.1 bc | 49.3 ± 9.5 d | 4.35 ± 0.69 c |
| D11.5-L1.2 | 8.3 ± 0.6 cd | 12.2 ± 0.6 bc | 5.9 ± 0.3 c | 7.0 ± 1.6 b | 4.1 ± 0.5 b | 53.2 ± 3.1 d | 4.76 ± 1.68 bc |
| D14.4-L1.2 | 10.7 ± 0.6 ab | 13.5 ± 1.0 b | 7.3 ± 0.3 b | 8.4 ± 0.6 a | 5.0 ± 0.4 a | 70.7 ± 7.2 b | 3.47 ± 0.83 cd |
| D17.3-L1.2 | 11.0 ± 1.0 a | 15.3 ± 0.7 a | 8.7 ± 0.9 a | 8.6 ± 0.8 a | 5.1 ± 0.6 a | 98.3 ± 5.6 a | 3.10 ± 0.57 d |
| D20.2-L1.2 | 9.3 ± 0.6 c | 13.5 ± 0.6 b | 7.9 ± 0.9 ab | 6.3 ± 0.3 c | 4.4 ± 0.6 ab | 75.8 ± 9.5 b | 2.89 ± 0.65 d |
| D11.5-L2.2 | 8.8 ± 0.5 cd | 11.9 ± 1.0 c | 6.9 ± 0.7 bc | 4.5 ± 0.9 d | 4.2 ± 1.4 b | 60.8 ± 5.7 c | 4.69 ± 0.89 bc |
| D14.4-L2.2 | 8.0 ± 1.4 d | 12.1 ± 1.4 bc | 6.8 ± 0.7 bc | 5.3 ± 1.2 cd | 3.8 ± 0.5 bc | 61.6 ± 10.1 c | 6.69 ± 1.18 a |
| D17.3-L2.2 | 9.3 ± 0.6 c | 12.4 ± 1.8 bc | 7.4 ± 0.7 b | 5.7 ± 1.1 cd | 4.6 ± 0.2 ab | 70.5 ± 16.8 b | 4.86 ± 2.16 bc |
| D20.2-L2.2 | 8.8 ± 0.4 cd | 10.8 ± 0.6 d | 6.8 ± 0.2 bc | 4.4 ± 1.1 d | 4.3 ± 0.7 ab | 53.1 ± 1.8 d | 4.20 ± 0.67 c |
| DLI | * | * | * | * | * | * | * |
| LQ | * | * | * | * | * | * | * |
| DLI × LQ | * | * | * | * | NS | * | * |
| Treatments | Power Consumption Based on Increased Total Fresh Weight (kWh per 100 g FW) | Energy Yield (g FW kWh−1) | LUE |
|---|---|---|---|
| D11.5-F1.8 | 4.50 ± 0.11 cd z | 17.91 ± 1.21 de | 0.018 ± 0.001 b |
| D14.4-F1.8 | 4.82 ± 0.43 c | 15.17 ± 1.75 e | 0.016 ± 0.003 bc |
| D17.3-F1.8 | 4.86 ± 0.17 c | 15.92 ± 0.82 e | 0.022 ± 0.002 ab |
| D20.2-F1.8 | 7.12 ± 0.37 a | 11.21 ± 0.54 f | 0.015 ± 0.001 bc |
| D11.5-L0.9 | 4.05 ± 0.32 d | 21.23 ± 2.08 d | 0.014 ± 0.003 c |
| D14.4-L0.9 | 3.82 ± 0.28 de | 21.46 ± 1.10 d | 0.013 ± 0.003 c |
| D17.3-L0.9 | 3.55 ± 0.31 de | 20.13 ± 2.40 d | 0.013 ± 0.004 c |
| D20.2-L0.9 | 5.65 ± 0.40 b | 14.13 ± 0.87 e | 0.012 ± 0.003 c |
| D11.5-L1.2 | 2.73 ± 0.10 f | 29.77 ± 1.00 c | 0.022 ± 0.008 ab |
| D14.4-L1.2 | 2.07 ± 0.03 g | 39.16 ± 0.62 b | 0.026 ± 0.003 a |
| D17.3-L1.2 | 1.73 ± 0.19 h | 46.82 ± 3.99 a | 0.025 ± 0.003 a |
| D20.2-L1.2 | 2.77 ± 0.07 f | 29.25 ± 1.16 c | 0.021 ± 0.006 ab |
| D11.5-L2.2 | 2.83 ± 0.06 f | 29.91 ± 1.72 c | 0.020 ± 0.003 ab |
| D14.4-L2.2 | 3.12 ± 0.30 e | 26.71 ± 2.47 cd | 0.013 ± 0.002 c |
| D17.3-L2.2 | 2.77 ± 0.15 f | 29.98 ± 1.92 c | 0.015 ± 0.002 bc |
| D20.2-L2.2 | 4.38 ± 0.04 cd | 18.66 ± 0.59 de | 0.012 ± 0.002 c |
| DLI | * | * | NS |
| LQ | * | * | * |
| DLI × LQ | * | * | NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; He, D.; Ji, F.; Zhang, S.; Zheng, J. Effects of Daily Light Integral and LED Spectrum on Growth and Nutritional Quality of Hydroponic Spinach. Agronomy 2020, 10, 1082. https://doi.org/10.3390/agronomy10081082
Gao W, He D, Ji F, Zhang S, Zheng J. Effects of Daily Light Integral and LED Spectrum on Growth and Nutritional Quality of Hydroponic Spinach. Agronomy. 2020; 10(8):1082. https://doi.org/10.3390/agronomy10081082
Chicago/Turabian StyleGao, Wei, Dongxian He, Fang Ji, Sen Zhang, and Jianfeng Zheng. 2020. "Effects of Daily Light Integral and LED Spectrum on Growth and Nutritional Quality of Hydroponic Spinach" Agronomy 10, no. 8: 1082. https://doi.org/10.3390/agronomy10081082
APA StyleGao, W., He, D., Ji, F., Zhang, S., & Zheng, J. (2020). Effects of Daily Light Integral and LED Spectrum on Growth and Nutritional Quality of Hydroponic Spinach. Agronomy, 10(8), 1082. https://doi.org/10.3390/agronomy10081082





