Effects of Additive Type on Fermentation and Aerobic Stability and Its Interaction with Air Exposure on Silage Nutritive Value
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ensiling
2.2. Laboratory Analysis
2.3. Calculations and Statistical Analysis
3. Results
3.1. Trial 1
3.2. Trial 2
3.3. Trial 3
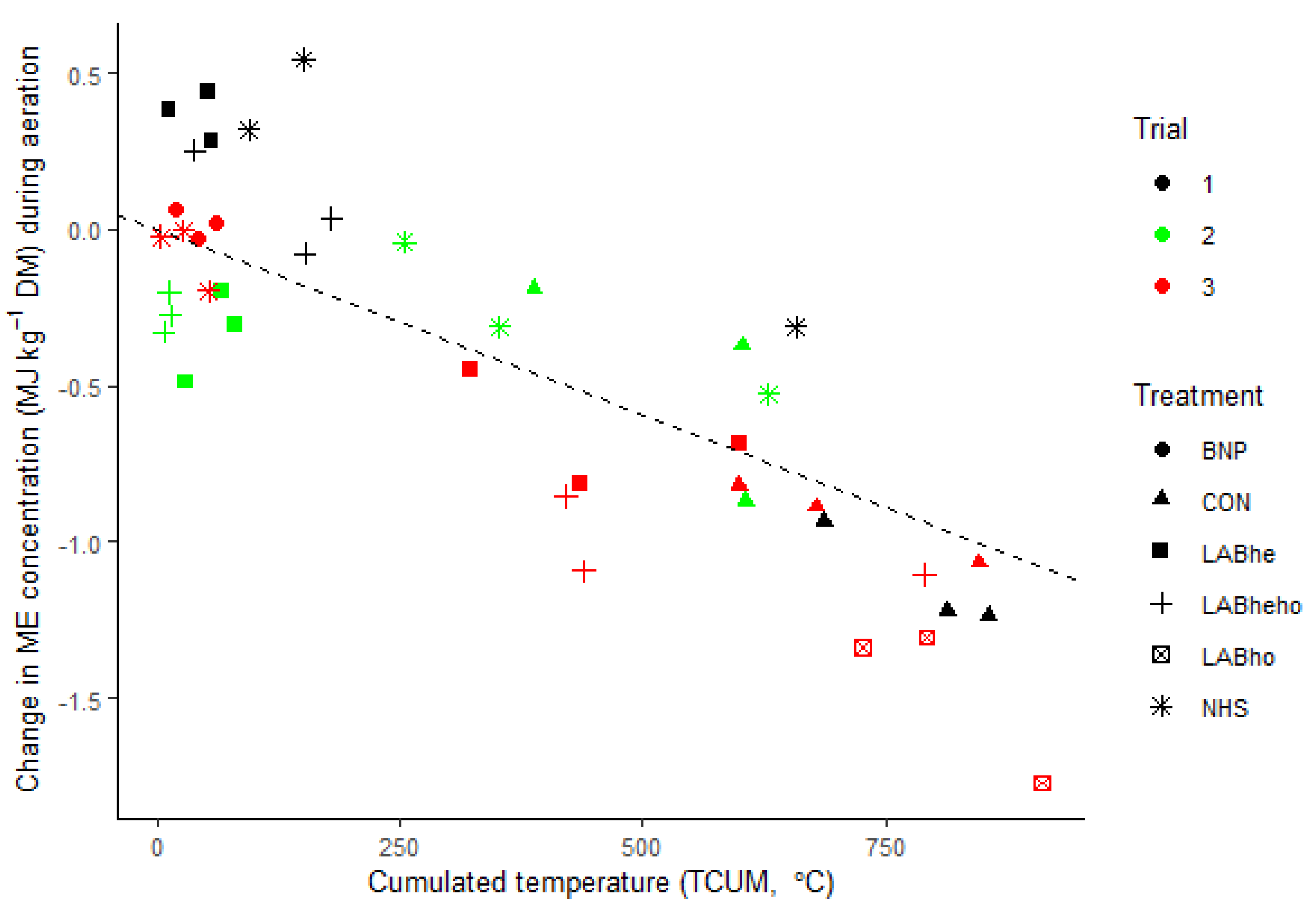
3.4. Relationship Between the Extent of Aerobic Deterioration and the Changes in Metabolisable Energy during Aeration
4. Discussion
4.1. Effects of Additives on Fermentation Characteristics, Yeast Count and Aerobic Stability
4.2. Effects of Aeration and Additives on Silage Nutritive Value
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage review: Factor affecting dry matter and qualitative losses in silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabacco, E.; Comino, L.; Borreani, G. Production efficiency, costs and environmental impacts of conventional and dynamic forage systems for dairy farms in Italy. Europ. J. Agron. 2018, 99, 1–12. [Google Scholar] [CrossRef]
- Åby, B.A.; Randby, A.T.; Bonesmo, H.; Aass, L. Impact of grass silage quality on greenhouse gas emissions from dairy and beef production. Grass Forage Sci. 2019, 74, 525–534. [Google Scholar] [CrossRef]
- Ranck, E.J.; Holden, L.A.; Dillon, J.A.; Rotz, C.A.; Soder, K.J. Economic and environmental effects of double cropping winter annuals and corn using the Integrated Farm System Model. J. Dairy Sci. 2020, 103, 3804–3815. [Google Scholar] [CrossRef] [PubMed]
- Rooke, J.A.; Hatfield, R.D. Biochemistry of ensiling. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Holmes, H.J., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 2003; pp. 95–139. [Google Scholar]
- Kung, L., Jr.; Sheperd, A.C.; Smagala, A.M.; Endres, K.M.; Bessett, C.A.; Ranjit, N.K.; Glancey, J.L. The effects of preservatives based on propionic acid on the fermentation aerobic stability of corn silage and a total mixed ration. J. Dairy Sci. 1998, 81, 1322–1330. [Google Scholar] [CrossRef]
- Salvo, P.A.R.; Schonell, E.P.; Daniel, J.L.P.; Santos, M.C.; Morais, G.; Winckler, J.P.; Silva, J.; Nussio, L.G. Effects of Pichia norvegensis and air exposure on the nutritive value of corn silages for dairy cows. In Proceedings of the XVIIth International Silage Conference, Piracicaba, Brazil, 1–3 July 2015; pp. 70–71. [Google Scholar]
- Windle, M.; Kung, L., Jr. The effect of a feed additive on the feeding value of a silage based TMR exposed to air. J. Dairy Sci. 2013, 96, 16. [Google Scholar]
- Fink-Gremmels, J. Mycotoxins in cattle feeds and carry-over to dairy milk: A review. Food Add. Contam. 2008, 25, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Ogunade, I.M.; Martinez-Tuppia, C.; Queiroz, O.C.M.; Jiang, Y.; Drouin, P.; Wu, F.; Vyas, D.; Adesogan, A.T. Silage review: Mycotoxins in silage: Occurrence, effects, prevention, mitigation. J. Dairy Sci. 2018, 101, 4034–4059. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Stokes, M.R.; Lin, C.J. Silage additives. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Holmes, H.J., Eds.; American Society of Agronomy, Inc. Publishers: Madison, WI, USA, 2003; pp. 305–360. [Google Scholar]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L., Jr. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Weinberg, Z.G.; Ogunade, I.M.; Cervantes, A.A.P.; Arriola, K.G.; Jiang, Y.; Kim, D.; Li, X.; Gonçalves, M.C.M.; Vyas, D.; et al. Meta-analysis of effects of inoculation with homofermentative and facultative heterofermentative lactic acid bacteria on silage fermentation, aerobic stability, and the performance of dairy cows. J. Dairy Sci. 2017, 100, 4587–4603. [Google Scholar] [CrossRef] [Green Version]
- Honig, H.; Thaysen, J. 10 years testing of silage additives by dlg—A comprehensive data evaluation. In Proceedings of the XIIIth International Silage Conference, Auchincruive, Scotland, 11–13 September 2002. [Google Scholar]
- Auerbach, H.; Nadeau, E. Chemical additives for silages: When to use it and what are the options? In Proceedings of the VIth International Symposium on Forage Quality and Conservation, Piracicaba, Brazil, 7–8 November 2019. [Google Scholar]
- Honig, H.; Schild, G.-J.; Weissbach, F.; Daenicke, R. Effect of a combination of lactic acid bacteria with formate and benzoate as a silage additive for grass under farm conditions. In Proceedings of the XIth International Silage Conference., Aberystwyth, UK, 8–11 September 1996. [Google Scholar]
- Muck, R.E.; Kung, L., Jr. Effects of silage additives on ensiling. In Proceedings of the Silage: Field to Feedbunk, North American Conference, Hershey, PA, USA, 11–13 February 1997. [Google Scholar]
- Bernardi, A.; Härter, C.J.; Silva, A.W.L.; Reis, R.A.; Rabelo, C.H.S. A meta-analysis examining lactic acid bacteria inoculants for maize silage: Effects on fermentation, aerobic stability, nutritive value and livestock production. Grass Forage Sci. 2019, 74, 596–612. [Google Scholar] [CrossRef]
- Kleinschmit, D.H.; Kung, L., Jr. A meta-analysis of the effects of Lactobacillus buchneri on the fermentation and aerobic stability of corn and grass and small-grain silages. J. Dairy Sci. 2006, 89, 4005–4013. [Google Scholar] [CrossRef]
- Driehuis, F.; Oude Elferink, S.J.W.H.; van Wikselaar, P.G. Fermentation characteristics and aerobic stability of grass silage inoculated with Lactobacillus buchneri, with or without homofermentative lactic acid bacteria. Grass Forage Sci. 2001, 56, 330–343. [Google Scholar] [CrossRef]
- Auerbach, H.; Weiss, K.; Theobald, P.; Nadeau, E. Effects of inoculant type on dry matter losses, fermentation pattern, yeast count and aerobic stability of green rye silages. In Proceedings of the 12. BOKU-Symposium Tierernährung, Vienna, Austria, 11 April 2013. [Google Scholar]
- Haigh, P.M.; Parker, J.W.G. Effect of silage additives and wilting on silage fermentation, digestibility and intake, and on live weight change in young cattle. Grass Forage Sci. 1985, 40, 429–436. [Google Scholar] [CrossRef]
- Steen, R.W.J. Recent advances in the use of silage additives for dairy cattle. In Proceedings of the Occasional Symposium No. 25—Management issues for the grassland farmer in the 1990’s, Malvern, UK, 26–27 November 1990. [Google Scholar]
- Auerbach, H.; Nadeau, E.; Weiss, K.; Theobald, P. 2016a. Effects of sodium nitrite-containing additives on dry matter losses, fermentation pattern and biogenic amine formation in lucerne and cocksfoot silage. In Proceedings of the 17th International Conference on Forage Conservation, Horny Smokovec, Slovak Republic, 27–29 September 2016. [Google Scholar]
- Auerbach, H.; Nadeau, E. Effects of chemical additives on whole-crop maize silage traits. In Proceedings of the 22nd International Grassland Congress, Sydney, NSW, Australia, 15–19 September 2013. [Google Scholar]
- Bernardes, T.F.; de Oliveira, I.L.; Lara, M.A.S.; Casagrande, D.R.; Avila, C.L.S.; Pereira, O.G. Effects of potassium sorbate and sodium benzoate at two application rates on fermentation and aerobic stability of maize silage. Grass Forage Sci. 2014, 70, 491–498. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Smith, M.L.; da Silva, E.B.; Windle, M.C.; da Silva, T.C.; Polukis, S.A. An evaluation of the effectiveness of a chemical additive based on sodium benzoate, potassium sorbate, and sodium nitrite on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 2018, 101, 5949–5960. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Weinberg, Z. Changes during aerobic exposure of wheat silages. Anim. Feed Sci. Technol. 2009, 154, 76–82. [Google Scholar] [CrossRef]
- Nadeau, E.; Svensson, E.; Zaralis, K.; Helander, C.; Pauly, T.; Arnesson, A. Effects of additive on aerobic stability and nutritive value of maize silage stored during different time periods when harvested at advancing maturity stages. In Proceedings of the Advances in Animal Biosciences—8th International Symposium on the nutrition of herbivores, Aberythwyth, UK, 6–9 September 2011. [Google Scholar]
- Tabacco, E.; Righi, F.; Quarantelli, A.; Borreani, G. Dry matter and nutritional losses during aerobic deterioration of corn and sorghum silages as influenced by different lactic acid bacteria inocula. J. Dairy Sci. 2011, 94, 1409–1419. [Google Scholar] [CrossRef]
- Weissbach, F.; Strubelt, C. Correcting the dry matter content of grass silages as a substrate for biogas production. J. Agric. Eng. 2008, 63, 210. [Google Scholar]
- Weissbach, F. A simple method for the correction of fermentation losses measured in laboratory silos. In Proceedings of the XIVth International Silage Conference, Belfast, UK, July 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Weiss, K.; Kaiser, E. Milchsäurebstimmung in Silageextrakten mit Hilfe der HPLC [Lactic acid determination in silage extracts by HPLC]. Das Wirtschaftseigene Futter 1995, 41, 69–80. [Google Scholar]
- Weiss, K.; Kroschewski, B.; Auerbach, H. Effects of air exposure, temperature and additives on fermentation characteristics, yeast count, aerobic stability and volatile organic compounds in corn silage. J. Dairy Sci. 2016, 99, 8053–8069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Lengerken, J.; Zimmermann, K. Handbuch Futtermittelprüfung [Handbook Feed Evaluation], 1st ed.; Deutscher Landwirtschaftsverlag: Berlin, Germany, 1991; pp. 206–267. (In German) [Google Scholar]
- ISO 21527-1. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95; International Organization for Standardization: Geneva, Switzerland, 2008.
- Honig, H. Evaluation of aerobic stability. In Proceedings of the EUROBAC Conference, Uppsala, Sweden, 12–16 August 1986. [Google Scholar]
- Lindgren, E. Vallfodrets Näringsvärde Bestämt In Vivo Och Med Olika Laboratoriemetoder; Report No. 45; Department of Animal Nutrition and Management, Swedish University of Agricultural Sciences: Uppsala, Sweden, 1979. (In Swedish) [Google Scholar]
- Lindgren, E. Nykalibrering av VOS Metoden för Bestämning av Energivärde Hos Vallfoder; Working Paper; Department of Animal Nutrition and Management, Swedish University of Agricultural Sciences: Uppsala, Sweden, 1983. (In Swedish) [Google Scholar]
- Lindgren, E. Fodrets Energivärde; Course Paper Feed Science HNU 3; Department of Animal Nutrition and Management, Swedish University of Agricultural Sciences: Uppsala, Sweden, 1988. (In Swedish) [Google Scholar]
- Oude-Elferink, S.J.W.H.; Krooneman, J.; Gotschal, J.C.; Spoelstra, S.F.; Faber, F.; Driehuis, F. Anaerobic degradation of lactic acid to acetic acid and 1,2-propandediol by Lactobacillus buchneri. Appl. Environm. Microbiol. 2001, 67, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kung, L., Jr.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.J.; Hu, W.; Mills, J.A.; Kung, L., Jr. The development of lactic acid bacteria and Lactobacillus buchneri and their effects on the fermentation of alfalfa silage. J. Dairy Sci. 2009, 92, 5005–5010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reich, L.J.; Kung, L., Jr. Effects of combining Lactobacillus buchneri 40788 with various lactic acid bacteria on the fermentation and aerobic stability of corn silage. Anim. Feed Sci. Technol. 2010, 159, 105–109. [Google Scholar] [CrossRef]
- Krooneman, J.; Faber, F.; Alderkamp, A.C.; Oude Elferink, S.J.H.W.; Driehuis, F.; Cleenwerck, I.; Swings, J.; Gottschal, J.C.; Vancanneyt, M. Lactobacillus diolivorans sp. nov., a 1,2-propanediol-degrading bacterium isolated from aerobically stable maize silage. Int. J. Syst. Evol. Microbiol. 2002, 52, 639–646. [Google Scholar] [CrossRef]
- Gomes, A.L.M.; Jacovaci, F.A.; Bolsson, D.C.; Nussio, L.G.; Jobim, C.C.; Daniel, J.L.P. Effect of light wilting and heterolactic inoculant on the formation of volatile organic compounds, fermentative losses and aerobic stability of oat silage. Anim. Feed Sci. Technol. 2019, 247, 194–198. [Google Scholar] [CrossRef]
- Lingvall, P.; Lättemäe, P. Influence of hexamine and sodium nitrite in combination with sodium benzoate and sodium propionate on fermentation and hygienic quality of wilted and long cut grass silage. J. Sci. Food Agric. 1999, 79, 257–264. [Google Scholar] [CrossRef]
- Jonsson, A.; Pahlow, G. Systematic classification and biochemical characterization of yeasts growing in grass silage inoculated with Lactobacillus cultures. Anim. Res. Dev. 1984, 20, 7–22. [Google Scholar]
- Schmidt, R.J.; Kung, L., Jr. The effects of Lactobacillus buchneri with or without a homolactic bacterium on the fermentation and aerobic stability of corn silages made at different locations. J. Dairy Sci. 2010, 93, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Woolford, M.K. Microbiological screening of the straight chain fatty acids (C1-C12) as potential silage additives. J. Sci. Food Agric. 1975, 26, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Woolford, M.K. Microbiological screening of food preservatives, cold sterilants and specific antimicrobial agents as silage additives. J. Sci. Food Agric. 1975, 26, 229–237. [Google Scholar] [CrossRef]
- Santos, M.C.; Golt, C.; Joerger, R.D.; Mechor, G.D.; Mourão, G.B.; Kung, L., Jr. Identification of the major yeasts isolated from high moisture corn and corn silages in the United States using genetic and biochemical methods. J. Dairy Sci. 2016, 100, 1151–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addah, W.; Baah, J.; Groenewegen, P.; Okine, E.K.; McAllister, T.A. Comparison of the fermentation characteristics, aerobic stability and nutritive value of barley and corn silages ensiled with or without a mixed bacterial inoculant. Can. J. Anim. Sci. 2011, 91, 133–146. [Google Scholar] [CrossRef]
- Brüsemeister, F.; Kalzendorf, C.; Losand, B.; Ruser, B.; Thaysen, J. Influence of treating grass with a ferulate esterase releasing inoculant (Pioneer® 11GFT) on preservation and total tract digestibility of ensilage. In Proceedings of the XVth International Silage Conference, Madison, WI, USA, 27–29 July 2009. [Google Scholar]
- Brüsemeister, F.; Kalzendorf, C.; Losand, B.; Ruser, B.; Thaysen, J. Influence of inoculating corn with a ferulate esterase releasing inoculant (Pioneer® brand 11CFT) on preservation and total tract digestibility of ensilage. In Proceedings of the XVth International Silage Conference, Madison, WI, USA, 27–29 July 2009. [Google Scholar]
- Pitt, R.E.; Muck, R.E.; Pickering, N.B. A model of aerobic fungal growth in silage. 2. Aerobic stability. Grass Forage Sci. 1991, 46, 301–312. [Google Scholar] [CrossRef]
- Middelhoven, W.J.; van Baalen, A.H.M. Development of the yeast flora of whole-crop maize during ensiling and during subsequent aerobiosis. J. Sci. Food Agric. 1988, 42, 199–207. [Google Scholar] [CrossRef]
- Auerbach, H.; (International Silage Consultancy, Wettin-Löbejün, Germany); Theobald, P.; (University of Nürtingen-Geislingen, Nürtingen, Germany). Personal communication, 2018.
- Auerbach, H.; Weber, U.; Weber, G.; Weiss, K.; Theobald, P. Effects of different chemical additives on the fermentation and aerobic stability of high-moisture corn ensiled in bags. In Proceedings of the XVIIth International Silage Conference, Piracicaba, Brazil, 1–3 July 2015. [Google Scholar]
- Adesogan, A.T.; Salawu, M.B.; Ross, A.B.; Davies, D.R.; Brooks, A.E. Effect of Lactobacillus buchneri, Lactobacillus fermentum, Leuconostoc mesenteroides inoculants, or a chemical additive on the fermentation, aerobic stability, and nutritive value of crimped wheat grains. J. Dairy Sci. 2003, 86, 1789–1796. [Google Scholar] [CrossRef] [Green Version]
| Trial | DM | Crude Ash | Crude Protein | aNDFom | ADFom | ADL | WSC |
|---|---|---|---|---|---|---|---|
| g kg−1 | g kg−1 DM | ||||||
| 1 | 419 | 90 | 112 | 549 | 306 | 28 | 138 |
| 2 | 351 | 108 | 128 | 578 | 322 | 39 | 106 |
| 3 | 216 | 88 | 195 | 462 | ND | ND | 108 |
| Parameter | CON | LABhe | LABheho | NHS | SEM | p |
|---|---|---|---|---|---|---|
| DM (g kg−1) | 407 b | 410 a,b | 413 a | 410 a,b | 1.4 | 0.026 |
| DM loss (%) | 5.0 c | 6.6 a | 5.3 b | 4.1 d | 0.03 | <0.001 |
| WSC | 74.1 b | 17.9 d | 30.8 c | 91.2 a | 1.71 | <0.001 |
| NH3-N (g kg−1 N) | 74 b | 82 a | 68 c | 54 d | 0.5 | <0.001 |
| pH | 4.16 c | 4.31 a | 4.07 d | 4.24 b | 0.005 | <0.001 |
| Lactic Acid | 43.4 b | 30.5 d | 46.7 a | 37.8 c | 0.47 | <0.001 |
| Acetic Acid | 11.0 b | 21.0 a | 14.2 b | 11.0 b | 1.43 | 0.004 |
| Propionic Acid | ND | ND | ND | ND | ||
| Butyric Acid | ND | ND | ND | ND | ||
| Ethanol | 5.9 a,b | 8.6 a | 2.4 b | 1.1 b | 1.14 | 0.006 |
| n-propanol | ND | ND | ND | ND | ||
| 1,2-propanediol | 4.1 b | 14.3 a | 9.9 a | 3.2 b | 1.12 | <0.001 |
| Yeast Count (log cfu g−1) | 6.4 a | 2.2 b | 3.3 b | 4.3 a,b | 0.57 | 0.004 |
| ASTA (hours) | 44 b | >276 a | >276 a | 201 a,b | 37.8 | 0.008 |
| TCUM (°C) | 786 a | 40 b | 123 b | 301 b | 96.0 | 0.002 |
| Parameter | CON | LABhe | LABheho | NHS | SEM | p |
|---|---|---|---|---|---|---|
| IVOMD (% of OM) | ||||||
| Before Aeration | 85.0 a | 82.6 a | 84.3 a | 84.3 a | 0.81 | <0.001 |
| After Aeration | 78.1 b | 85.2 a | 84.9 a | 85.8 a | ||
| Treatment Mean | 81.6 b | 83.9 a,b | 84.6 a,b | 85.1 a | 0.68 | 0.027 |
| Metabolisable Energy | ||||||
| (MJ kg−1 DM) | ||||||
| Before Aeration | 10.8 a | 10.4 a | 10.7 a | 10.7 a | 0.13 | <0.001 |
| After Aeration | 9.6 b | 10.8 a | 10.7 a | 10.8 a | ||
| Treatment Mean | 10.2 b | 10.6 a,b | 10.7 a | 10.8 a | 0.10 | 0.020 |
| Parameter | CON | LABhe | LABheho | NHS | SEM | p |
|---|---|---|---|---|---|---|
| DM (g kg−1) | 325 b | 348 a | 342 a,b | 359 a | 5.1 | 0.009 |
| DM loss (%) | 6.8 b | 8.6 a | 7.0 b | 5.5 c | 0.12 | <0.001 |
| WSC | 9.5 b | 4.1 c | 7.7 b,c | 33.9 a | 0.89 | <0.001 |
| NH3-N (g kg−1 N) | 168 a | 131 b | 104 c | 83 d | 3.7 | <0.001 |
| pH | 4.75 a | 4.67 a | 4.40 b | 4.36 b | 0.018 | <0.001 |
| Lactic Acid | 42.5 a | 28.2 b | 43.6 a | 45.6 a | 0.96 | <0.001 |
| Acetic Acid | 15.9 b | 32.0 a | 29.0 a | 19.8 b | 1.40 | <0.001 |
| Propionic Acid | 0.2 b | 1.9 a | 0 c | 0 c | 0.04 | <0.001 |
| Butyric Acid | 1.5 a | 0.3 b | 0.3 b | 0 c | 0.01 | <0.001 |
| Ethanol | 13.7 a | 18.4 a | 6.6 b | 3.4 b | 1.05 | <0.001 |
| n-propanol | 0 b | 2.9 a | 0 b | 0.3 b | 0.12 | <0.001 |
| 1,2-propanediol | 1.8 d | 7.4 c | 18.2 a | 11.0 b | 0.40 | <0.001 |
| Yeast Count (log cfu g−1) | 6.3 a | 1.7 c | 2.1 c | 3.9 b | 0.30 | <0.001 |
| ASTA (hours) | 37 b | >288 a | >288 a | 164 a,b | 29.6 | <0.001 |
| TCUM (°C) | 532 a | 58 b | 10 b | 412 a | 67.2 | 0.001 |
| Parameter | CON | LABhe | LABheho | NHS | SEM | p |
|---|---|---|---|---|---|---|
| IVOMD (% of OM) | ||||||
| Before Aeration | 77.8 | 79.0 | 79.2 | 80.3 | 0.57 | 0.786 |
| After Aeration | 75.4 | 77.0 | 77.5 | 79.0 | ||
| Treatment Mean | 76.6 b | 78.0 a,b | 78.3 a,b | 79.7 a | 0.42 | 0.006 |
| Metabolisable energy | ||||||
| (MJ kg−1 DM) | ||||||
| Before Aeration | 9.8 | 10.0 | 10.0 | 10.2 | 0.10 | 0.690 |
| After Aeration | 9.3 | 9.6 | 9.7 | 9.9 | ||
| Treatment Mean | 9.5 b | 9.8 a,b | 9.8 a | 10.0 a | 0.07 | 0.008 |
| Parameter | CON | LABho | LABhe | LABheho | NHS | BNP | SEM | p |
|---|---|---|---|---|---|---|---|---|
| DM (g kg−1) | 229 b | 230 b | 231 a,b | 231 a,b | 232 a,b | 234 a | 0.8 | 0.005 |
| DM loss (%) | 6.0 b | 5.6 c | 6.3 a | 6.2 a,b | 5.7 c | 5.5 c | 0.06 | <0.001 |
| WSC | 10.3 c | 14.4 a,b | 7.8 d | 8.8 c,d | 12.6 b | 16.0 a | 0.41 | <0.001 |
| NH3-N (g kg−1 N) | 73 a,b | 68 c,d | 77 a | 72 b,c | 60 e | 66 c,d | 1.1 | <0.001 |
| pH | 4.17 a | 4.06 b | 4.18 a | 4.19 a | 4.19 a | 4.18 a | 0.014 | <0.001 |
| Lactic Acid | 87.4 a,b | 95.4 a | 80.5 b | 83.9 b | 81.3 b | 82.3 b | 2.35 | 0.007 |
| Acetic Acid | 16.3 c,d | 15.1 d | 20.8 b | 19.0 b,c | 23.1 a | 22.0 a,b | 0.68 | <0.001 |
| Propionic Acid | ND | ND | ND | ND | ND | ND | ||
| Butyric Acid | ND | ND | ND | ND | ND | ND | ||
| Ethanol | 5.2 a | 4.2 a | 5.3 a | 4.6 a | 2.6 b | 2.1 b | 0.30 | <0.001 |
| n-propanol | ND | ND | ND | ND | ND | ND | ||
| 1,2-propanediol | 2.3 c | 2.2 c | 6.0 a | 5.1 a,b | 4.9 a,b | 4.2 b | 0.27 | <0.001 |
| Yeast Count (log cfu g−1) | 3.3 b | 5.7 a | 2.2 b,c | 1.7 c | 1.7 c | 1.8 c | 0.28 | <0.001 |
| ASTA (hours) | 164 c | 105 d | 238 b | 228 b | >336 a | >336 a | 8.1 | <0.001 |
| TCUM (°C) | 708 a,b | 811 a | 452 b | 550 a,b | 27 c | 40 c | 70.2 | <0.001 |
| Parameter | CON | LABho | LABhe | LABheho | NHS | BNP | SEM | p |
|---|---|---|---|---|---|---|---|---|
| IVOMD (% of OM) | ||||||||
| Before Aeration | 91.1 a,A | 91.6 a,A | 90.7 a,A | 91.7 a,A | 90.6 a,A | 91.5 a,A | 0.45 | <0.001 |
| After Aeration | 88.1 c,d,B | 85.7 d,B | 88.4 b,c,A | 87.4 c,d,B | 90.3 a,b,A | 91.7 a,A | ||
| Treatment Mean | 89.6 b,c | 88.7 c | 89.5 b,c | 89.6 b,c | 90.5 a,b | 91.6 a | 0.32 | <0.001 |
| Metabolisable Energy | ||||||||
| (MJ kg−1 DM) | ||||||||
| Before Aeration | 11.4 a,A | 11.5 a,A | 11.3 a,A | 11.5 a,A | 11.3 a,A | 11.4 a,A | 0.07 | <0.001 |
| After Aeration | 10.5 b,B | 10.0 c,B | 10.7 b,B | 10.4 b,B | 11.2 a,A | 11.4 a,A | ||
| Treatment Mean | 10.9 b | 10.7 b | 11.0 b | 10.9 b | 11.3 a | 11.4 a | 0.06 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auerbach, H.; Nadeau, E. Effects of Additive Type on Fermentation and Aerobic Stability and Its Interaction with Air Exposure on Silage Nutritive Value. Agronomy 2020, 10, 1229. https://doi.org/10.3390/agronomy10091229
Auerbach H, Nadeau E. Effects of Additive Type on Fermentation and Aerobic Stability and Its Interaction with Air Exposure on Silage Nutritive Value. Agronomy. 2020; 10(9):1229. https://doi.org/10.3390/agronomy10091229
Chicago/Turabian StyleAuerbach, Horst, and Elisabet Nadeau. 2020. "Effects of Additive Type on Fermentation and Aerobic Stability and Its Interaction with Air Exposure on Silage Nutritive Value" Agronomy 10, no. 9: 1229. https://doi.org/10.3390/agronomy10091229
APA StyleAuerbach, H., & Nadeau, E. (2020). Effects of Additive Type on Fermentation and Aerobic Stability and Its Interaction with Air Exposure on Silage Nutritive Value. Agronomy, 10(9), 1229. https://doi.org/10.3390/agronomy10091229





