Phytomanagement of Chromium-Contaminated Soils Using Cannabis sativa (L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Layout
2.2. Plants Management
2.3. Plant Harvest
2.4. Biomass Analysis and Elements Mass Balance
2.5. Statistical Analysis
3. Results
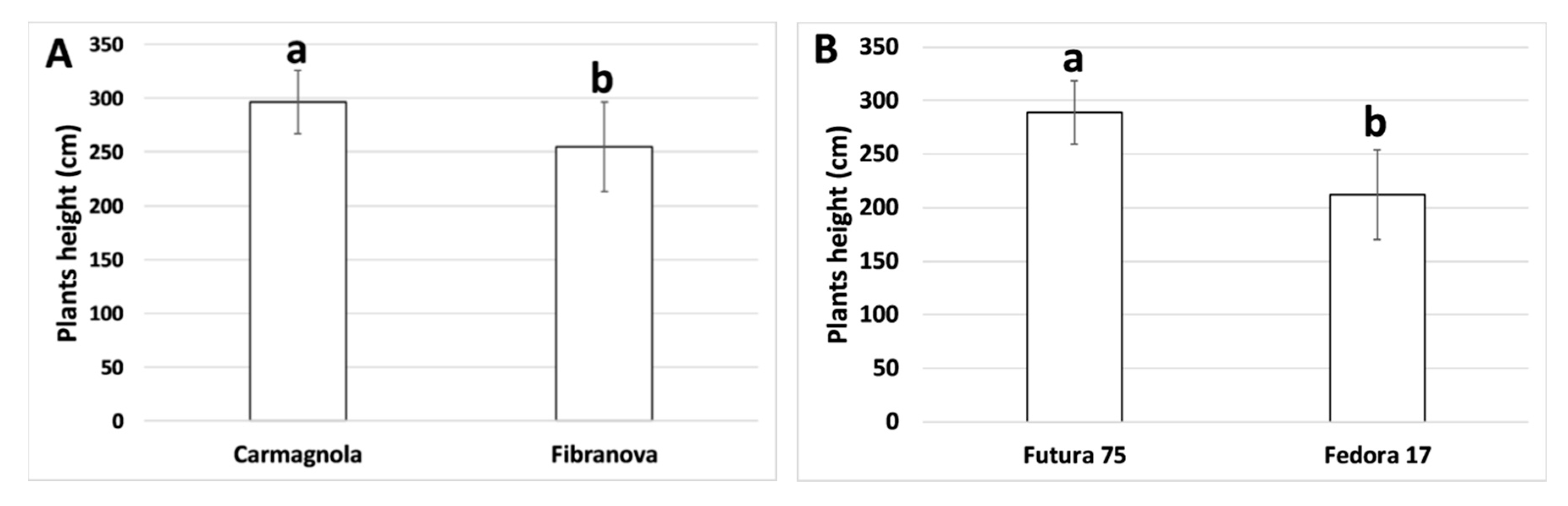
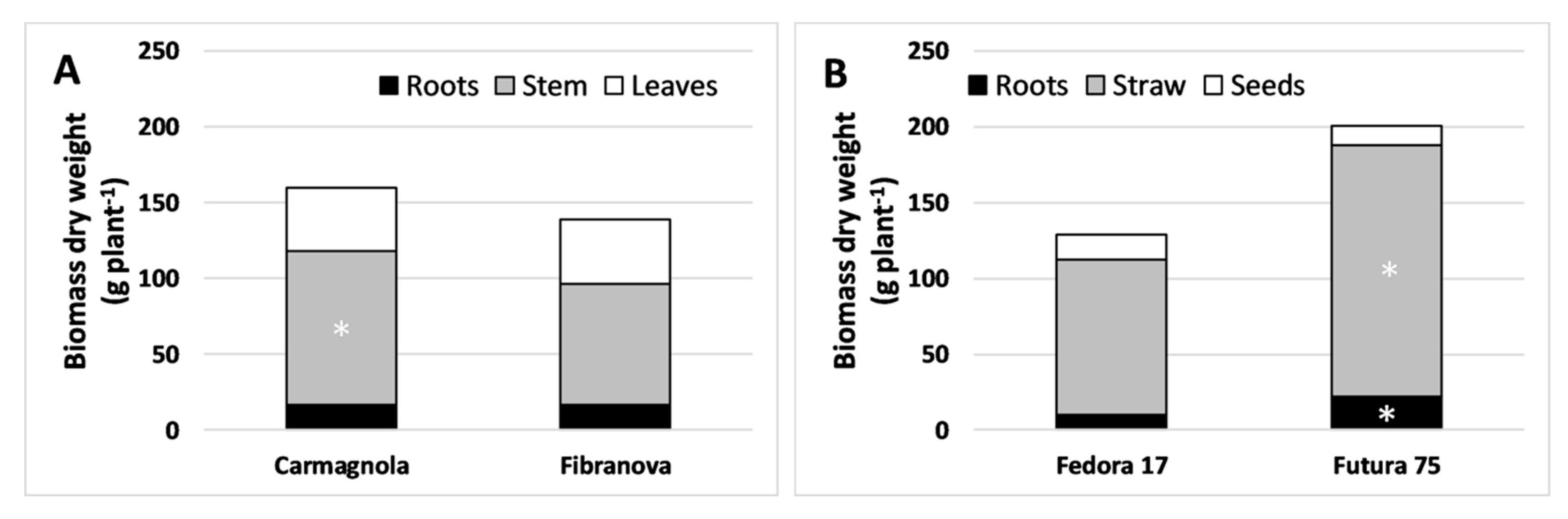
3.1. Morpho-Biometric Characteristics
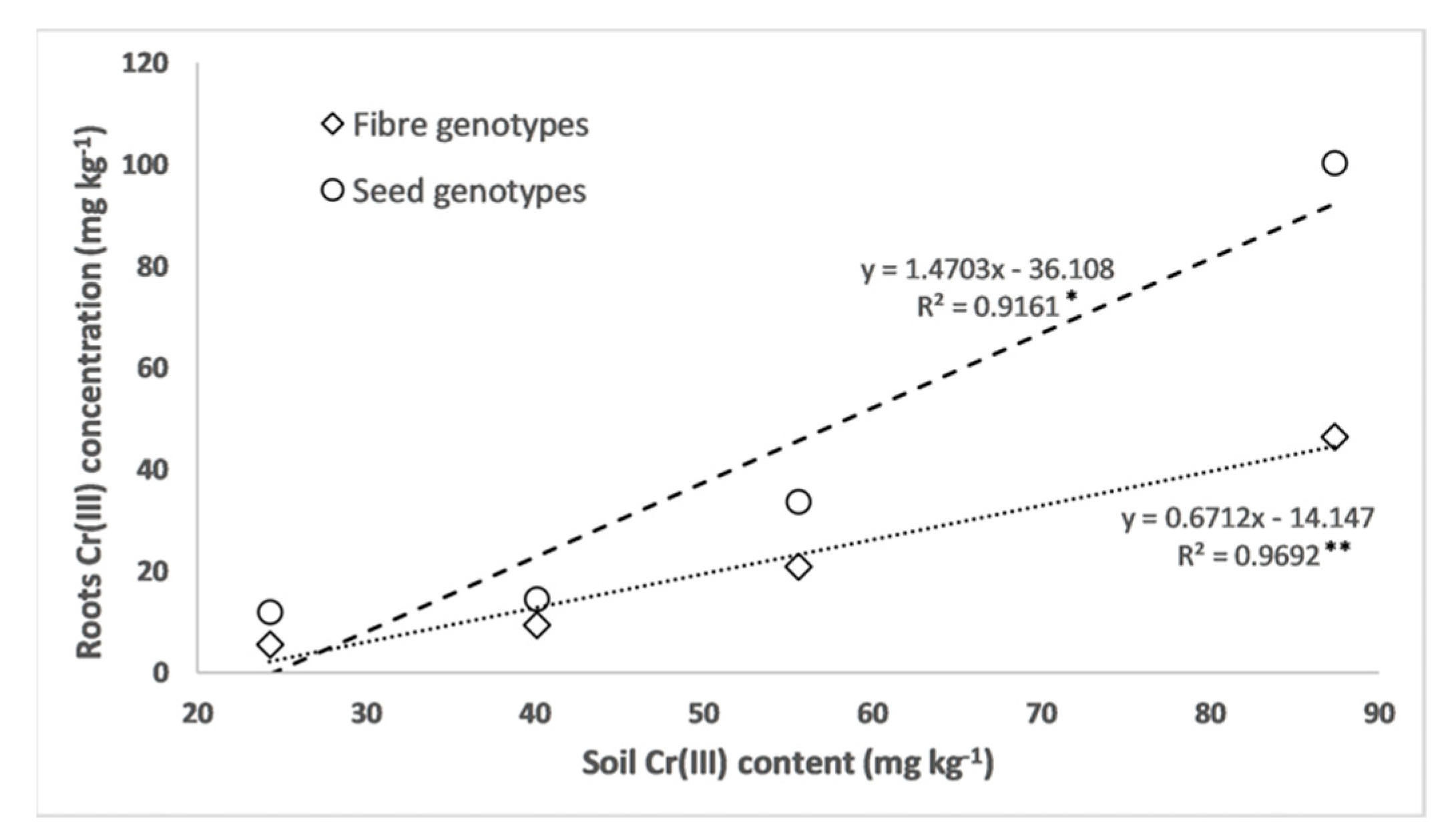
3.2. Chromium and Macronutrient Concentrations in the Biomass
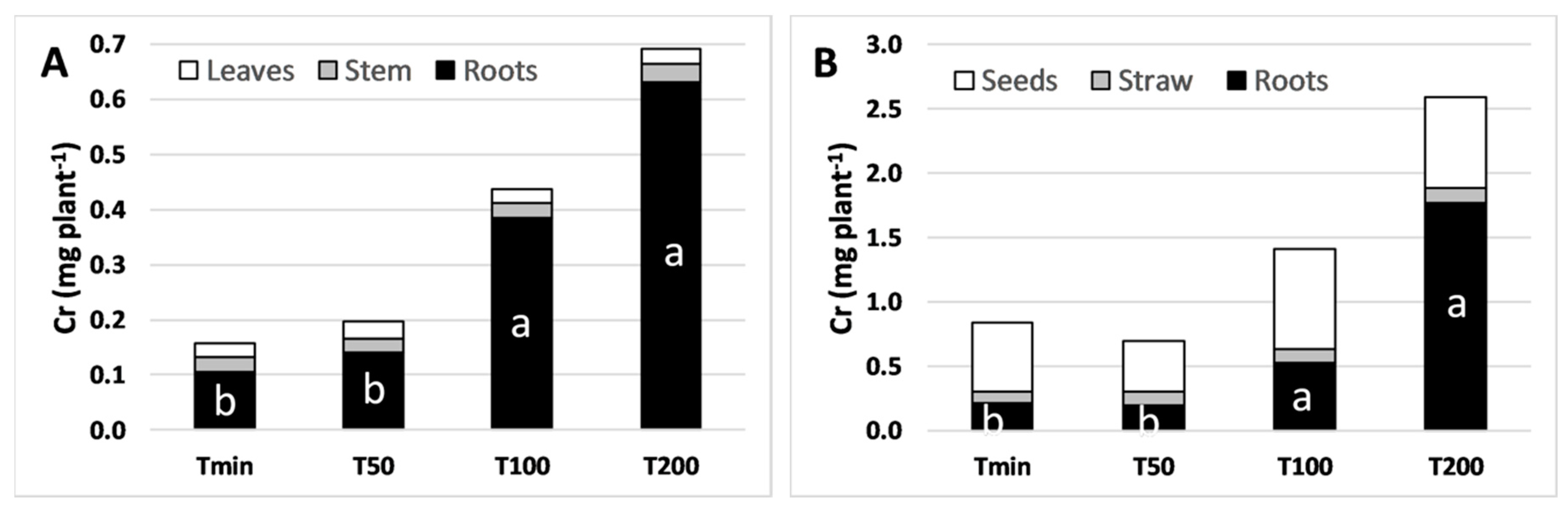
3.3. Chromium and Macronutrients Plant Mass Balance
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schultes, R.E.; Klein, W.M.; Plowman, T.; Lockwood, T.E. Cannabis: An example of taxonomic neglect. Bot. Mus. leaf. Harv. Univ. 1974, 23, 337–367. [Google Scholar]
- Giupponi, L.; Leoni, V.; Carrer, M.; Ceciliani, G.; Sala, S.; Panseri, S.; Pavlovic, R.; Giorgi, A. Overview on Italian hemp production chain, related productive and commercial activities and legislative framework. Ital. J. Agron. 2020. [Google Scholar] [CrossRef]
- Hu, R.; Lim, J.K. Fabrication and mechanical properties of completely biodegradable hemp fiber reinforced polylactic acid composites. J. Compos. Mater. 2007, 41, 1655–1666. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Deferne, J.; Pate, D.W. Hemp seed oil: A source of valuable essential fatty acids. J. Hemp Assoc. 1996, 3, 4–7. [Google Scholar]
- Amaducci, S.; Zatta, A.; Pelatti, F.; Venturi, G. Influence of agronomic factors on yield and quality of hemp (Cannabis sativa L.) fibre and implication for an innovative production system. Field Crop. Res. 2008, 107, 161–169. [Google Scholar] [CrossRef]
- Prade, T.; Svensson, S.E.; Andersson, A.; Mattsson, J.E. Biomass and energy yield of industrial hemp grown for biogas and solid fuel. Biomass Bioenergy 2011, 35, 3040–3049. [Google Scholar] [CrossRef]
- Vamerali, T.; Bandiera, M.; Coletto, L.; Zanetti, F.; Dickinson, N.M.; Mosca, G. Phytoremediation trials on metal-and arsenic-contaminated pyrite wastes (Torviscosa, Italy). Environ. Pollut. 2009, 157, 887–894. [Google Scholar] [CrossRef]
- Linger, P.; Müssig, J.; Fischer, H.; Kobert, J. Industrial hemp (Cannabis sativa L.) growing on heavy metal contaminated soil: Fibre quality and phytoremediation potential. Ind. Crop. Prod. 2002, 16, 33–42. [Google Scholar] [CrossRef]
- Angelova, V.; Ivanova, R.; Delibaltova, V.; Ivanov, K. Bio-accumulation and distribution of heavy metals in fibre crops (flax, cotton and hemp). Ind. Crop. Prod. 2004, 19, 197–205. [Google Scholar] [CrossRef]
- Shi, G.; Cai, Q. Cadmium tolerance and accumulation in eight potential energy crops. Biotechnol. Adv. 2009, 27, 555–561. [Google Scholar] [CrossRef]
- Citterio, S.; Santagostino, A.; Fumagalli, P.; Prato, N.; Ranalli, P.; Sgorbati, S. Heavy metal tolerance and accumulation of Cd, Cr and Ni by Cannabis sativa L. Plant Soil 2003, 256, 243–252. [Google Scholar] [CrossRef]
- Ullahm, R.; Hadi, F.; Ahmad, S.; Jan, A.U.; Rongliang, Q. Phytoremediation of lead and chromium contaminated soil improves with the endogenous phenolics and proline production in Parthenium, Cannabis, Euphorbia, and Rumex species. Water Air Soil Pollut. 2019, 230, 40. [Google Scholar] [CrossRef]
- Burges, A.; Alkorta, I.; Epelde, L.; Garbisu, C. From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. Int. J. Phytoremediat. 2018, 20, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, M.W.H.; Papazoglou, E.G.; Robinson, B.H.; Schulin, R. Phytomanagement: Phytoremediation and the production of biomass for economic revenue on contaminated land. In Phytoremediation, Management of Environmental Contaminants; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 1, pp. 115–132. [Google Scholar]
- Turner, M.A.; Rust, R.H. Effects of chromium on growth and mineral nutrition of soybeans. Soil Sci. Soc. Am. J. 1971, 35, 755–758. [Google Scholar] [CrossRef]
- Khan, A.G. Relationships between chromium biomagnification ratio, accumulation factor, and mycorrhizae in plants growing on tannery effluent-polluted soil. Environ. Int. 2001, 26, 417–423. [Google Scholar] [CrossRef]
- Dube, B.K.; Tewari, K.; Chatterjee, J.; Chatterjee, C. Excess chromium alters uptake and translocation of certain nutrients in citrullus. Chemosphere 2003, 53, 1147–1153. [Google Scholar] [CrossRef]
- Sundaramoorthy, P.; Chidambaram, A.; Ganesh, K.S.; Unnikannan, P.; Baskaran, L. Chromium stress in paddy: (i) nutrient status of paddy under chromium stress; (ii) phytoremediation of chromium by aquatic and terrestrial weeds. C. R. Biol. 2010, 333, 597–607. [Google Scholar] [CrossRef]
- Fässler, E.; Robinson, B.H.; Stauffer, W.; Gupta, S.K.; Papritz, A.; Schulin, R. Phytomanagement of metal-contaminated agricultural land using sunflower, maize and tobacco. Agric. Ecosyst. Environ. 2010, 136, 49–58. [Google Scholar] [CrossRef]
- Nsanganwimana, F.; Pourrut, B.; Waterlot, C.; Louvel, B.; Bidar, G.; Labidi, S.; Fontaine, J.; Muchembled, J.; Lounès-Hadj Sahraoui, A.; Fourrier, H.; et al. Metal accumulation and shoot yield of Miscanthus × giganteus growing in contaminated agricultural soils: Insights into agronomic practices. Agric. Ecosyst. Environ. 2015, 213, 61–71. [Google Scholar] [CrossRef]
- Jeannin, T.; Yung, L.; Evon, P.; Labonne, L.; Ouagne, P.; Lecourt, M.; Cazaux, D.; Chalot, M.; Placet, V. Native stinging nettle (Urtica dioica L.) growing spontaneously under short rotation coppice for phytomanagement of trace element contaminated soils: Fibre yield, processability and quality. Ind. Crop. Prod. 2020, 145, 111997. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Deram, A. Phytomanagement: A realistic approach to soil remediating phytotechnologies with new challenges for plant science. Int. J. Plant Biol. Res. 2014, 2, 1023. [Google Scholar]
- Aubin, M.P.; Seguin, P.; Vanasse, A.; Tremblay, G.F.; Mustafa, A.F.; Charron, J.B. Industrial hemp response to nitrogen, phosphorus, and potassium fertilization. Crop. Forage Turfgrass Manag. 2015, 1, 1–10. [Google Scholar] [CrossRef]
- Struik, P.C.; Amaducci, S.; Bullard, M.J.; Stutterheim, N.C.; Venturi, G.; Cromack, H.T.H. Agronomy of fibre hemp (Cannabis sativa L.) in Europe. Ind. Crop. Prod. 2000, 11, 107–118. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Riggi, E.; Testa, G.; Scordia, D.; Copani, V. Evaluation of European developed fibre hemp genotypes (Cannabis sativa L.) in semi-arid Mediterranean environment. Ind. Crop. Prod. 2013, 50, 312–324. [Google Scholar] [CrossRef]
- Tang, K.; Struik, P.C.; Yin, X.; Calzolari, D.; Musio, S.; Thouminot, C.; Bjelková, M.; Stramkale, V.; Magagnini, G.; Amaducci, S. A comprehensive study of planting density and nitrogen fertilization effect on dual-purpose hemp (Cannabis sativa L.) cultivation. Ind. Crop. Prod. 2017, 107, 427–438. [Google Scholar] [CrossRef]
- Faux, A.M.; Draye, X.; Lambert, R.; d’Andrimont, R.; Raulier, P.; Bertin, P. The relationship of stem and seed yields to flowering phenology and sex expression in monoecious hemp (Cannabis sativa L.). Eur. J. Agron. 2013, 47, 11–22. [Google Scholar] [CrossRef]
- Stafecka, I.; Stramkale, V.; Stramkalis, A.; Kroica, I.; Ivanovs, S. Impact of the agro-environmental factors on the seed yield and yields components productivity of Latvian original hemp. J. Agric. Eng. Res. 2016, 61, 164–167. [Google Scholar]
- Baker, A.J. Accumulators and excluders-strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef]
- Ertani, A.; Mietto, A.; Borin, M.; Nardi, S. Chromium in agricultural soils and crops: A review. Water Air Soil Pollut. 2017, 228, 190. [Google Scholar] [CrossRef]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Shanker, A.K.; Djanaguiraman, M.; Sudhagar, R.; Chandrashekar, C.N.; Pathmanabhan, G. Differential antioxidative response of ascorbate glutathione pathway enzymes and metabolites to chromium speciation stress in green gram (Vigna radiata (L.) R. Wilczek. cv CO 4) roots. Plant Sci. 2004, 166, 1035–1043. [Google Scholar] [CrossRef]
- Caldelas, C.; Bort, J.; Febrero, A. Ultrastructure and subcellular distribution of Cr in Iris pseudacorus L. using TEM and X-ray microanalysis. Cell Biol. Toxicol. 2012, 28, 57–68. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; De la Rosa, G.; Peralta-Videa, J.R.; Montes, M.; Cruz-Jimenez, G.; Cano-Aguilera, I. Differential uptake and transport of trivalent and hexavalent chromium by tumbleweed (Salsola kali). Arch. Environ. Contam. Toxicol. 2005, 48, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowski, M.; Radziemska, M. Effects of chromium (III and VI) on spring barley and maize biomass yield and content of nitrogenous compounds. Toxicol. Env. Heal. A 2010, 73, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Tóth, G.; Hermann, T.; Szatmári, G.; Pásztor, L. Maps of heavy metals in the soils of the European Union and proposed priority areas for detailed assessment. Sci. Total Environ. 2016, 565, 1054–1062. [Google Scholar] [CrossRef]
- Ministry of the Environment, Finland. Government Decree on the Assessment of Soil Contamination and Remediation Needs (214/2007). 2007. Available online: http://faolex.fao.org/docs/pdf/fin113198.pdf (accessed on 18 August 2020).
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) 2014. Scientific opinion on dietary reference values for chromium. EFSA J. 2014, 12, 3845. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| Dry matter | 89.5% |
| Chromium(III) * | 27.5 g kg−1 |
| Total nitrogen * | 55 g kg−1 |
| Total phosphorus * | 10.0 g kg−1 |
| Potassium * | 15 g kg−1 |
| Variety Types | Plant Fractions | Elements | Tmin | T50 | T100 | T200 |
|---|---|---|---|---|---|---|
| Fibre | Roots | Cr (mg Kg−1 dw) | 5.68 ± 7.03 b | 9.55 ± 5.26 b | 20.98 ± 18.42 ab | 46.41 ± 34.51 a |
| N (% dw) | 1.22 ± 0.32 a | 0.87 ± 0.13 b | 0.76 ± 0.23 b | 0.89 ± 0.18 b | ||
| K (mg Kg−1 dw) | 12,951.48 ± 2704.58 a | 5323.26 ± 1568.75 b | 6641.23 ± 4162.18 b | 7258.03 ± 3209.18 b | ||
| P (mg Kg−1 dw) | 1994.87 ± 436.56 a | 1316.11 ± 150.19 b | 1275.63 ± 210.54 b | 1542.95 ± 300.87 b | ||
| Stem | Cr (mg Kg−1 dw) | 0.30 ± 0.10 | 0.27 ± 0.09 | 0.30 ± 0.13 | 0.41 ± 0.18 | |
| N (% dw) | 0.80 ± 0.13 | 0.66 ± 0.12 | 0.65 ± 0.07 | 0.75 ± 0.18 | ||
| K (mg Kg−1 dw) | 10,163.56 ± 2557.41 | 9932.75 ± 1882.04 | 10,724.10 ± 2211.84 | 11,908.36 ± 4572.6 | ||
| P (mg Kg−1 dw) | 1335.28 ± 318.24 | 1422.45 ± 281.29 | 1588.31 ± 442.97 | 1538.28 ± 351.57 | ||
| Leaves | Cr (mg Kg−1 dw) | 0.55 ± 0.10 | 0.72 ± 0.18 | 0.58 ± 0.04 | 0.66 ± 0.11 | |
| N (% dw) | 2.50 ± 0.44 | 2.08 ± 0.41 | 2.11 ± 0.35 | 2.44 ± 0.38 | ||
| K (mg Kg−1 dw) | 14,426.02 ± 1298.27 | 15,983.73 ± 3276.28 | 16,070.84 ± 2967.53 | 17,450.22 ± 4685.19 | ||
| P (mg Kg−1 dw) | 2489.66 ± 445.04 | 2594.71 ± 392.26 | 2487.89 ± 327.08 | 2838.39 ± 545.75 | ||
| Seed | Roots | Cr (mg Kg−1 dw) | 11.92 ± 4.01 b | 14.56 ± 8.94 b | 33.57 ± 30.38 ab | 100.46 ± 109.24 a |
| N (% dw) | 0.83 ± 0.20 | 0.86 ± 0.16 | 0.73 ± 0.20 | 0.86 ± 0.13 | ||
| K (mg Kg−1 dw) | 5495.77 ± 2009.73 | 5239.02 ± 1932.79 | 5017.82 ± 1932.66 | 5724.70 ± 1379.44 | ||
| P (mg Kg−1 dw) | 1778.97 ± 508.31 | 1708.05 ± 377.79 | 1573.90 ± 530.46 | 1649.56 ± 330.49 | ||
| Straw | Cr (mg Kg−1 dw) | 0.74 ± 0.11 | 0.95 ± 0.33 | 0.84 ± 0.34 | 0.78 ± 0.16 | |
| N (% dw) | 1.33 ± 0.11 | 1.31 ± 0.34 | 1.08 ± 0.19 | 1.25 ± 0.29 | ||
| K (mg Kg−1 dw) | 15,320.60 ± 2316.58 | 16,656.38 ± 5158.17 | 1338.93 ± 3832.62 | 15,116.10 ± 3688.81 | ||
| P (mg Kg−1 dw) | 3820.72 ± 659.72 | 4505.96 ± 604.59 | 3912.06 ± 581.18 | 3669.96 ± 1100.12 | ||
| Seeds | Cr (mg Kg−1 dw) | 33.70 ± 16.13 | 36.17 ± 16.45 | 49.30 ± 30.19 | 41.74 ± 22.87 | |
| N (% dw) | 2.62 ± 0.76 | 2.44 ± 0.65 | 2.68 ± 0.62 | 2.55 ± 0.67 | ||
| K (mg Kg−1 dw) | 6621.09 ± 508.78 | 6296.05 ± 681.80 | 6445.32 ± 718.98 | 6865.66 ± 1368.48 | ||
| P (mg Kg−1 dw) | 8510.30 ± 1079.98 | 8909.49 ± 1213.61 | 9097.48 ± 1132.58 | 9352.20 ± 1903.70 |
| Variety Types | Plant Fractions | Elements | Tmin | T50 | T100 | T200 |
|---|---|---|---|---|---|---|
| Fibre | Roots | N (mg plant−1) | 234.5 ± 70.8 a | 128.7 ± 27.8 b | 135.2 ± 61.9 b | 132.9 ± 48.6 b |
| K (mg plant−1) | 253.6 ± 76.3 a | 79.5 ± 28.3 b | 124.0 ± 102.3 b | 114.6 ± 70.1 b | ||
| P (mg plant−1) | 38.6 ± 10.5 a | 19.6 ± 4.5 b | 22.8 ± 8.0 b | 22.5 ± 6.5 b | ||
| Stem | N mg plant−1) | 712.8 ± 103.8 | 630.9 ± 181.2 | 586.9 ± 126.7 | 613.7 ± 162.9 | |
| K (mg plant−1) | 894.2 ± 158.5 | 929.6 ± 204.6 | 954.3 ± 223.6 | 926.2 ± 214.4 | ||
| P (mg plant−1) | 120.8 ± 38.9 | 136.4 ± 45.0 | 139.1 ± 29.0 | 126.1 ± 36.9 | ||
| Leaves | N mg plant−1) | 1094.3 ± 141.4 | 871.3 ± 215.6 | 903.9 ± 163.0 | 918.3 ± 213.3 | |
| K (mg plant−1) | 635.9 ± 70.6 | 660.3 ± 117.5 | 684.0 ± 100.0 | 637.9 ± 138.1 | ||
| P (mg plant−1) | 108.9 ± 15.9 | 108.1 ± 19.6 | 106.1 ± 10.7 | 107.3 ± 30.4 | ||
| Seed | Roots | N (mg plant−1) | 150.7 ± 90.0 | 101.6 ± 56.8 | 125.2 ± 118.1 | 142.3 ± 57.2 |
| K (mg plant−1) | 108.8 ± 86.2 | 69.7 ± 53.4 | 100.7 ± 113.2 | 95.1 ± 43.1 | ||
| P (mg plant−1) | 33.1 ± 22.2 | 20.5 ± 10.9 | 28.0 ± 27.6 | 27.3 ± 10.9 | ||
| Straw | N (mg plant−1) | 1760.6 ± 600.3 | 1534.6 ± 524.9 | 1439.7 ± 506.2 | 1819.7 ± 811.0 | |
| K (mg plant−1) | 1987.0 ± 605.9 | 1908.0 ± 502.3 | 1835.1 ± 505.1 | 2157.2 ± 981.3 | ||
| P (mg plant−1) | 498.4 ± 179.4 | 554.3 ± 226.0 | 517.1 ± 163.2 | 541.1 ± 277.9 | ||
| Seeds | N (mg plant−1) | 422.6 ± 268.5 | 265.5 ± 154.0 | 397.4 ± 266.5 | 467.7 ± 226.5 | |
| K (mg plant−1) | 105.9 ± 49.9 | 65.8 ± 31.7 | 90.5 ± 54.0 | 120.7 ± 49.8 | ||
| P (mg plant−1) | 135.1 ± 57.9 | 94.0 ± 49.0 | 130.3 ± 79.9 | 164.7 ± 63.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raimondi, G.; Rodrigues, J.; Maucieri, C.; Borin, M.; Bona, S. Phytomanagement of Chromium-Contaminated Soils Using Cannabis sativa (L.). Agronomy 2020, 10, 1223. https://doi.org/10.3390/agronomy10091223
Raimondi G, Rodrigues J, Maucieri C, Borin M, Bona S. Phytomanagement of Chromium-Contaminated Soils Using Cannabis sativa (L.). Agronomy. 2020; 10(9):1223. https://doi.org/10.3390/agronomy10091223
Chicago/Turabian StyleRaimondi, Giorgia, Joana Rodrigues, Carmelo Maucieri, Maurizio Borin, and Stefano Bona. 2020. "Phytomanagement of Chromium-Contaminated Soils Using Cannabis sativa (L.)" Agronomy 10, no. 9: 1223. https://doi.org/10.3390/agronomy10091223
APA StyleRaimondi, G., Rodrigues, J., Maucieri, C., Borin, M., & Bona, S. (2020). Phytomanagement of Chromium-Contaminated Soils Using Cannabis sativa (L.). Agronomy, 10(9), 1223. https://doi.org/10.3390/agronomy10091223






