Abstract
The increase of bacterial community tolerance to Cu, and of cotolerance to the antibiotics tetracycline (TC), oxytetracycline (OTC) and chlortetracycline (CTC), was studied in three soils spiked with six different Cu concentrations (resulting in 0, 125, 250, 500, 750 and 1000 mg kg−1 into soils) in a laboratory experiment, after 42 days of incubation. The results show significant increases of bacterial community tolerance to the metal when soil Cu concentrations were between 125 and 500 mg kg−1. Moreover, Cu soil pollution also caused cotolerance to the three antibiotics studied but for higher Cu concentrations (1000 mg kg−1).
1. Introduction
Soil pollution due to Cu is widely extended worldwide, mainly caused by human activities such as agriculture, mining or industry. The use of Cu-based fungicides is of special importance in agriculture, especially in crops with high economic value, such as fruits and vineyards [1]. However, the use of Cu fungicides in agriculture is currently under discussion due to its high persistence and potential harmful effects on soil microbes [2,3,4,5]. In fact, the European Commission restricted the amounts of Cu per hectare and year that can be used in agriculture [6]. Therefore, an increase in knowledge regarding the impact of Cu accumulation on soil microbes is of key importance for clarifying the discussions about Cu uses in agriculture. One understudied effect, especially regarding allowable threshold for Cu concentrations, is the increase of bacterial community tolerance to antibiotics in Cu polluted soils. It is well known that the increase of the concentration of any pollutant in a soil may suppose a selection pressure on soil bacterial communities, causing tolerance to that pollutant [7]. This effect is called pollution-induced community tolerance (PICT) and may be useful to quantify the harmful effects produced by pollutants on soil bacteria. In previous studies, various authors have observed increases in bacterial community tolerance to Cu in soils polluted with this metal [8,9,10,11]. Moreover, Cu pollution may increase the bacterial community tolerance to other pollutants, such as different heavy metals or antibiotics [5,12,13]. This effect of cotolerance for metals and antibiotics is a matter of high concern [14], since metal pollution in the environment may play an important role in the proliferation of antibiotic resistance [15,16,17].
Taking into account that the increase of bacterial community resistance to antibiotics has become a crucial threat to public health worldwide, the study of eventual increases in bacterial community resistance to antibiotics in Cu polluted soils is of major importance. The studies about this topic may be addressed using DNA fingerprints (such as the presence of resistant genes) or from a functional point of view (PICT), i.e., direct measurements of the bacterial community tolerance to antibiotics. Although DNA techniques (for resistant genes) may be more sensitive than functional endpoints, they do not directly imply confirmation of toxicity. In addition, the interpretation of the data on changes in resistance genes is not always easy [18]. Therefore, functional endpoints such as bacterial community tolerance (PICT) to antibiotics are also needed. However, up to date, many studies focused on resistance genes [19,20,21], but only few studies dealing with functional tolerance (PICT) are available. Among them, Berg et al. [12] studied cotolerance to antibiotics (chloramphenicol, nalidixic acid, olaquindox, streptomycin, tetracycline, ampicillin and vancomycin) in a Cu polluted soil; Fernández-Calviño and Bååth [5] studied cotolerance to bronopol, streptomycin, chloramphenicol, vancomycin, tetracycline and tylosin, while Li et al. [10] studied cotolerance to tylosin and vancomycin, both studies being also performed in Cu-polluted soils; Liu et al. [22] studied the cotolerance to tylosin in a Cu polluted soil; and Song et al. [13] studied cotolerance to tetracycline in a Cu and Zn polluted soil. However, no studies have been performed focusing on bacterial community tolerance to oxytetracycline and chlortetracycline in Cu polluted soils. The family of tetracycline antibiotics is of special concern because they are the antibiotics most used in veterinary in the European Union [23], and among them the most widely used are tetracycline (TC), oxytetracycline (OTC) and chlortetracycline (CTC) [24]. Therefore, increases in bacterial community tolerance to these antibiotic represent and important risk for livestock and also for human heath if these bacteria reach drinking water or are present in irrigation water used for fruits and vegetables [25,26,27].
In the current work we hypothesize that soil contamination with Cu will induce functional bacterial community tolerance to this metal but also functional cotolerance to the antibiotics tetracycline (TC), oxytetracycline (OTC) and chlortetracycline (CTC). Therefore, the aims of this study are: firstly, to verify the eventual development of tolerance to Cu and cotolerance to TC, OTC and CTC in soil bacterial communities exposed to different Cu concentrations; secondly, to determine the Cu concentrations from which bacterial communities develop tolerance to Cu and cotolerance to antibiotics (relevant to stablish Cu threshold levels in soils); thirdly, to evaluate eventual differences in tolerance and cotolerance development for soils differing in texture, total carbon content and effective cation exchange capacity.
2. Materials and Methods
2.1. Chemicals
Tetracycline hydrochloride (TC, CAS. 64-75-5; ≥95% in purity), oxytetracycline hydrochloride (OTC, CAS 2058-46-0; ≥95% in purity) and chlortetracycline hydrochloride (CTC, CAS 64-72-2; ≥97% in purity) were supplied by Sigma–Aldrich (Steinheim, Germany). Copper (as CuSO4 5H2O, CAS: 7758-99-8, 99% in purity), was supplied by Panreac (Barcelona, Spain).
2.2. Soil Samples
Three soils from A Limia area, located in Southeast of Galicia (NW Spain), were selected form a set of soils previously analyzed by Conde-Cid et al. [28]. The three soils were classified as Mollic Umbrisols (Anthric) according to IUSS Working Group WRB [29]. Soil samples were taken with a soil auger (0–20 cm depth) to an overall amount of 2 kg (10 subsamples) along each sampling plot. Soil subsamples were subsequently mixed into a single composite soil sample for each of the three soils. Once in the laboratory, composite soil samples were air-dried, sieved through a 2 mm mesh and stored in polyethylene bottles until analysis.
The proportions of sand (particle size 2–0.05 mm), silt (0.05–0.002 mm) and clay (<0.002 mm) of the soils were determined by wet sieving for the size fractions greater than 0.05 mm and using the international pipette method for all others. The pH in water (pHw) was measured at a soil/water ratio of 1:2.5 after 10 min using a glass electrode. Total carbon and nitrogen contents were determined on a ThermoFinnigan 1112 Series NC elemental analyzer. The effective cation exchange capacity at soil pH (eCEC) was estimated as the sum of exchangeable basic cations (K, Na, Ca, Mg) extracted with 0.2 M NH4Cl [30], and exchangeable Al extracted with 1 M KCl [31]. Available phosphorus was extracted using 0.5 M NaHCO3 and determined using the phosphomolybdic complex method [32]. Total concentrations of Na, K, Ca, Mg, Al, Fe, Mn, as well as As, Cd, Cr, Cu, Ni, Pb, and Zn, were determined using ICP-mass spectrometry (820-NS, Varian, Palo Alto, CA, USA), after nitric acid (65%) microwave assisted digestion.
The studied soils present different textures [sandy loam (soil 1), clay loam (soil 2) and sandy clay loam (soil 3)], total carbon contents (between 1.1 and 10.9%) and effective cation exchange capacity (4.1–11.7 cmolc kg−1), while similar pH (4.5–4.8) and available P (136–262 mg kg−1). In addition, the studied soils present similar (and low) Cu concentrations (between 10.7 and 21.6 mg kg−1). These and other soil characteristics are shown in Table 1.

Table 1.
General characteristics of the three soils used.
2.3. Experimental Design
The three soils were spiked with Cu, incubated during a 42 days period (in order to have enough time for microbial communities adaptation to the new conditions), and then, the bacterial community tolerance to Cu and the three antibiotics (TC, OTC and CTC) was estimated.
The procedure used for soil spiking with Cu was as follows: dry soil samples were weighted in 54 polypropylene jars (5 g of soil on each jar), rewetted until 40% of water holding capacity to recover the microbial activity, and incubated at 22 °C for 15 days. Then, soil samples were spiked by triplicate with different Cu concentrations (Cu added as CuSO4 5H2O), giving soil Cu concentrations of 0, 125, 250, 500, 750 and 1000 mg kg−1) and reaching a moisture content of 80% of the water holding capacity. Then, the resulting 54 microcosms (3 soils × 6 Cu concentrations × 3 replications) were incubated at 22 °C in darkness during 42 days. Each microcosm was incubated in 50 mL polypropylene bottles in systems allowing air-exchange, at constant moisture content, adding water when necessary.
PICT, i.e., bacterial community tolerance to Cu and antibiotics (TC, OTC and CTC) was estimated essentially according to Bååth [2] and Díaz-Raviña et al. [33]. In brief, after 42 days of incubation, soil bacteria were extracted from soil microcosms using homogenization and centrifugation techniques [34], i.e., mixing soil samples with distilled water (ratio soil:water 1:20) using a multivortex shaker for 3 min at maximum intensity. The resulting soil suspensions were then centrifuged for 10 min at 1000× g to obtain a bacterial suspension (supernatant). Then, aliquots (1.35 mL) of the bacterial suspension were transferred to 2 mL microcentrifugation tubes and mixed with 0.15 mL of different concentrations of Cu or antibiotics (TC, OTC or CTC). Seven different concentrations of each compound (Cu, TC, OTC or CTC), plus a control with only distilled water, were used for each compound and soil sample. The final concentrations varied between 10−5 and 10−2 mol L−1 for Cu; and between 0.01 and 400 mg L−1 for TC, OTC and CTC. On each microcentrifugation tube, bacterial community growth was estimated using the [3H]leucine incorporation method [35]: 0.2 µL of [3H]Leu (37 MBq mL−1 and 5.74 TBq mmol−1; Amersham) were added with nonlabeled Leu to each microcentrifugation tube, resulting in 275 nM Leu in the bacterial suspensions. After 2 h of incubation at 22 °C, growth was terminated by adding 75 mL of 100% trichloroacetic acid. Washing was performed as described by Bååth et al. [35], and subsequent measurement of radioactivity was performed using a liquid scintillation counter (Tri-Carb 2810 TR, PerkinElmer, Waltham, MA, USA). The resulting [3H] leucine incorporation data (an estimation of bacterial community growth) were used to perform dose-response curves (leucine incorporation vs. Cu or antibiotic concentration), from which bacterial community tolerance to Cu (or antibiotics) indexes (log IC50) were estimated.
2.4. Data Analyses
The tolerance of the bacterial community to Cu and the three antibiotics (TC, OTC and CTC) was estimated as log IC50, the logarithm of the concentration that resulted in 50% inhibition of bacterial community growth (leucine incorporation) in the dose-response curves. A higher value of log IC50 indicates a higher community tolerance, while a lower value indicates that Cu or the antibiotics are more toxic to the bacterial community, i.e., it is less tolerant. Log IC50 was calculated using a logistic model, a model commonly used for IC50 estimations from dose-response curves [36,37,38]: Y = c/[1 + eb(X−a)], where Y is the measured level of Leu incorporation, X is the logarithm of the substance (Cu, TC, OTC or CTC) added to the bacterial suspension, a is the log IC50, c the bacterial growth rate in absence of the toxic substance, and b is a slope parameter indicating the inhibition rate.
The distribution of the data was tested for normality by the Kolmogorov-Smirnov (K-S) test and for variance homogeneity by the Levene’s Test. Significant increases of bacterial community tolerance to Cu or to antibiotics were estimated by analysis of variance (ANOVA) followed by Dunnett’s posthoc test. This test allowed comparison of the statistical differences between control soils (no Cu added) and soils spiked with Cu.
3. Results
Results showed clear dose-response curves for all soils and substances tested (Figure 1). However, for soil 1 (with low carbon content), it was not possible to obtain dose-response curves, due to the high inhibition of bacterial growth taking place for soil Cu concentrations higher than 125 mg kg−1.
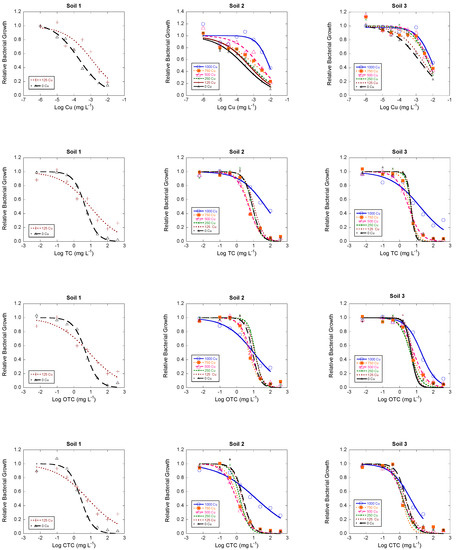
Figure 1.
Dose-response curves showing the inhibition of bacterial community growth in response to increases of Cu or antibiotics (TC, OTC and CTC) concentrations in the bacterial suspensions, expresses as log concentration (mg L−1). Each line represents one of the six microcosms tested in the soil (0, 125, 250, 500, 750 and 1000 mg Cu kg−1 of soil). Dose-response curves were modeled using a logistic model. A curve shifted to the right indicates increases in bacterial community tolerance to Cu or antibiotics (TC, OTC or CTC). TC: tetracycline; OTC: oxytetracycline; CTC: chlortetracycline.
Clear increases in bacterial community tolerance to Cu (PICT) were found as a function of Cu concentration rise, i.e., dose-response curves shifted to the right as Cu concentration in the soil increased (Figure 1). In order to check the magnitude of bacterial community tolerance to Cu increases, log IC50 values were estimated from dose-response curves using the logistic model (Figure 2), with R2 values > 0.9 in most cases. Looking to log IC50 values (Figure 2), significant increases of bacterial community tolerance to Cu were found in soil samples polluted with Cu concentrations ≥125 mg kg−1 (for soil 1), ≥500 mg kg−1 (for soil 2), and ≥250 mg kg−1 (for soil 3). For 125 mg kg−1 of Cu, bacterial community tolerance to Cu (PICT) increased in 0.81, 0.27 and 0.18 log units in soils 1, 2 and 3, while for the highest Cu concentration (1000 mg kg−1) PICT increased 1.45 and 0.63 log units for soil 2 and soil 3, respectively.
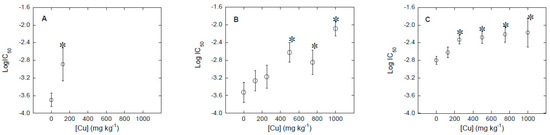
Figure 2.
Variation of bacterial community tolerance to Cu (expressed as log IC50), as a function of Cu concentration in the soil. (A): soil 1; (B): soil 2; and (C): soil 3. * Indicates significant increases in log IC50 compared to the control log IC50 (p < 0.05).
The results obtained in this study also show that Cu pollution may cause increases in bacterial community tolerance to the antibiotics TC, OTC and CTC (Figure 1; Figure 3). However, according to log IC50 values, the increase in bacterial community tolerance to antibiotics was only significant for the highest dose of Cu (1000 mg kg−1) in soils 2 and 3 (in the case of TC and CTC), while it was only significant for OTC in soil 3. For soils 2 and 3, the magnitude of increases in bacterial community tolerance to antibiotics for soil samples polluted with 1000 mg kg−1 of Cu were (expressed in log units): for TC, 0.53 (soil 2) and 0.79 (soil 3); for OTC, 0.07 (soil 2; not significant) and 0.61 (soil 3); and for CTC, 0.77 (soil 2) and 0.27 (soil 3). Although not significant, in soil 1 the magnitude of bacterial community tolerance to antibiotics (expressed as log IC50) was relatively high, reaching values of 0.29, 0.19 and 0.40 log unit for TC, OTC and CTC (respectively) in the sample polluted with Cu at a dose of 125 mg kg−1. Figure 3 also shows that, for intermediate Cu concentrations, the bacterial community tolerance to antibiotics decreased in some cases.
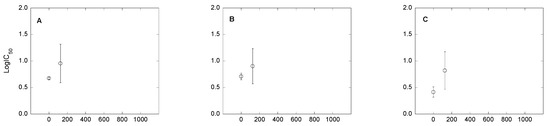
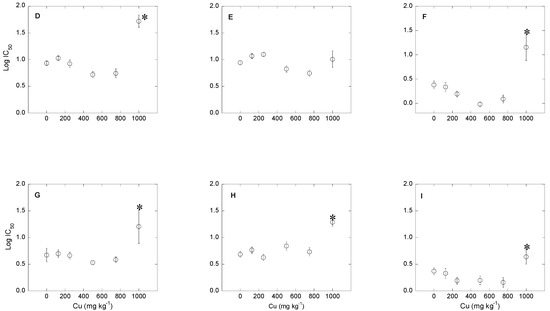
Figure 3.
Variation of bacterial community tolerance to antibiotics (expressed as log IC50), as a function of Cu concentration in the soil. (A–C): soil 1; (D–F): soil 2; and (G–I): soil 3. (A,D,G): tetracycline; (B,E,H): oxytetracycline; and (C,F,I): chlortetracycline. * Indicates significant increases in log IC50 compared to the control log IC50 (p < 0.05).
4. Discussion
The addition of Cu to the soils studied caused a significant increase in bacterial community tolerance to Cu in Cu-polluted soils (with higher tolerance for higher Cu concentrations), which is an expected result, previously found by other authors [5,13,33,39,40,41]. However, the present work shows that the magnitude of those increases was different for different soils, suggesting that increases in bacterial community tolerance to Cu in Cu-polluted soils may be highly influenced by soil characteristics. Thus, soil management may modulate the increases of bacterial community tolerance to Cu (and hence Cu toxicity). Therefore, further studies would be needed to clarify the influence of soil properties on bacterial community tolerance to Cu, and hence improve soil management in Cu-polluted soils, such as those devoted to vineyards [42].
Cu pollution also causes increases in bacterial community tolerance to tetracycline (TC) antibiotic, as previously reported [5,12,13]. Bearing in mind that bacterial community tolerance to TC in Cu-polluted soils has been found only for three tested soils in previous works, the results of the present work for three more soils support the previous research. Moreover, this work is the first one showing the increase of bacterial community tolerance for oxytetracycline (OTC) and chlortetracycline (CTC) in Cu-polluted soils, enhancing the previous short list of antibiotics for which it had been shown that Cu pollution may cause bacterial community tolerance: tetracycline, vancomycin and tylosin [5,12]. In relation to coselection mechanisms, there are a great variety that may be involved, such as: coresistance, cross-resistance or coregulation [14], but their relative importance is still unknown [13]. However, irrespective of the cotolerance mechanisms involved, increasing the knowledge about Cu concentrations that may induce bacterial community tolerance to antibiotics is of key importance. The cotolerance to the three antibiotics here studied was found for Cu concentrations higher than those that increased tolerance to Cu (Figure 2), in accordance with results previously found for TC in a Cu-polluted soil [5] but also for cotolerance to metals [33] or phenols [43]. In the present work, significant increases in bacterial community tolerance to TC, OTC and CTC were found for 1000 mg kg−1 of Cu. In relation with these data, Fernández-Calviño and Bååth [5] found that, for a Cu concentration in soil ≥500 mg kg−1, there was bacterial community tolerance to TC, while Song et al. [13] found significant bacterial community tolerance to TC for Cu concentration in the soil ≥365 mg kg−1. Together with the magnitude of the changes, these differences found among different works suggest that increases in bacterial community tolerance to TC in Cu-polluted soils are dependent on soil type. The most important parameters for Cu availability in soils, and hence for Cu toxicity, are soil pH and organic matter content [44,45]. Therefore, increasing the soil pH and/or organic matter content in soils may reduce the risk of increases of bacterial community tolerance to tetracycline antibiotics in these soils. However, the increases of bacterial community tolerance to tetracycline antibiotics found in the present work did not show a clear pattern for the different soils, suggesting that other factors may affect those increases. Therefore, in order to clarify the effect of soil properties on the increase of bacterial community tolerance to tetracycline antibiotics, further research using a higher number of soil samples is needed.
The increases of bacterial community tolerance to TC, OTC and CTC antibiotics for Cu concentrations around 1000 mg kg−1, indicate that, in fact, real increases of this kind are relatively improbable in current vineyard soils worldwide, since actual Cu concentrations in vineyards generally range between 100 and 200 mg kg−1 [42]. However, in some vineyard soils, values higher than 1000 mg kg−1 were found [46,47]. Moreover, in vineyard soils with common and lower Cu values (100–200 mg kg−1), it is probable that some hotspots with Cu concentrations higher than 1000 mg kg−1 occur, due to the high horizontal [48] and vertical [49] variability found for Cu in vineyards soil. Therefore, those soil need an especial control to avoid potential environmental problems and human health risks. In addition, results found for soil 1 in the current study, although not significant, suggest that increases in bacterial community tolerance to antibiotics may occur in soils with Cu concentrations around 100 and 200 mg kg−1, and therefore many soils worldwide may present this type of problem. Therefore, more research is needed to identify the most sensitive soils to this potential issue, together with soil characteristics that may be modified to minimize it.
Finally, the decreases in the bacterial community tolerance to antibiotics found in some cases for intermediate Cu concentrations (250–500 mg kg−1) (Figure 3) may be due to the development of bacterial community tolerance to Cu for those intermediate Cu concentrations, together with more sensitivity to antibiotics. However, at high Cu concentrations (1000 mg kg−1), bacterial communities developed tolerance to both Cu and antibiotics. These results suggest that a different tolerance mechanism may take place at intermediate and high Cu concentrations, but further research would be needed to deepen understanding and clarify this possibility.
5. Conclusions
Soil spiking with Cu caused bacterial community tolerance to this metal in the three soils studied for Cu concentrations ranging between 125 and 500 mg kg−1. Moreover, the addition of Cu to the soils also caused cotolerance to the three antibiotics tested: tetracycline, oxytetracycline and chlortetracycline. However, the Cu concentration needed to achieve cotolerance to these three tetracycline antibiotics was much higher (around 1000 mg kg−1). In addition, the results suggest that the magnitude of bacterial community tolerance to tetracycline antibiotics in Cu polluted soils may be different in soils with different characteristics. These results have environmental implications and could be taken into account when programming management practices for soils with high Cu levels in order to reduce risks of damage to soil microbiota, the surrounding environment and livestock and human health.
Author Contributions
Conceptualization, M.A.-E., A.N.-D. and E.Á.-R.; methodology, M.D.-R., D.F.-C.; formal analysis, V.S.-M. and M.J.F.-S.; investigation, V.S.-M. and D.F.-C.; writing—original draft preparation, V.S.-M. and M.D.-R.; writing—review and editing, A.N.-D. and D.F.-C.; funding acquisition, M.A.-E., M.J.F.-S., E.Á.-R. and A.N.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This study has been funded by Xunta de Galicia (Consellería de Economía, Emprego e Industria) through the project ED431F 2018/06 and by the Spanish Ministry of Economy and Competitiveness through the projects CGL2015-67333-C2-1-R and -2-R (FEDER Funds). Research group was also funded by Xunta de Galicia via CITACA Strategic Partnership (ED431E 2018/07) and BV1 research group (ED431C 2017/62-GRC). David Fernández Calviño holds a Ramón y Cajal contract (RYC-2016-20411), financed by the Spanish Ministry of Economy, Industry and Competitiveness. Vanesa Santás Miguel holds a predoctoral fellowship founded by the University of Vigo.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Fernández-Calviño, D.; Soler-Rovira, P.; Polo, A.; Díaz-Raviña, M.; Arias-Estevez, M.; Plaza, C. Enzyme activities in vineyard soils long-term treated with copper-based fungicides. Soil Boil. Biochem. 2010, 42, 2119–2127. [Google Scholar] [CrossRef]
- Bååth, E. Measurement of heavy metal tolerance of soil bacteria using thymidine incorporation into bacteria extracted after homogenization-centrifugation. Soil Boil. Biochem. 1992, 24, 1167–1172. [Google Scholar] [CrossRef]
- Bruins, M.R.; Kapil, S.; Oehme, F.W. Microbial Resistance to Metals in the Environment. Ecotoxicol. Environ. Saf. 2000, 45, 198–207. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Schwenke, G.; Van Zwieten, L. Impact of agricultural inputs on soil organisms—A review. Soil. Res. 2006, 44, 379–406. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Bååth, E. Co-selection for antibiotic tolerance in Cu-polluted soil is detected at higher Cu-concentrations than increased Cu-tolerance. Soil Boil. Biochem. 2013, 57, 953–956. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2018/1981 of 13 December 2018 Renewing the Approval of the Active Substances Copper Compounds, as Candidates for Substitution, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market, and Amending the Annex to Commission Implementing Regulation (EU) No 540/2011; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Blanck, H. A Critical Review of Procedures and Approaches Used for Assessing Pollution-Induced Community Tolerance (PICT) in Biotic Communities. Hum. Ecol. Risk Assess. Int. J. 2002, 8, 1003–1034. [Google Scholar] [CrossRef]
- Díaz-Raviña, M.; De Anta, R.C.; Bååth, E. Tolerance (PICT) of the Bacterial Communities to Copper in Vineyards Soils from Spain. J. Environ. Qual. 2007, 36, 1760–1764. [Google Scholar] [CrossRef]
- Wakelin, S.; Gerard, E.; Black, A.; Hamonts, K.; Condron, L.; Yuan, T.; Van Nostrand, J.D.; Zhou, J.; O’Callaghan, M. Mechanisms of pollution induced community tolerance in a soil microbial community exposed to Cu. Environ. Pollut. 2014, 190, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, B.; Zhang, X.; Gao, M.; Wang, J. Effects of Cu exposure on enzyme activities and selection for microbial tolerances during swine-manure composting. J. Hazard. Mater. 2015, 283, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calviño, D.; Bååth, E. Interaction between pH and Cu toxicity on fungal and bacterial performance in soil. Soil Boil. Biochem. 2016, 96, 20–29. [Google Scholar] [CrossRef]
- Berg, J.; Thorsen, M.K.; Holm, P.E.; Jensen, J.; Nybroe, O.; Brandt, K.K. Cu Exposure under Field Conditions Coselects for Antibiotic Resistance as Determined by a Novel Cultivation-Independent Bacterial Community Tolerance Assay. Environ. Sci. Technol. 2010, 44, 8724–8728. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Rensing, C.; Holm, P.E.; Virta, M.; Brandt, K.K. Comparison of Metals and Tetracycline as Selective Agents for Development of Tetracycline Resistant Bacterial Communities in Agricultural Soil. Environ. Sci. Technol. 2017, 51, 3040–3047. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Wright, M.S.; Stepanauskas, R.; McArthur, J. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006, 14, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Summers, A.O.; Wireman, J.; Vimy, M.J.; Lorscheider, F.L.; Marshall, B.; Levy, S.B.; Bennett, S.; Billard, L. Mercury released from dental “silver” fillings provokes an increase in mercury-and antibiotic-resistant bacteria in oral and intestinal floras of primates. Antimicrob. Agents Chemother. 1993, 37, 825–834. [Google Scholar] [CrossRef]
- Alonso, A.; Sánchez, P.; Martínez, J.L. Environmental selection of antibiotic resistance genes. Environ. Microbiol. 2001, 3, 1–9. [Google Scholar] [CrossRef]
- Cao, J.; Yang, G.; Mai, Q.; Zhuang, Z.; Zhuang, L. Co-selection of antibiotic-resistant bacteria in a paddy soil exposed to as (III) contamination with an emphasis on potential pathogens. Sci. Total Environ. 2020, 725, 138367. [Google Scholar] [CrossRef]
- Milenkovski, S.; Bååth, E.; Lindgren, P.-E.; Berglund, O. Toxicity of fungicides to natural bacterial communities in wetland water and sediment measured using leucine incorporation and potential denitrification. Ecotoxicology 2010, 19, 285–294. [Google Scholar] [CrossRef]
- Poole, K. At the Nexus of Antibiotics and Metals: The Impact of Cu and Zn on Antibiotic Activity and Resistance. Trends Microbiol. 2017, 25, 820–832. [Google Scholar] [CrossRef]
- Guo, T.; Lou, C.; Zhai, W.; Tang, X.; Hashmi, M.Z.; Murtaza, R.; Li, Y.; Liu, X.; Xu, J. Increased occurrence of heavy metals, antibiotics and resistance genes in surface soil after long-term application of manure. Sci. Total Environ. 2018, 635, 995–1003. [Google Scholar] [CrossRef]
- Liu, K.; Sun, M.; Ye, M.; Chao, H.; Zhao, Y.; Xia, B.; Jiao, W.; Feng, Y.; Zheng, X.; Liu, M.; et al. Coexistence and association between heavy metals, tetracycline and corresponding resistance genes in vermicomposts originating from different substrates. Environ. Pollut. 2019, 244, 28–37. [Google Scholar] [CrossRef]
- Liu, B.; Li, Y.; Gao, S.; Chen, X. Copper exposure to soil under single and repeated application: Selection for the microbial community tolerance and effects on the dissipation of antibiotics. J. Hazard. Mater. 2017, 325, 129–135. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. European Medicines Agency. European surveillance of veterinary antimicrobial consumption. In Sales of Veterinary Antimicrobial Agents in 29 European Countries in 2014, (EMA/61769/2016); European Union: Hague, The Netherlands, 2016. [Google Scholar]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Stackelberg, P.E.; Gibs, J.; Furlong, E.T.; Meyer, M.; Zaugg, S.D.; Lippincott, R.L. Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds. Sci. Total Environ. 2007, 377, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Peñalver, J.J.; Pacheco, C.V.G.; Sánchez-Polo, M.; Utrilla, J.R. Degradation of tetracyclines in different water matrices by advanced oxidation/reduction processes based on gamma radiation. J. Chem. Technol. Biotechnol. 2012, 88, 1096–1108. [Google Scholar] [CrossRef]
- Arikan, O.A.; Rice, C.; Codling, E. Occurrence of antibiotics and hormones in a major agricultural watershed. Desalination 2008, 226, 121–133. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Álvarez-Esmorís, C.; Paradelo, R.; Nóvoa-Muñoz, J.C.; Arias-Estevez, M.; Álvarez-Rodriguez, E.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A. Occurrence of tetracyclines and sulfonamides in manures, agricultural soils and crops from different areas in Galicia (NW Spain). J. Clean. Prod. 2018, 197, 491–500. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods of Soil Analysis Part 3: Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Bertsch, P.M.; Bloom, P.R. Aluminium. In Methods of Soil Analysis Part 3: Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Propertie; Page, A.L., Miller, L.H., Keeney, D.R., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Díaz-Raviña, M.; Bååth, E.; Frostegård, Å. Multiple Heavy Metal Tolerance of Soil Bacterial Communities and Its Measurement by a Thymidine Incorporation Technique. Appl. Environ. Microbiol. 1994, 60, 2238–2247. [Google Scholar] [CrossRef]
- Bååth, E. Thymidine and leucine incorporation in soil bacteria with different cell size. Microb. Ecol. 1994, 27, 267–278. [Google Scholar] [CrossRef]
- Bååth, E.; Pettersson, M.; Söderberg, K. Adaptation of a rapid and economical microcentrifugation method to measure thymidine and leucine incorporation by soil bacteria. Soil Boil. Biochem. 2001, 33, 1571–1574. [Google Scholar] [CrossRef]
- Rousk, K.; Elyaagubi, F.K.; Jones, D.L.; Godbold, D.L. Bacterial salt tolerance is unrelated to soil salinity across an arid agroecosystem salinity gradient. Soil Boil. Biochem. 2011, 43, 1881–1887. [Google Scholar] [CrossRef]
- Sebaugh, J.L. Guidelines for accurate EC50/IC50 estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Rath, K.M.; Maheshwari, A.; Bengtson, P.; Rousk, K. Comparative Toxicities of Salts on Microbial Processes in Soil. Appl. Environ. Microbiol. 2016, 82, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Kunito, T.; Senoo, K.; Saeki, K.; Oyaizu, H.; Matsumoto, S. Usefulness of the Sensitivity–Resistance Index to Estimate the Toxicity of Copper on Bacteria in Copper-Contaminated Soils. Ecotoxicol. Environ. Saf. 1999, 44, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Witter, E.; Gong, P.; Bååth, E.; Marstorp, H. A study of the structure and metal tolerance of the soil microbial community six years after cessation of sewage sludge applications. Environ. Toxicol. Chem. 2000, 19, 1983–1991. [Google Scholar] [CrossRef]
- Niklińska, M.; Chodak, M.; Laskowski, R. Pollution-induced community tolerance of microorganisms from forest soil organic layers polluted with Zn or Cu. Appl. Soil Ecol. 2006, 32, 265–272. [Google Scholar] [CrossRef]
- Komárek, M.; Čadková, E.; Chrastný, V.; Bordas, F.; Bollinger, J.-C. Contamination of vineyard soils with fungicides: A review of environmental and toxicological aspects. Environ. Int. 2010, 36, 138–151. [Google Scholar] [CrossRef]
- Demoling, L.A.; Bååth, E. Use of pollution-induced community tolerance of the bacterial community to detect phenol toxicity in soil. Environ. Toxicol. Chem. 2008, 27, 334–340. [Google Scholar] [CrossRef]
- Spark, K.M.; Wells, J.D.; Johnson, B.B. Sorption of heavy metals by mineral-humic acid substrates. Soil Res. 1997, 35, 113. [Google Scholar] [CrossRef]
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef]
- Flores-Vélez, L.; Ducaroir, J.; Jaunet, A.; Robert, M. Study of the distribution of copper in an acid sandy vineyard soil by three different methods. Eur. J. Soil Sci. 1996, 47, 523–532. [Google Scholar] [CrossRef]
- Mirlean, N.; Roisenberg, A.; Chies, J.O. Metal contamination of vineyard soils in wet subtopics (southern Brazil). Environ. Pollut. 2007, 149, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calviño, D.; Garrido-Rodríguez, B.; López-Periago, E.; Paradelo, M.; Arias-Estevez, M. Spatial distribution of copper fractions in a vineyard soil. Land Degrad. Dev. 2011, 24, 556–563. [Google Scholar] [CrossRef]
- Pietrzak, U.; McPhail, D. Copper accumulation, distribution and fractionation in vineyard soils of Victoria, Australia. Geoderma 2004, 122, 151–166. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).