Effect of Nitrification Inhibitors on N2O Emissions after Cattle Slurry Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design and Crop Management
2.3. Nitrification Inhibitor Application Rates
2.4. Trace Gas Sampling and Flux Calculation
2.5. Weather Data, Soil and Plant Sampling, and Laboratory Analyses
2.6. Calculation of CO2 Equivalents
2.7. Statistical Analyses
3. Results
3.1. Weather Conditions
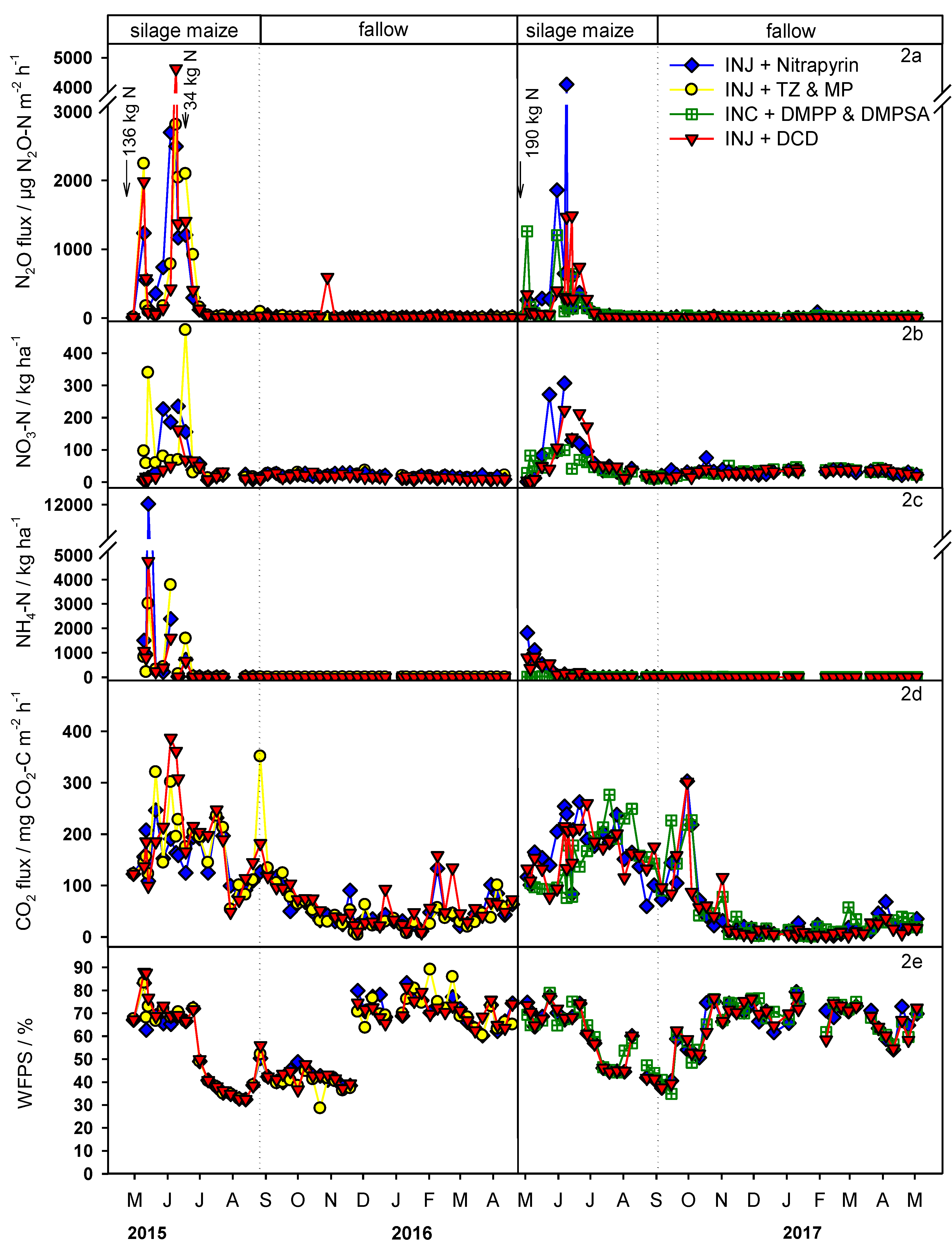
3.2. N2O Fluxes and Drivers
3.3. Annual N2O Emission and EFs
3.4. Maize Yield and N Removal
3.5. CO2 Footprint
4. Discussion
4.1. Effect of Environmental Condition and NIs on N2O Flux Rates
4.2. Effect of Environmental Conditions and NIs on Nnnual N2O Emission and Corresponding EFs
4.3. Impact of Different NIs on N Removal and Silage Maize Yield
4.4. Atmospheric Burden
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chadwick, D.; Sommer, S.; Thorman, R.; Fangueiro, D.; Cardenas, L.; Amon, B.; Misselbrook, T. Manure management: Implications for greenhouse gas emissions. Anim. Feed Sci. Technol. 2011, 166, 514–531. [Google Scholar] [CrossRef]
- Sanz-Cobena, A.; Lassaletta, L.; Estellés, F.; Del Prado, A.; Guardia, G.; Abalos, D.; Aguilera, E.; Pardo, G.; Vallejo, A.; Sutton, M.A.; et al. Yield-scaled mitigation of ammonia emission from N fertilization: The Spanish case. Environ. Res. Lett. 2014, 9, 125005. [Google Scholar] [CrossRef]
- Webb, J.; Pain, B.; Bittman, S.; Morgan, J. The impacts of manure application methods on emissions of ammonia, nitrous oxide and on crop response—A review. Agric. Ecosyst. Environ. 2010, 137, 39–46. [Google Scholar] [CrossRef]
- Mosier, A.R. Exchange of gaseous nitrogen compounds between terrestrial systems and the atmosphere. Plant Soil 2001, 228, 17–27. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portman, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef]
- Pozzer, A.; Tsimpidi, A.P.; Karydis, V.A.; de Meij, A.; Lelefield, J. Impact of agricultural emission reductions on fine-particulate matter and public health. Atmos. Chem. Phys. 2017, 17, 12813–12826. [Google Scholar] [CrossRef]
- Cassman, K.G.; Dobermann, A.R.; Walters, D. Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 2002, 31, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.C.; Huggins, D.R.; Jones, S.S. Characterizing nitrogen use efficiency in natural and agricultural ecosystems to improve the performance of cereal crops in low-input and organic agricultural systems. Field Crop. Res. 2008, 107, 89–101. [Google Scholar] [CrossRef]
- Flessa, H.; Ruser, R.; Dörsch, P.; Kamp, T.; Jimenez, M.A.; Munch, J.C.; Beese, F. Integrated evaluation of greenhouse gas emissions (CO2, CH4, N2O) from two farming systems in southern Germany. Agric. Ecosyst. Environ. 2002, 91, 175–189. [Google Scholar] [CrossRef]
- UBA (German Environment Agency). Submission under the United Nations Framework Convention on Climate Change and the Kyoto Protocol 2017. National Inventory Report for the German Greenhouse Gas Inventory 1990–2015. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/1410/publikationen/2017-05-02_climate-change_14-2017_nir-2017_unfccc_eng.pdf (accessed on 5 August 2020).
- Mannheim, T.; Braschkat, J.; Marschner, H. Reduktion von Ammoniakemissionen nach Ausbringung von Rinderflüssigmist auf Acker- und Grünlandstandorten: Vergleichende Untersuchungen mit Prallteller, Schleppschlauch und Injektion. J. Plant Nutr. Soil Sci. 1995, 158, 535–542. [Google Scholar]
- Sommer, S.G.; Hutchings, N.J. Ammonia emission from field applied manure and its reduction—Invited paper. Eur. J. Agron. 2001, 15, 1–15. [Google Scholar] [CrossRef]
- Wulf, S.; Maeting, M.; Clemens, J. Application technique and slurry co-fermentation effects on ammonia, nitrous oxide, and methane emissions after spreading: I. Ammonia volatilization. J. Environ. Qual. 2002, 31, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Horlacher, D.; Marschner, H. Schätzrahmen zur Beurteilung von Ammoniakverlusten nach Ausbringung von Rinderflüssigmist. J. Plant Nutr. Soil Sci. 1990, 153, 107–115. [Google Scholar] [CrossRef]
- Sommer, S.G.; Génermont, S.; Cellier, P.; Hutchings, N.J.; Olesen, J.E.; Morvan, T. Processes controlling ammonia emission from livestock slurry in the field. Eur. J. Agron. 2003, 19, 465–486. [Google Scholar] [CrossRef]
- Mattila, P.K. Ammonia Emissions from Pig and Cattle Slurry in the Field and Utilization of Slurry Nitrogen in Crop Production. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2006. [Google Scholar]
- Comfort, S.D.; Kelling, K.A.; Keeney, D.R.; Converse, J.C. Nitrous oxide production from injected liquid dairy manure. Soil Sci. Soc. Am. J. 1990, 54, 421–427. [Google Scholar] [CrossRef]
- Flessa, H.; Beese, F. Laboratory estimates of trace gas emissions following surface application and injection of cattle slurry. J. Environ. Qual. 2000, 29, 262–268. [Google Scholar] [CrossRef]
- Herr, C.; Mannheim, T.; Müller, T.; Ruser, R. Effect of cattle slurry application techniques on N2O and NH3 emissions from a loamy soil. J. Plant Nutr. Soil Sci. 2019, 182, 964–979. [Google Scholar] [CrossRef]
- Akiyama, H.; Yan, X.; Yagi, K. Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: Meta-analysis. Glob. Chang. Biol. 2010, 16, 1837–1846. [Google Scholar] [CrossRef]
- Ruser, R.; Schulz, R. The effect of nitrification inhibitors on the nitous oxide (N2O) release from agricultural soils—A review. J. Plant Nutr. Soil Sci. 2015, 178, 171–188. [Google Scholar] [CrossRef]
- Arp, D.J.; Sayavedra-Soto, L.A.; Hommes, N.G. Molecular biology and biochemistry of ammonia oxidation by Nitrosomonas europaea. Arch. Microbiol. 2002, 178, 250–255. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Ito, O.; Sahrawat, K.L.; Berry, W.L.; Nakahara, K.; Ishikawa, T.; Watanabe, T.; Suenaga, K.; Rondon, M.; Rao, I. Scope and strategies for regulation of nitrification in agricultural systems—Challenges and opportunities. Crit. Rev. Plant Sci. 2006, 25, 303–335. [Google Scholar] [CrossRef]
- Bateman, E.J.; Baggs, E.M. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol. Fertil. Soils 2005, 41, 379–388. [Google Scholar] [CrossRef]
- Baggs, E.M.; Rees, R.M.; Smith, K.A.; Vinten, A.J.A. Nitrous oxide emission from soils after incorporating crop residues. Soil Use Manag. 2000, 16, 82–87. [Google Scholar] [CrossRef]
- Smith, K.A. A model of the extent of anaerobic zones in aggregated soils, and its potential application to estimates of denitrification. J. Soil Sci. 1980, 31, 263–277. [Google Scholar] [CrossRef]
- Weier, K.L.; Doran, J.W.; Power, J.F.; Walters, D.T. Denitrification and the dinitrogen/nitrous oxide ratio as affected by soil water, available carbon, and nitrate. Soil Sci. Soc. Am. J. 1993, 57, 66–72. [Google Scholar] [CrossRef]
- Lin, S.; Hernandez-Ramirez, G.; Kryzanowski, L.; Wallace, T.; Grant, R.; Degenhardt, R.; Berger, N.; Sprout, C.; Lohstraeter, G.; Powers, L.-A. Timing of manure injection and nitrification inhibitors impacts on nitrous oxide emissions and nitrogen transformations in a barley crop. Soil Sci. Soc. Am. J. 2017, 81, 1595–1605. [Google Scholar] [CrossRef]
- Lin, S.; Hernandez-Ramirez, G. Nitrous oxide emissions from manured soils as a function of various nitrification inhibitor rates and soil moisture contents. Sci. Total Environ. 2020, 738, 139669. [Google Scholar] [CrossRef]
- Guardia, G.; Cangani, M.T.; Sanz-Cobena, A.; Junior, J.L.; Vallejo, A. Management of pig manure to mitigate NO and yield-scaled N2O emissions in an irrigated Mediterranean crop. Agric. Ecosyst. Environ. 2017, 238, 55–66. [Google Scholar] [CrossRef]
- Misselbrook, T.H.; Cardenas, L.M.; Camp, V.; Thorman, R.E.; Williams, J.R.; Rollett, A.J.; Chambers, B.J. An assessment of nitrification inihibitors to reduce nitrous oxide emissiosn from UK agriculture. Environ. Res. Lett. 2014, 9, 115006. [Google Scholar] [CrossRef]
- Vallejo, A.; García-Torres, L.; Díez, J.A.; Arce, A.; López-Fernández, S. Comparison of N losses (NO3−, N2O, NO) from surface applied, injected or amended (DCD) pig slurry of an irrigated soil in a Mediterranean climate. Plant Soil 2005, 272, 313–325. [Google Scholar] [CrossRef]
- Dittert, K.; Bol, R.; King, R.; Chadwick, D.; Hatch, D. Use of a novel nitrification inhibitor to reduce nitrous oxide emission from 15N-labelled dairy slurry injected into soil. Rapid Commun. Mass Spectrom. 2001, 15, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Jungkunst, H.F.; Freibauer, A.; Neufeldt, H.; Bareth, G. Nitrous oxide emissions from agricultural land use in Germany—A synthesis of available annual field data. J. Plant Nutr. Soil Sci. 2006, 169, 341–351. [Google Scholar] [CrossRef]
- Kaiser, E.-A.; Ruser, R. Nitrous oxide emissions from arable soils in Germany—An evaluation of six long-term field experiments. J. Plant Nutr. Soil Sci. 2000, 163, 249–259. [Google Scholar] [CrossRef]
- Pfab, H.; Palmer, I.; Buegger, F.; Fiedler, S.; Müller, T.; Ruser, R. Influence of a nitrification inhibitor and of placed N-fertilization on N2O fluxes from a vegetable cropped loamy soil. Agric. Ecosyst. Environ. 2012, 150, 91–101. [Google Scholar] [CrossRef]
- Agrarmeteorologie Baden-Württemberg: Wetterstation Hohenheim. Available online: http://www.wetter-bw.de/Internet/AM/NotesBwAM.nsf/bwweb/85f6baa2d299db4ac1257ca7003d9ab9?OpenDocument&TableRow=3.1.1%2C3.5#3.1 (accessed on 4 August 2020).
- DüV (German Legislation on Fertilization). Verordnung über die Anwendung von Düngemitteln, Bodenhilfsstoffen, Kultursubstraten und Pflanzenhilfsmitteln nach den Grundsätzen der guten fachlichen Praxis beim Düngen (Düngeverordnung—DüV). Bundesgesetzblatt Jahrg. 2006, 2, 22. [Google Scholar]
- EuroChem AGRO. Sicherheitsdatenblatt ENTEC® FL; EuroChem Agro GmbH: Mannheim, Germany, 2014. [Google Scholar]
- The Dow Chemical Company. Product Safety Assessment Nitrapyrin; The Dow Chemical Company, 2012. Available online: https://msdssearch.dow.com/PublishedLiteratureDOWCOM/dh_08c1/0901b803808c186f.pdf (accessed on 4 December 2014).
- SKW Stickstoffwerke Pisteritz GmbH. Sicherheitsdatenblatt Piadin®; SKW Stickstoffwerke Pisteritz GmbH, 2007. [Google Scholar]
- Alzchem. Safety Data Sheet, Dicyandiamide DCD; Alzchem, 2019. Available online: https://www.alzchem.com/sites/default/files/import/datenblatt/AlzChemSicherheitsdatenblatt_000000132103_EU_EN_5.8_.pdf (accessed on 17 July 2020).
- Hutchinson, G.; Mosier, A. Improved soil cover method for field measurement of nitrous oxide fluxes. Soil Sci. Soc. Am. J. 1981, 45, 311–316. [Google Scholar] [CrossRef]
- Gassner, M. Eignung von Runden Gassammelhauben zur Flächenrepräsentativen Erfassung von THG-Emissionen und Einfluss des Einsatzes eines Injektionsschars auf N2O- und CH4-Flüsse aus einer Parabraunerde. Bachelor’s Thesis, University of Hohenheim, Stuttgart, Germany, 2017. [Google Scholar]
- Pfab, H.; Palmer, I.; Buegger, F.; Fiedler, S.; Müller, T.; Ruser, R. N2O fluxes from a Haplic Luvisol under intensive production of lettuce and cauliflower as affected by different N-fertilization strategies. J. Plant Nutr. Soil Sci. 2011, 174, 545–553. [Google Scholar] [CrossRef]
- Fuss, R.; Asger, R.P. Gasfluxes: Greenhouse gas flux calculation from chamber measurements. R Package Version 0.1. 2014. Available online: https://bitbucket.org/ecoRoland/gasfluxes/src/master/ (accessed on 4 December 2014).
- R Core Team. A Language and Environment for Statistical Computing; R Core Team, 2016; Available online: http://www.r-project.org/ (accessed on 11 August 2020).
- Pedersen, A.R.; Petersen, S.O.; Schelde, K. A comprehensive approach to soil-atmosphere trace gas flux estimation with static chambers. Eur. J. Soil Sci. 2010, 61, 888–902. [Google Scholar] [CrossRef]
- Huber, P.J. Robust Statistics; Wiley: Cambridge, MA, USA, 1981. [Google Scholar]
- Ruser, R.; Fuß, R.; Andres, M.; Hegewald, H.; Kesenheimer, K.; Köbke, S.; Räbiger, T.; Quinones, T.S.; Augustin, J.; Christen, O.; et al. Nitrous oxide emissions from winter oilseed rape cultivation. Agric. Ecosyst. Environ. 2017, 249, 57–69. [Google Scholar] [CrossRef]
- Velthof, G.L.; Mosquera, J. The impact of slurry application technique on nitrous oxide emission from agricultural soils. Agric. Ecosyst. Environ. 2011, 140, 298–308. [Google Scholar] [CrossRef]
- Kramer, K.J.; Moll, H.C.; Nonhebel, S. Total greenhouse gas emissions related to the Dutch crop production system. Agric. Ecosyst. Environ. 1999, 72, 9–16. [Google Scholar] [CrossRef]
- IPCC. 2006 IPCC Guidelines for National Greenhouse Gas Inventories, Prepared by the National Greenhouse Gas Inventories Programme; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; IGES: Hayama, Japan, 2006. [Google Scholar]
- IPCC. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Calvo Buendia, E., Tanabe, K., Kranjc, A., Baasansuren, J., Fukuda, M., Ngarize, S., Osako, A., Pyrozhenko, Y., Shermanau, P., Federici, S., Eds.; IPCC: Kanagawa, Japan, 2019. [Google Scholar]
- Quemada, M.; Baranski, M.; Nobel-de Lange, M.N.J.; Vallejo, A.; Cooper, J.M. Meta-analysis of strategies to control nitrate leaching in irrigated agricultural systems and their effects on crop yield. Agric. Ecosyst. Environ. 2013, 174, 1–10. [Google Scholar] [CrossRef]
- Statista: Ertrag je Hektar Anbaufläche von Silomais in Deutschland in den Jahren 2005 bis 2019. Available online: https://de.statista.com/statistik/daten/studie/261750/umfrage/hektarertrag-von-silomais-in-deutschland/ (accessed on 6 June 2020).
- Dosch, P.; Gutser, R. Reducing N losses (NH3, N2O, N2) and immobilization from slurry through optimized application techniques. Fertil. Res. 1996, 43, 165–171. [Google Scholar] [CrossRef]
- Zhu, K.; Larsen, M.; Glud, R.N.; Jensen, L.S. Heterogeneity of O2 dynamics in soil amended with animal manureand implications for greenhouse gas emissions. Soil Biol. Biochem. 2015, 84, 96–106. [Google Scholar] [CrossRef]
- Heincke, M.; Kaupenjohann, M. Effects of soil solution on the dynamics of N2O emissions: A review. Nutr. Cycl. Agroecosyst. 1999, 55, 133–157. [Google Scholar] [CrossRef]
- Laville, P.; Neri, S.; Continanza, D.; Ferrante, V.; Luca, F.; Bosco, S.; Virgili, G. Cross-validation of a mobile N2O flux prototype (IPNOA) using micrometeorological and chamber methods. J. Energy Power Eng. 2015, 9, 375–385. [Google Scholar]
- Leitner, S.; Homyak, P.; Blankinship, J.; Eberwein, J.; Jenerette, G.; Zechmeister-Boltenstern, S.; Schimel, J. Linking NO and N2O emission pulses with the mobilization of mineral and organic N upon rewetting dry soils. Soil Biol. Biochem. 2017, 115, 461–466. [Google Scholar] [CrossRef]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J.C. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Agaki, J.; Zsolnay, A.; Bastida, F. Quantity and spectroscopic properties of soil dissolved organic matter (DOM) as a function of soil sample treatments: Air-drying and pre-incubation. Chemosphere 2007, 69, 1040–1046. [Google Scholar] [CrossRef]
- Lundquist, E.J.; Jackson, L.E.; Scow, K.M. Wet-dry cycles affect dissolved organic carbon in two California agricultural soils. Soil Biol. Biochem. 1999, 31, 1031–1038. [Google Scholar] [CrossRef]
- Davidson, E.A. Fluxes of nitrous oxide and nitric oxide following wetting of dry soil. Soil Sci. Soc. Am. J. 1992, 56, 95–102. [Google Scholar] [CrossRef]
- Calahan, E.; Ernfors, M.; Müller, C.; Devaney, D.; Laughlin, R.J.; Watson, C.J.; Hennessy, D.; Grant, J.; Khalil, M.I.; McGeough, K.L.; et al. The effect of nitrification inhibitor dicyandiamide (DCD) on nitrous oxide and methane emissions after cattle slurry application to Irish grassland. Agric. Ecosyst. Environm. 2015, 199, 339–349. [Google Scholar] [CrossRef]
- McGeough, K.L.; Laughlin, R.J.; Watson, C.J.; Müller, C.; Ernfors, M.; Cahalan, E.; Richards, K.G. The effect of cattle slurry in combination with nitrate and thenitrification inhibitor dicyandiamide on in situ nitrous oxide and dinitrogen emissions. Biogeosciences 2012, 9, 4909–4919. [Google Scholar] [CrossRef]
- ten Huf, M.; Olfs, H.-W. Effect of the nitrification inhibitor DMPP on nitrous oxide emissions and the stabilization of ammonium following the injection of dairy slurry and digestate in a soil-column experiment. J. Plant Nutr. Soil Sci. 2020, 183, 129–135. [Google Scholar] [CrossRef]
- Granli, T.; Bøckman, O.C. Nitrous oxide from agriculture. Norw. J. Agric. Sci. 1994, 12, 1–127. [Google Scholar]
- Chen, D.; Suter, H.C.; Islam, A.; Edis, R. Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emission from a clay loam soil fertilized with urea. Soil Biol. Biochem. 2010, 42, 660–664. [Google Scholar] [CrossRef]
- Guardia, G.; Vallejo, A.; Cardenas, L.M.; Dixon, E.R.; García-Marco, S. Fate of 15N-labelled ammonium nitrate with or without the new nitrification inhibitor DMPSA in an irrigated maize crop. Soil Biol. Biochem. 2018, 116, 193–202. [Google Scholar] [CrossRef]
- Irigoyen, I.; Muro, J.; Azpilikueta, M.; Aparicio-Tejo, P.; Lamsfus, C. Ammonium oxidation kinetics in the presence of nitrification inhibitors DCD and DMPP at various temperatures. Aust. J. Soil Res. 2003, 41, 1177–1183. [Google Scholar] [CrossRef]
- Mahmood, T.; Ali, R.; Latif, Z.; Ishaque, W. Dicyandiamide increases the fertilizer N loss from an alkaline calcareous soil treated with 15N-labelled urea under warm climate and under different crops. Biol. Fertil. Soils 2011, 47, 619–631. [Google Scholar] [CrossRef]
- McGeough, K.L.; Watson, C.J.; Müller, C.; Laughlin, J.R.; Chadwick, D.R. Evidence that the efficacy of the nitrification inhibitor dicyandiamide (DCD) is affected by soil properties in UK soils. Soil Biol. Biochem. 2016, 94, 222–232. [Google Scholar] [CrossRef]
- Slangen, J.H.G.; Kerkhoff, P. Nitrification inhibitors in agriculture and horticulture: A literature review. Fertil. Res. 1984, 5, 1–76. [Google Scholar] [CrossRef]
- Zerulla, W.; Barth, T.; Dressel, J.; Erhardt, K.; Horchler von Locquenghien, K.; Pasda, G.; Rädle, M.; Wissemeier, A. 3,4-Dimethylpyrazole phosphate (DMPP)—A new nitrification inhibitor for agriculture and horticulture. An introduction. Biol. Fertil. Soils 2001, 34, 79–84. [Google Scholar] [CrossRef]
- Sørensen, P.; Amato, M. Remineralisation and residual effects of N after application of pig slurry to soil. Eur. J. Agron. 2002, 16, 81–95. [Google Scholar] [CrossRef]
- Seiz, P.; Guzman-Bustamante, I.; Schulz, R.; Müller, T.; Ruser, R. Effect of crop residue removal and straw addition on nitrous oxide emissions from a horticulturally used soil in South Germany. Soil Sci. Soc. Am. J. 2019, 83, 1399–1409. [Google Scholar] [CrossRef]
- Guzman-Bustamante, I.; Winkler, T.; Schulz, R.; Müller, T.; Mannheim, T.; Laso Bayas, J.C.; Ruser, R. N2O emissions from a loamy soil cropped with winter wheat as affected by N-fertilizer amount and nitrification inhibitor. Nutr. Cycl. Agroecosyst. 2019, 114, 173–191. [Google Scholar] [CrossRef]
- Risk, N.; Snider, D.; Wagner-Riddle, C. Mechanisms leading to enhanced soil nitrous oxide fluxes induced by freeze-thaw cycles. Can. J. Soil Sci. 2013, 93, 401–414. [Google Scholar] [CrossRef]
- Wagner-Riddle, C.; Furon, A.; McLaughlin, N.L.; Lee, I.; Barbeau, J.; Jayasundara, S.; Parkin, G.; von Bertoldi, P.; Warland, J. Intensive measurement of nitrous oxide emissions from a corn-soybean-wheat rotation under two contrasting management systems over 5 years. Glob. Chang. Biol. 2007, 13, 1722–1736. [Google Scholar] [CrossRef]
- Xu, X.K.; Duan, C.T.; Wu, H.H.; Li, T.S.; Cheng, W.G. Effect of intensity and duration of freezing on soil microbial biomass extractable C and N pools, and N2O and CO2 emissions from a forest soil in cold temperate region. Sci. China Earth Sci. 2016, 59, 156–169. [Google Scholar] [CrossRef]
- Pacholski, A.; Berger, N.; Bustamante, I.; Ruser, R.; Guillermo, G.; Mannheim, T. Effects of the novel nitrification inhibitor DMPSA on yield, mineral N dynamics and N2O emissions. In Proceedings of the International Nitrogen Initiative Conference “Solutions to Improve Nitrogen Use Efficiency for the World”, Melbourne, Australia, 4–8 December 2016; pp. 4–8. [Google Scholar]
- Weiske, A.; Benckiser, G.; Ottow, J.C.G. Effect of the new nitrification inhibitor DMPP in comparison to DCD on nitrous oxide (N2O) emissions and methane (CH4) oxidation during 3 years of repeated applications in field experiments. Nutr. Cycl. Agorecosyst. 2001, 60, 57–64. [Google Scholar] [CrossRef]
- Marsden, K.A.; Marín-Martínez, A.J.; Vallejo, A.; Hill, P.W.; Jones, D.L.; Chadwick, D.R. The mobility of nitrification inhibitors under simulated ruminant urine deposition and rainfall: A comparison between DCD and DMPP. Biol. Fertil. Soils 2016, 52, 491–503. [Google Scholar] [CrossRef]
- Wang, H.; Köbke, S.; Dittert, K. Use of urease and nitrification inhibitors to reduce gaseous nitrogen emissions from fertilizers containing ammonium nitrate and urea. Glob. Ecol. Conserv. 2020, 22, e00933. [Google Scholar] [CrossRef]
- Wolf, U.; Fuß, R.; Höppner, F.; Flessa, H. Contribution of N2O and NH3 to total greenhouse gas emission from fertilization: Results from a sandy soil fertilized with nitrate and biogas digestate with and without nitrification inhibitor. Nutr. Cycl. Agorecosyst. 2014, 100, 121–134. [Google Scholar] [CrossRef]
- Duncan, E.W.; Dell, C.J.; Kleinmann, P.J.A.; Beegle, D.B. Nitrous oxide and ammonia emissions from injected and broadcast-applied dairy slurry. J. Environ. Qual. 2016, 46, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, E.; Lassaletta, L.; Sanz-Cobena, A.; Garnier, J.; Vallejo, A. The potential of organic fertilizers and water management to reduce N2O emissions in Mediterranean climate cropping systems. A review. Agric. Ecosyst. Environ. 2013, 164, 32–52. [Google Scholar] [CrossRef]
- Louro, A.; Cárdenas, L.M.; García, M.I.; Báez, D. Greenhouse gas fluxes from a grazed grassland soil after slurry injectionsand mineral fertilizer applications under the Atlantic climatic conditions of NW Spain. Sci. Total Environ. 2016, 573, 258–269. [Google Scholar] [CrossRef]
- Severin, M.; Fuß, R.; Well, R.; Garlipp, F.; Van den Weghe, H. Soil, slurry and application effects on greenhouse gas emissions. Plant Soil Environ. 2015, 61, 344–351. [Google Scholar] [CrossRef]
- Seidel, A.; Pacholski, A.; Nyord, T.; Vestergaard, A.; Pahlmann, I.; Herrmann, A.; Kage, H. Effects of acidification and injection of pasture applied cattle slurry on ammonia losses, N2O emissions and crop N uptake. Agric. Ecosyst. Environ. 2017, 247, 23–32. [Google Scholar] [CrossRef]
- Charles, A.; Rochette, P.; Whalen, J.K.; Angers, D.A.; Chantigny, M.H.; Bertrand, N. Global nitrous oxide emission factors from agricultural soils after addition of organic amendments: A meta-analysis. Agric. Ecosyst. Environ. 2017, 236, 88–98. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Boumans, L.J.M.; Batjes, N.H. Modeling global annual N2O and NO emissions from fertilized fields. Glob. Biogeochem. Cycles 2002, 16, 28-1–28-9. [Google Scholar] [CrossRef]
- Van Nguyen, Q.; Wu, D.; Kong, X.; Bol, R.; Petersen, S.O.; Jensen, L.S.; Liu, S.; Brüggemann, N.; Glud, R.N.; Larsen, M.; et al. Effects of cattle slurry and nitrification inhibitor application on spatial soil O2 dynamics and N2O production pathways. Soil Biol. Biochem. 2017, 114, 200–209. [Google Scholar] [CrossRef]
- Fangmeier, A.; Hadwiger-Fangmeier, A.; Van der Eerden, L.; Jager, H.-J. Effect of atmospheric ammonia on vegetation-A review. Environ. Pollut. 1994, 86, 43–82. [Google Scholar] [CrossRef]
- McCormick, R.A.; Nelson, D.W.; Sutton, A.L.; Huber, D.M. Increased N efficiency from nitrapyrin added to liquid swine manure used as a fertilizer for corn. Agron. J. 1984, 76, 1010–1014. [Google Scholar] [CrossRef]
- Sutton, A.L.; Huber, D.M.; Jones, D.D.; Kelly, D.T.; Bache, D.H. Use of nitrification inhibitors with summer applications of swine manure. Appl. Eng. Agric. ASAE 1986, 2, 179–185. [Google Scholar] [CrossRef]
- Federolf, C.P.; Westerschulte, M.; Olfs, H.-W.; Broll, G.; Trautz, D. Enhanced nutrient use efficiencies from liquid manure by positioned injection in maize cropping in northwest Germany. Eur. J. Agron. 2016, 75, 130–138. [Google Scholar] [CrossRef]
- Schmitt, M.A.; Evans, S.D.; Randall, G.W. Effect of liquid manure application methods on soil nitrogen and corn grain yields. J. Prod. Agric. 1995, 8, 186–189. [Google Scholar] [CrossRef]
- Gaines, T.P.; Gaines, S.T. Soil texture effect on nitrate leaching in soil percolates. Commun. Soil Sci. Plant Anal. 1994, 25, 2561–2570. [Google Scholar] [CrossRef]
- Huang, J.; Chen, Y.; Pan, J.; Liu, W.; Yang, G.; Xiao, X.; Zheng, H.; Tang, W.; Tang, H.; Zhou, L. Carbon footprint of different agricultural systems in China estimated by different evaluation metrics. J. Clean. Prod. 2019, 225, 939–948. [Google Scholar] [CrossRef]
- Börjesson, P.; Prade, T.; Lantz, M.; Björnsson, L. Energy crop-based biogas as vehicle fuel—The impact of crop selection on energy efficiency and greenhouse gas performance. Energies 2015, 8, 6033–6058. [Google Scholar] [CrossRef]
- Smeets, E.M.W.; Bouwman, L.F.; Stehfest, E.; van Vuuren, D.P.; Posthuma, A. Contribution of N2O to the greenhouse gas balance of first-generation biofuels. Glob. Chang. Biol. 2009, 15, 1–23. [Google Scholar] [CrossRef]
- KTBL (German Association for Technology and Structures in Agriculture). Faustzahlen für die Landwirtschaft, 14th ed.; Scheuermanndruck GmbH: Darmstadt, Germany, 2009. [Google Scholar]
- Cherrier, V.; Grebot, B.; Kirhensteine, I.; Brutus, J.; Taylor, K.; Berman, S.; Sarteel, M. Collection and Analysis of Data for the Control of Emissions from the Spreading of Manure. Final AMEC Report for the European Commission. Available online: https://ec.europa.eu/environment/air/pdf/Final%20Report.pdf (accessed on 28 June 2020).

| Treatment | N Fertilizer | Application Technique | Nitrification Inhibitor | Sampling Period | ||
|---|---|---|---|---|---|---|
| 1st Year | 2nd Year | |||||
| CON * | unfertilized | - | - | X | X | |
| INC * | cattle slurry | trail hose + incorp. | - | X | X | |
| INC | +DMPP&DMPSA | cattle slurry | trail hose + incorp. | 3,4-dimethylpyrazole phosphate & 3,4-dimethylpyrazole succinic acid | X | |
| INJ * | cattle slurry | injection | - | X | X | |
| INJ | +DMPP | cattle slurry | injection | 3,4-dimethylpyrazole phosphate | X | X |
| INJ | +DMPSA | cattle slurry | injection | 3,4-dimethylpyrazole succinic acid | X | X |
| INJ | +DMPP&DMPSA | cattle slurry | injection | 3,4-dimethylpyrazole phosphate & 3,4-dimethylpyrazole succinic acid | X | X |
| INJ | +nitrapyrin | cattle slurry | injection | Nitrapyrin | X | X |
| INJ | +DCD | cattle slurry | injection | dicyandiamide | X | X |
| INJ | +TZ&MP | cattle slurry | injection | 1,2,4 triazole & 3-methylpyrazole | X | |
| Treatment | N2O Emission | EF | ||||
|---|---|---|---|---|---|---|
| [kg N2O-N ha−1 yr−1] | [% of N Applied] | |||||
| 1st Year | 2nd Year | Mean | 1st Year | 2nd Year | Mean | |
| CON | 2.3 b | 3.3 c | 2.8 | |||
| INC | 2.1 b | 6.7 b,c | 4.4 | 0.0 | 1.8 | 0.9 |
| INC + DMPP&DMPSA | n.d. | 5.4 b,c | 5.4 | n.d. | 1.1 | 1.1 |
| INJ | 16.2 a | 11.5 a | 13.9 | 8.4 | 4.4 | 6.4 |
| INJ + DMPP | 12.8 a | 5.5 b,c | 9.2 | 6.3 | 1.2 | 3.8 |
| INJ + DMPSA | 12.4 a | 8.4 b | 10.4 | 6.1 | 2.7 | 4.4 |
| INJ + DMPP&DMPSA | 9.6 a | 4.9 b,c | 7.3 | 4.4 | 0.9 | 2.7 |
| INJ + nitrapyrin | 12.8 a | 7.9 b | 10.4 | 6.3 | 2.4 | 4.4 |
| INJ + DCD | 11.0 a | 4.4 b,c | 7.7 | 5.2 | 0.6 | 2.9 |
| INJ + TZ&MP | 13.4 a | n.d. | 13.4 | 6.7 | n.d. | 6.7 |
| Year | Treatment | Percentage of the Total CO2 Equivalents | Area-Related CO2e | Yield-Related CO2e | ||||
|---|---|---|---|---|---|---|---|---|
| N2O Emissions | NH3 Emissions | CH4 Emissions | NO3− Leaching | Fuel Consumption | [kg ha−1 year−1] | [kg Mg−1 DM−1] | ||
| 1st | INJ | 90.9 | 0.5 | 2.1 | 3.1 | 3.5 | 8362 a | 585 a |
| INJ + DMPP | 89.8 | 0.7 | 1.4 | 3.8 | 4.3 | 6668 a | 574 a | |
| INJ + DMPSA | 89.6 | 0.7 | 1.3 | 4.0 | 4.5 | 6481 a | 459 a | |
| INJ + DMPP&DMPSA | 86.8 | 0.9 | 1.8 | 4.9 | 5.6 | 5194 a | 348 a | |
| INJ + nitrapyrin | 89.9 | 0.7 | 1.2 | 3.9 | 4.3 | 6680 a | 436 a | |
| INJ + DCD | 88.5 | 0.8 | 1.3 | 4.4 | 5.0 | 5814 a | 394 a | |
| INJ + TZ&MP | 90.3 | 0.7 | 1.2 | 3.7 | 4.2 | 6955 a | 514 a | |
| 2nd | INJ | 89.4 | 1.0 | 0.3 | 4.7 | 4.6 | 6005 A | 395 A |
| INJ + DMPP | 82.0 | 1.9 | 0.5 | 6.8 | 8.9 | 3138 B | 206 B | |
| INJ + DMPSA | 87.4 | 1.3 | 0.3 | 4.8 | 6.2 | 4491 AB | 283 AB | |
| INJ + DMPP&DMPSA | 80.5 | 2.1 | 0.2 | 7.5 | 9.8 | 2851 B | 182 B | |
| INJ + nitrapyrin | 86.8 | 1.4 | 0.2 | 5.0 | 6.6 | 4235 AB | 279 AB | |
| INJ + DCD | 80.7 | 2.0 | 0.4 | 7.3 | 9.5 | 2919 B | 198 B | |
| INC + DMPP&DMPSA | 83.7 | 1.9 | −0.9 | 6.8 | 8.4 | 3029 B | 239 B | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herr, C.; Mannheim, T.; Müller, T.; Ruser, R. Effect of Nitrification Inhibitors on N2O Emissions after Cattle Slurry Application. Agronomy 2020, 10, 1174. https://doi.org/10.3390/agronomy10081174
Herr C, Mannheim T, Müller T, Ruser R. Effect of Nitrification Inhibitors on N2O Emissions after Cattle Slurry Application. Agronomy. 2020; 10(8):1174. https://doi.org/10.3390/agronomy10081174
Chicago/Turabian StyleHerr, Christina, Thomas Mannheim, Torsten Müller, and Reiner Ruser. 2020. "Effect of Nitrification Inhibitors on N2O Emissions after Cattle Slurry Application" Agronomy 10, no. 8: 1174. https://doi.org/10.3390/agronomy10081174
APA StyleHerr, C., Mannheim, T., Müller, T., & Ruser, R. (2020). Effect of Nitrification Inhibitors on N2O Emissions after Cattle Slurry Application. Agronomy, 10(8), 1174. https://doi.org/10.3390/agronomy10081174





