Molecular Assisted Selection for Pollination-Constant and Non-Astringent Type without Male Flowers in Spanish Germplasm for Persimmon Breeding
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.1.1. Validation of AST and DlSx-AF4S Markers
2.1.2. Marker Assisted Selection
2.2. Methods
2.2.1. DNA Isolation
2.2.2. Molecular Markers Analysis
3. Results and Discussion
3.1. Marker Assisted Selection Validation: Production of Male Flowers
3.2. Marker Assisted Selection Validation: Selection of PCNA
3.3. Marker Assisted Selection of Both Traits in the IVIA Breeding Program
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dellaporta, S.L.; Calderon-Urrea, A. Sex determination in flowering plants. Plant Cell 1993, 5, 1241–1251. [Google Scholar] [PubMed]
- Yonemori, K.; Sugiura, A.; Tanaka, K.; Kameda, K. Floral Ontogeny and Sex Determination in Monoecious-type Persimmons. J. Am. Soc. Hortic. Sci. 1993, 118, 293–297. [Google Scholar] [CrossRef]
- Xu, L.Q.; Zhang, Q.L.; Luo, Z.R. Occurrence and cytological mechanism of 2n pollen formation in Chinese Diospyros spp. (Ebenaceae) staminate germplasm. J. Hortic. Sci. Biotechnol. 2008, 83, 668–672. [Google Scholar] [CrossRef]
- Yakushiji, H.; Yamada, M.; Yonemori, K.; Sato, A.; Kimura, N. Staminate flower production on shoots of Fuyu’ and “Jiro” persimmon (Diospyros kaki Thunb.). J. Jpn. Soc. Hortic. Sci. 1995, 64, 41–46. [Google Scholar] [CrossRef]
- Giordani, E.; Picardi, E.; Radice, S. Morfologia y Fisiologia. In El Cultivo del Caqui; Badenes, M.L., Intrigliolo, D.S., Salvador, A., Vicent, A., Eds.; Generalitat Valenciana: Valencia, Spain, 2015; pp. 17–33. ISBN 9788448260187. [Google Scholar]
- Kajiura, M.; Blumenfeld, A. Diospyros kaki. In CRC Handbook of Flowering; Halevy, A.H., Ed.; CRC Press: Boca Raton, FL, USA, 1989; Volume 6, pp. 298–306. ISBN 9781315893464. [Google Scholar]
- Yamada, M.; Giordani, E.; Yonemori, K. Persimmon. In Fruit Breeding; Badanes, M.L., Byrne, D.H., Eds.; Springer: New York, NY, USA, 2012; Volume 8, pp. 663–693. ISBN 9781441907639. [Google Scholar]
- Sugiura, A.; Yonemori, K.; Harada, H.; Tomana, T. Changes of ethanol and acetaldehyde contents in Japanese persimmon fruits and their relation to natural deastringency. Stud. Inst. Hortic. Kyoto Univ. 1979, 9, 41–47. [Google Scholar]
- Sugiura, A.; Tomana, T. Relationships of ethanol production by seeds of different types of Japanese persimmons and their tannin content. Hort. Sci. 1983, 18, 319–321. [Google Scholar]
- Akagi, T.; Kajita, K.; Kibe, T.; Morimura, H.; Tsujimoto, T.; Nishiyama, S.; Kawai, T.; Yamane, H.; Tao, R. Development of molecular markers associated with sexuality in Diospyros lotus L. and their application in D. kaki Thunb. J. Jpn. Soc. Hortic. Sci. 2014, 83, 214–221. [Google Scholar] [CrossRef]
- Akagi, T.; Henry, I.M.; Tao, R.; Comai, L. A Y-chromosome-encoded small RNA acts as a sex determinant in persimmons. Science 2014, 346, 646–650. [Google Scholar] [CrossRef]
- Yonemori, K.; Matsushima, J. Property of Development of the Tannin Cells in Non-Astringent Type Fruits of Japanese Persimmon (Diospyros kaki) and Its Relationship to Natural Deastringency. J. Jpn. Soc. Hortic. Sci. 1985, 54, 201–208. [Google Scholar] [CrossRef]
- Ikeda, I.; Yamada, M.; Kurihara, A.; Nishida, T. Inheritance of Astringency in Japanese Persimmon. J. Jpn. Soc. Hortic. Sci. 1985, 54, 39–45. [Google Scholar] [CrossRef]
- Kanzaki, S.; Yamada, M.; Sato, A.; Mitani, N.; Ustunomiya, N.; Yonemori, K. Conversion of RFLP markers for the selection of pollination-constant and non-astringent type persimmons (Diospyros kaki Thunb.) into PCR-based markers. J. Jpn. Soc. Hortic. Sci. 2009, 78, 68–73. [Google Scholar] [CrossRef]
- Kono, A.; Kobayashi, S.; Onoue, N.; Sato, A. Characterization of a highly polymorphic region closely linked to the AST locus and its potential use in breeding of hexaploid persimmon (Diospyros kaki Thunb.). Mol. Breed. 2016, 36, 1–13. [Google Scholar] [CrossRef]
- Kanzaki, S.; Yonemori, K.; Sugiura, A.; Sato, A.; Yamada, M. Identification of molecular markers linked to the trait of natural astringency loss of Japanese persimmon (Diospyros kaki) fruit. J. Jpn. Soc. Hortic. Sci. 2001, 126, 51–55. [Google Scholar] [CrossRef]
- Ikegami, A.; Yonemori, K.; Sugiura, A.; Sato, A.; Yamada, M. Segregation of astringency in F1 progenies derived from crosses between pollination-constant, nonastringent persimmon cultivars. HortScience 2004, 39, 371–374. [Google Scholar] [CrossRef]
- Akagi, T.; Takeda, Y.; Yonemori, K.; Ikegami, A.; Kono, A.; Yamada, M.; Kanzaki, S. Quantitative genotyping for the astringency locus in hexaploid persimmon cultivars using quantitative real-time PCR. J. Jpn. Soc. Hortic. Sci. 2010, 135, 59–66. [Google Scholar] [CrossRef]
- Kanzaki, S.; Akagi, T.; Masuko, T.; Kimura, M.; Yamada, M.; Sato, A.; Mitani, N.; Ustunomiya, N.; Yonemori, K. SCAR markers for practical application of marker-assisted selection in persimmon (Diospyros kaki Thunb.) breeding. J. Jpn. Soc. Hortic. Sci. 2010, 79, 150–155. [Google Scholar] [CrossRef]
- Onoue, N.; Kobayashi, S.; Kono, A.; Sato, A. SSR-based molecular profiling of 237 persimmon (Diospyros kaki Thunb.) germplasms using an ASTRINGENCY-linked marker. Tree Genet. Genomes 2018, 14. [Google Scholar] [CrossRef]
- Martínez-Calvo, J.; Naval, M.; Zuriaga, E.; Llácer, G.; Badenes, M.L. Morphological characterization of the IVIA persimmon (Diospyros kaki Thunb.) germplasm collection by multivariate analysis. Genet. Resour. Crop Evol. 2013, 60, 233–241. [Google Scholar] [CrossRef]
- Del Naval, M.M.; Zuriaga, E.; Pecchioli, S.; Llácer, G.; Giordani, E.; Badenes, M.L. Analysis of genetic diversity among persimmon cultivars using microsatellite markers. Tree Genet. Genomes 2010, 6, 677–687. [Google Scholar] [CrossRef][Green Version]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytolog. Bull 1987, 19, 11–15. [Google Scholar] [CrossRef]
- Soriano, J.M.; Pecchioli, S.; Romero, C.; Vilanova, S.; Llácer, G.; Giordani, E.; Badenes, M.L. Development of microsatellite markers in polyploid persimmon (Diospyros kaki Thunb) from an enriched genomic library. Mol. Ecol. Notes 2006, 6, 368–370. [Google Scholar] [CrossRef]
- Besada, C.; Novillo, P.; Navarro, P.; Salvador, A. Causes of flesh browning in persimmon–A review. Acta Hortic. 2018, 1195, 203–210. [Google Scholar] [CrossRef]
- Munera, S.; Besada, C.; Blasco, J.; Cubero, S.; Salvador, A.; Talens, P.; Aleixos, N. Astringency assessment of persimmon by hyperspectral imaging. Postharvest Biol. Technol. 2017, 125, 35–41. [Google Scholar] [CrossRef]
- Yamada, M. Persimmon Breeding in Japan. Jpn. Agric. Res. Q. 1993, 27, 33–37. [Google Scholar]
- Yamada, M.; Sato, A.; Yakushiji, H.; Yoshinaga, K.; Yamane, H.; Endo, M. Characteristics of “Luo Tian Tian Shi”, a non-astringent cultivar of oriental persimmon (Diospyros kaki Thunb.) of Chinese origin in relation to non-astringent cultivars of Japanese origin. Bull. Fruit Tree Res. Stn. 1993, 25, 19–32. [Google Scholar]
- Bellini, E.; Giordani, E. Germplasm and breeding of persimmon in Europe. Acta Hortic. 2005, 685, 65–74. [Google Scholar] [CrossRef]
- Badenes, M.L.; Martinez-Calvo, J.; Naval, M.M. The persimmon breeding program at IVIA: Alternatives to conventional breeding of persimmon. Acta Hortic. 2013, 996, 71–76. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, Q.; Luo, C.; Xie, F. Recent advances of persimmon research and industry in China. Acta Hortic. 2013, 996, 43–48. [Google Scholar] [CrossRef]
- Ma, K.B.; Lee, I.B.; Kim, Y.K.; Won, K.H.; Cho, K.S.; Choi, J.J.; Lee, B.H.N.; Kim, M.S. ‘Jowan’, an early maturing PCNA (pollination constant non-astringent) persimmon (Diospyros kaki Thunb.). Acta Hortic. 2018, 1195, 61–64. [Google Scholar] [CrossRef]
- Mitani, N.; Kono, A.; Yamada, M.; Sato, A.; Kobayashi, S.; Ban, Y.; Ueno, T.; Shiraishi, M.; Kanzaki, S.; Tsujimoto, T.; et al. Application of marker-assisted selection in persimmon breeding of PCNA offspring using SCAR markers among the population from the cross between Non-PCNA ‘taigetsu’ and PCNA ‘kanshu’. HortScience 2014, 49, 1132–1135. [Google Scholar] [CrossRef]
- Zhu, Q.G.; Xu, Y.; Yang, Y.; Guan, C.F.; Zhang, Q.Y.; Huang, J.W.; Grierson, D.; Chen, K.S.; Gong, B.C.; Yin, X.R. The persimmon (Diospyros oleifera Cheng) genome provides new insights into the inheritance of astringency and ancestral evolution. Hortic. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
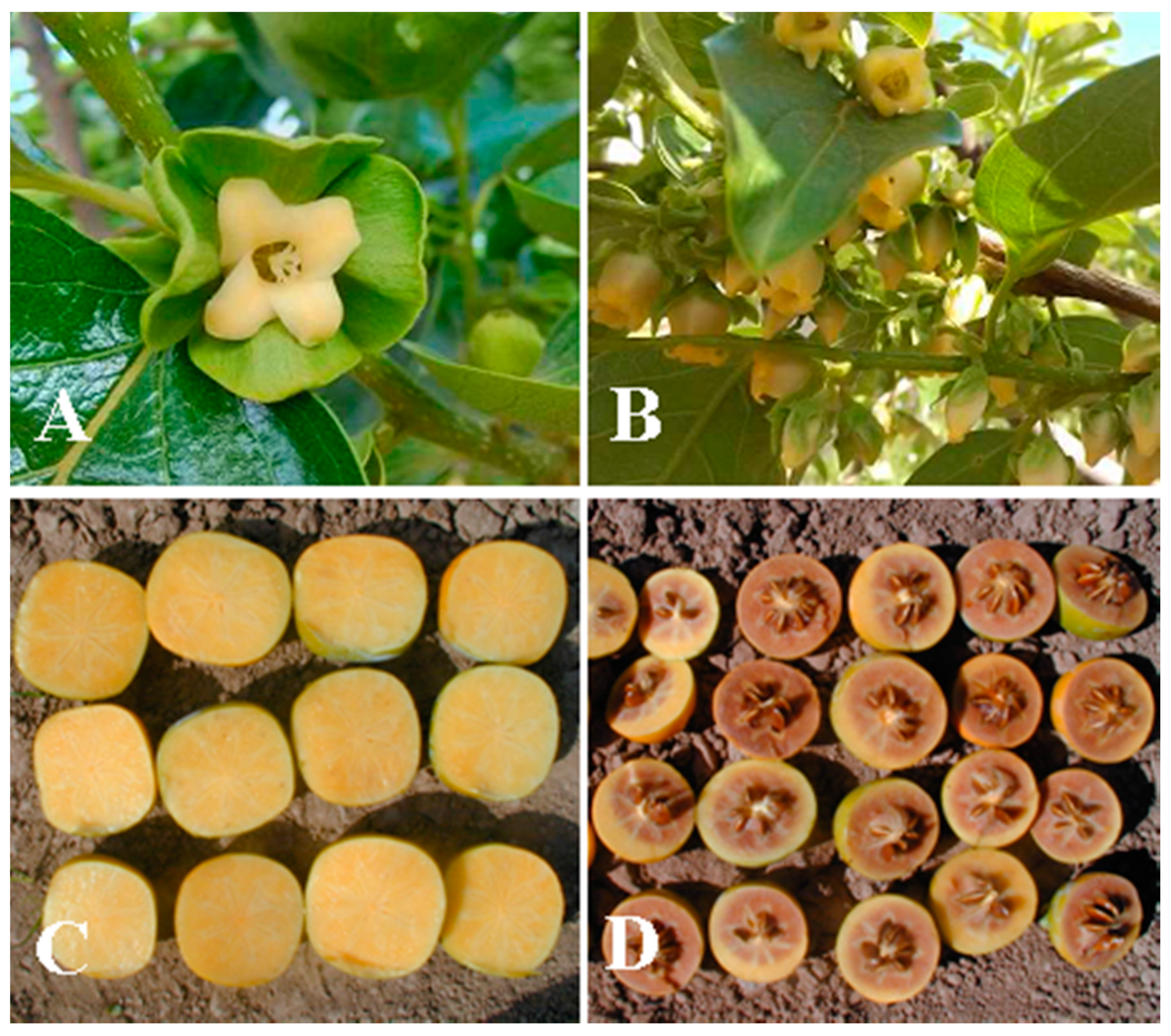
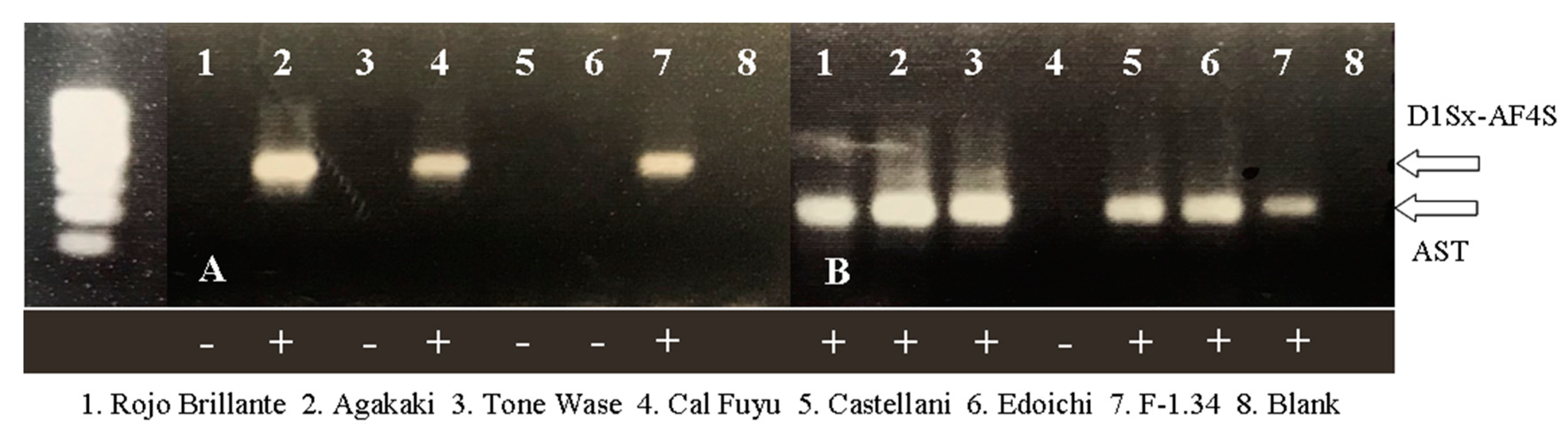


| Variety | Origin | Type 1 | AST 2 | Flowers 1 | D1Sx-AF4S 2 | Variety | Origin | Type 1 | AST 2 | Flower 1 | D1Sx-AF4S 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anheca | Spain | PCA | + | ♀ | − | Kawabata | Japan | PCNA | − | ♀ | − |
| Ferrán-12 | Spain | PCA | + | ♀ | − | Koda Gosho | Japan | PCNA | − | ♀ | − |
| Reus-6 | Spain | PCA | + | ♀ | − | Maekawa jiro | Japan | PCNA | − | ♀ | − |
| Tomatero | Spain | PCA | + | ♀ | − | Mukaku jiro | Japan | PCNA | − | ♀ | − |
| Costata | Italy | PCA | + | ♀ | − | O’Gosho | Japan | PCNA | − | ♀ | − |
| Lycopersicon | Italy | PCA | + | ♀ | − | Suruga | Japan | PCNA | − | ♀ | − |
| Aizumishirazu-A | Japan | PCA | + | ♀ | − | Yamato Gosho | Japan | PCNA | − | ♀ | − |
| Fuji | Japan | PCA | + | ♀ | − | Bétera-2 | Spain | PVA | + | ♀ | − |
| Korea Kaki | Japan | PCA | + | ♀ | − | Reus-15 | Spain | PVA | + | ♀ | − |
| Takura | Japan | PCA | + | ♀ | − | Rojo Brillante | Spain | PVA | + | ♀ | − |
| Yokono | Japan | PCA | + | ♀ | − | Xato de Bonrepós | Spain | PVA | + | ♀ | − |
| Cal Fuyu | Japan | PCNA | − | ♀/♂ | + | Aizumishirazu-B | Japan | PVA | + | ♀ | − |
| Fau Fau | Japan | PCNA | − | ♀/♂ | + | Atago | Japan | PVA | + | ♀ | − |
| Fukuro Gosho | Japan | PCNA | − | ♀/♂ | + | Hiratanekaki | Japan | PVA | + | ♀ | − |
| Fuyu | Japan | PCNA | − | ♀ | − | Hiratanenashi | Japan | PVA | + | ♀ | − |
| Giant Fuyu | Japan | PCNA | − | ♀ | − | Maru | Japan | PVA | + | ♀/♂ | + |
| Hana fuyu | Japan | PCNA | − | ♀ | − | Tone Wase | Japan | PVA | + | ♀ | − |
| Hana Gosho | Japan | PCNA | − | ♀/♂ | + | Pakistan Seedless | Pakistan | PVA | + | ♀ | − |
| Ichikikei Jiro | Japan | PCNA | − | ♀ | − | Agakaki | Japan | PVNA | + | ♀/♂ | + |
| Isahaya | Japan | PCNA | − | ♀ | − | Castellani | Italy | PVNA | + | ♀ | − |
| Jiro | Japan | PCNA | − | ♀ | − | Edoichi | Italy | PVNA | + | ♀ | − |
| Progeny | Total | Number of Offspring | |||
|---|---|---|---|---|---|
| AST + | D1Sx-AF4S + | AST + D1Sx-AF4S + | AST − D1Sx-AF4S − | ||
| F-1.34 | 99 | 61 (61.6) | 39 (39.4) | 22 (22.2) | 21 (21.2) |
| F-1.50 | 65 | 39 (60.0) | 43 (66.2) | 28 (43.1) | 11 (16.9) |
| F-1.52 | 47 | 13 (27.7) | 24 (51.1) | 4 (8.5) | 14 (29.8) |
| F-2.27 | 5 | 4 (80.0) | 3 (60.0) | 3 (60.0) | 1 (20.0) |
| F-4.19 | 11 | 8 (72.7) | 9 (81.8) | 7 (63.6) | 1 (9.1) |
| F-4.24 | 61 | 20 (32.8) | 31 (50.8) | 10 (16.4) | 20 (32.8) |
| F-4.35 | 38 | 14 (36.8) | 9 (23.7) | 4 (10.5) | 19 (50.0) |
| F-4.49 | 52 | 14 (26.9) | 17 (32.7) | 3 (5.8) | 24 (46.2) |
| F-5.32 | 12 | 10 (83.3) | 6 (50.0) | 4 (33.3) | 0 (0.0) |
| F-5.34 | 27 | 11 (40.7) | 16 (59.3) | 5 (18.5) | 5 (18.5) |
| F-5.36 | 17 | 12 (70.6) | 9 (52.9) | 4 (23.5) | 0 (0.0) |
| F-5.41 | 7 | 5 (71.4) | 3 (42.9) | 3 (42.9) | 2 (28.6) |
| total | 441 | 211 (47.8) | 209 (47.4) | 97 (22.0) | 118 (26.8) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blasco, M.; Gil-Muñoz, F.; Naval, M.d.M.; Badenes, M.L. Molecular Assisted Selection for Pollination-Constant and Non-Astringent Type without Male Flowers in Spanish Germplasm for Persimmon Breeding. Agronomy 2020, 10, 1172. https://doi.org/10.3390/agronomy10081172
Blasco M, Gil-Muñoz F, Naval MdM, Badenes ML. Molecular Assisted Selection for Pollination-Constant and Non-Astringent Type without Male Flowers in Spanish Germplasm for Persimmon Breeding. Agronomy. 2020; 10(8):1172. https://doi.org/10.3390/agronomy10081172
Chicago/Turabian StyleBlasco, Manuel, Francisco Gil-Muñoz, María del Mar Naval, and María Luisa Badenes. 2020. "Molecular Assisted Selection for Pollination-Constant and Non-Astringent Type without Male Flowers in Spanish Germplasm for Persimmon Breeding" Agronomy 10, no. 8: 1172. https://doi.org/10.3390/agronomy10081172
APA StyleBlasco, M., Gil-Muñoz, F., Naval, M. d. M., & Badenes, M. L. (2020). Molecular Assisted Selection for Pollination-Constant and Non-Astringent Type without Male Flowers in Spanish Germplasm for Persimmon Breeding. Agronomy, 10(8), 1172. https://doi.org/10.3390/agronomy10081172





