The Influence of Soil Physical and Chemical Properties on Saffron (Crocus sativus L.) Growth, Yield and Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Agronomic Trial
2.2. Physical-Chemical Analysis of Soil
2.3. Spectrophotometric Analysis of Saffron Extract
2.4. Color Measurement of Dry Stigma and Fresh Tepal
2.5. Statistical Analysis
3. Results
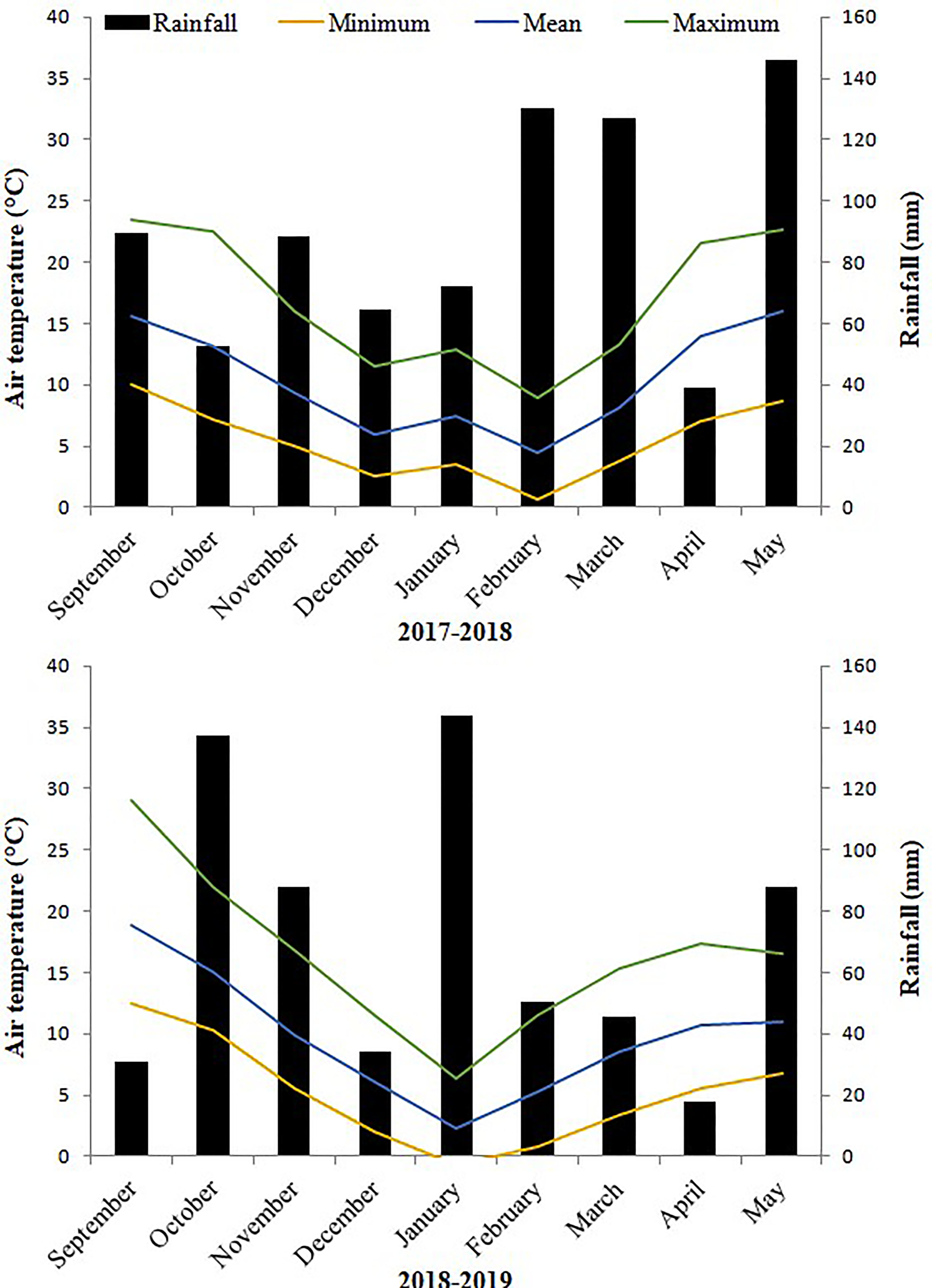
3.1. Climatic Data
3.2. Morphological and Colorimetric Traits of Flower
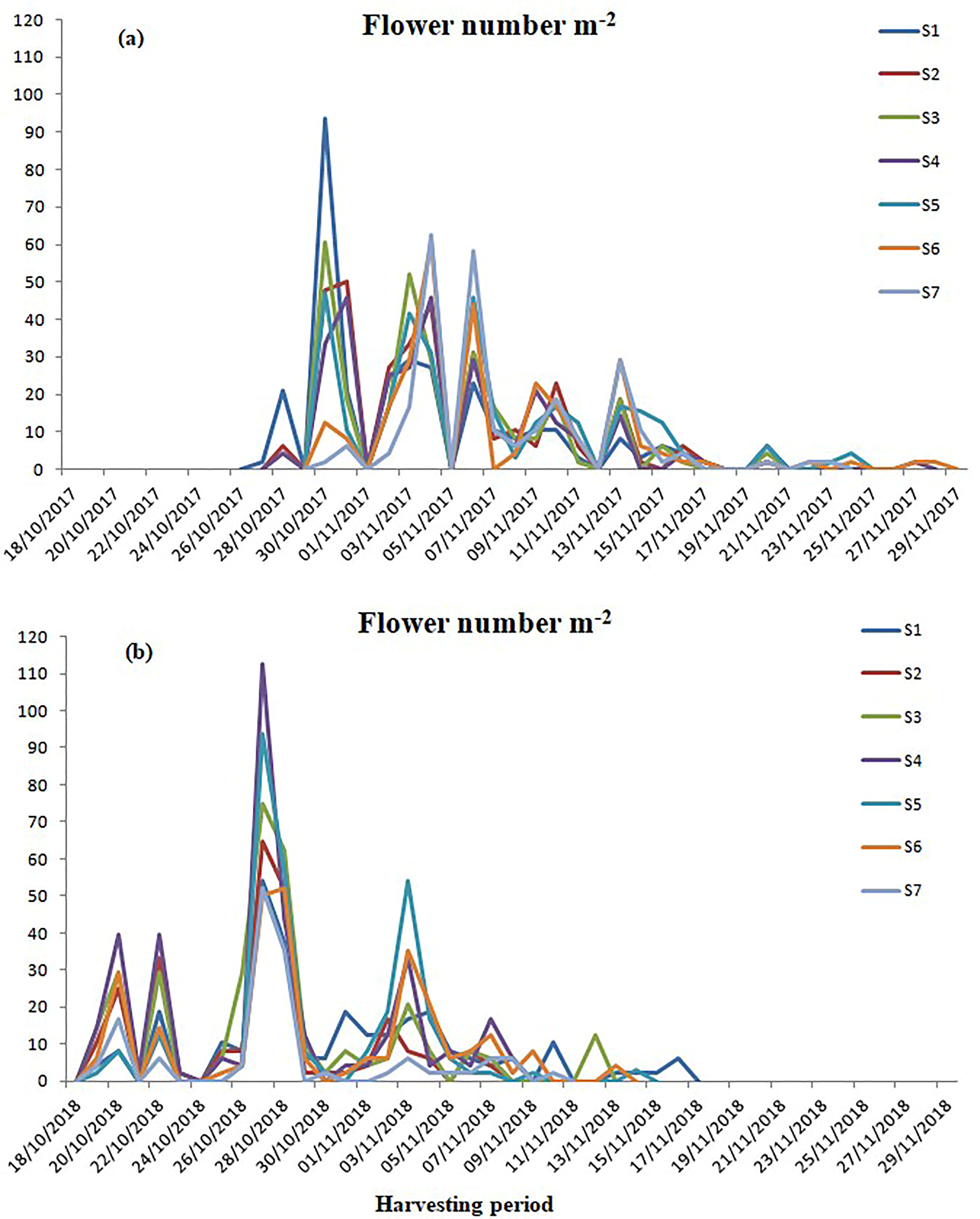
3.3. Flowering Period and Stigma Yield
3.4. Qualitative and Colorimetric Traits of Spice
3.5. Leaf Traits and Daughter Corm Yield
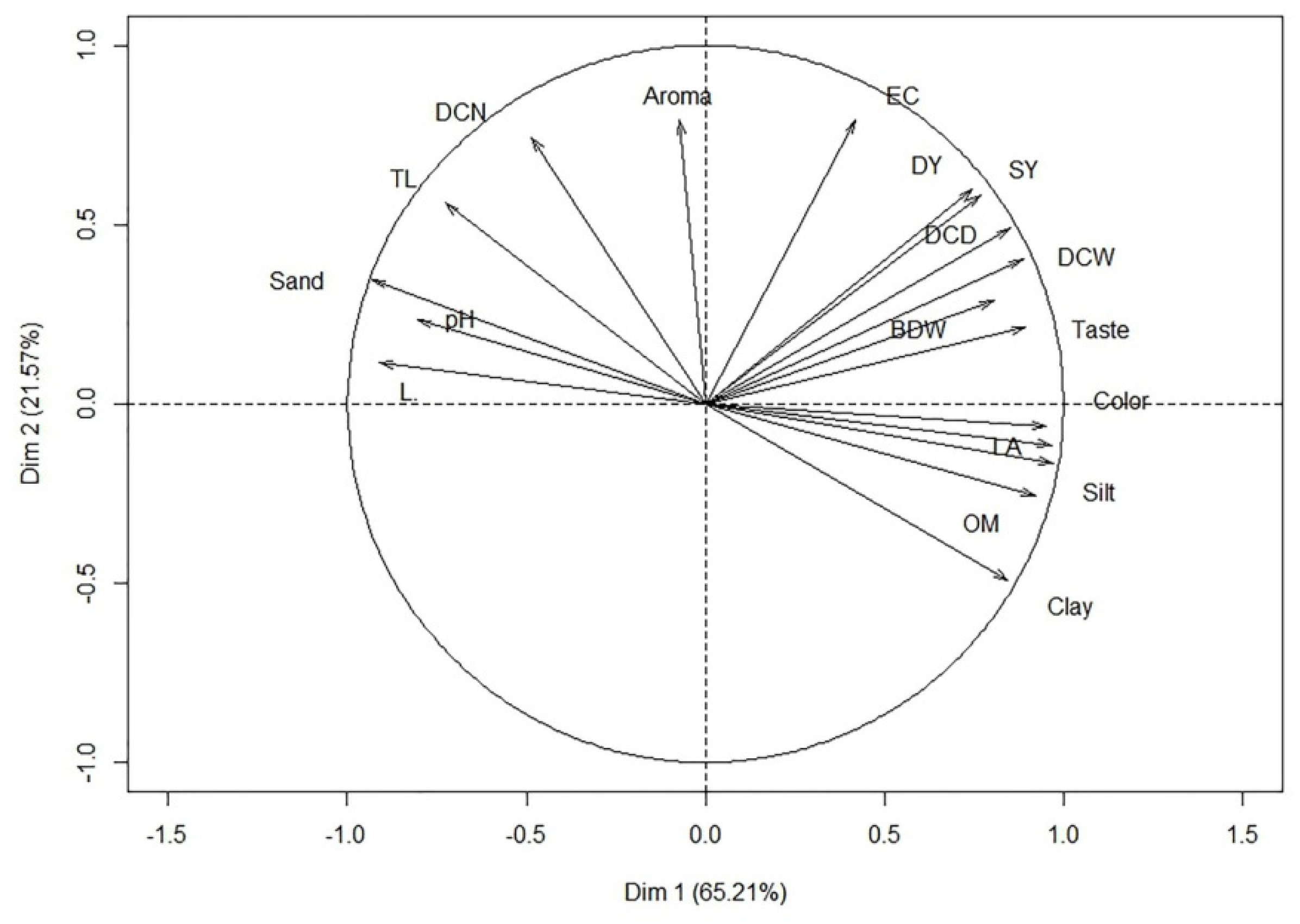
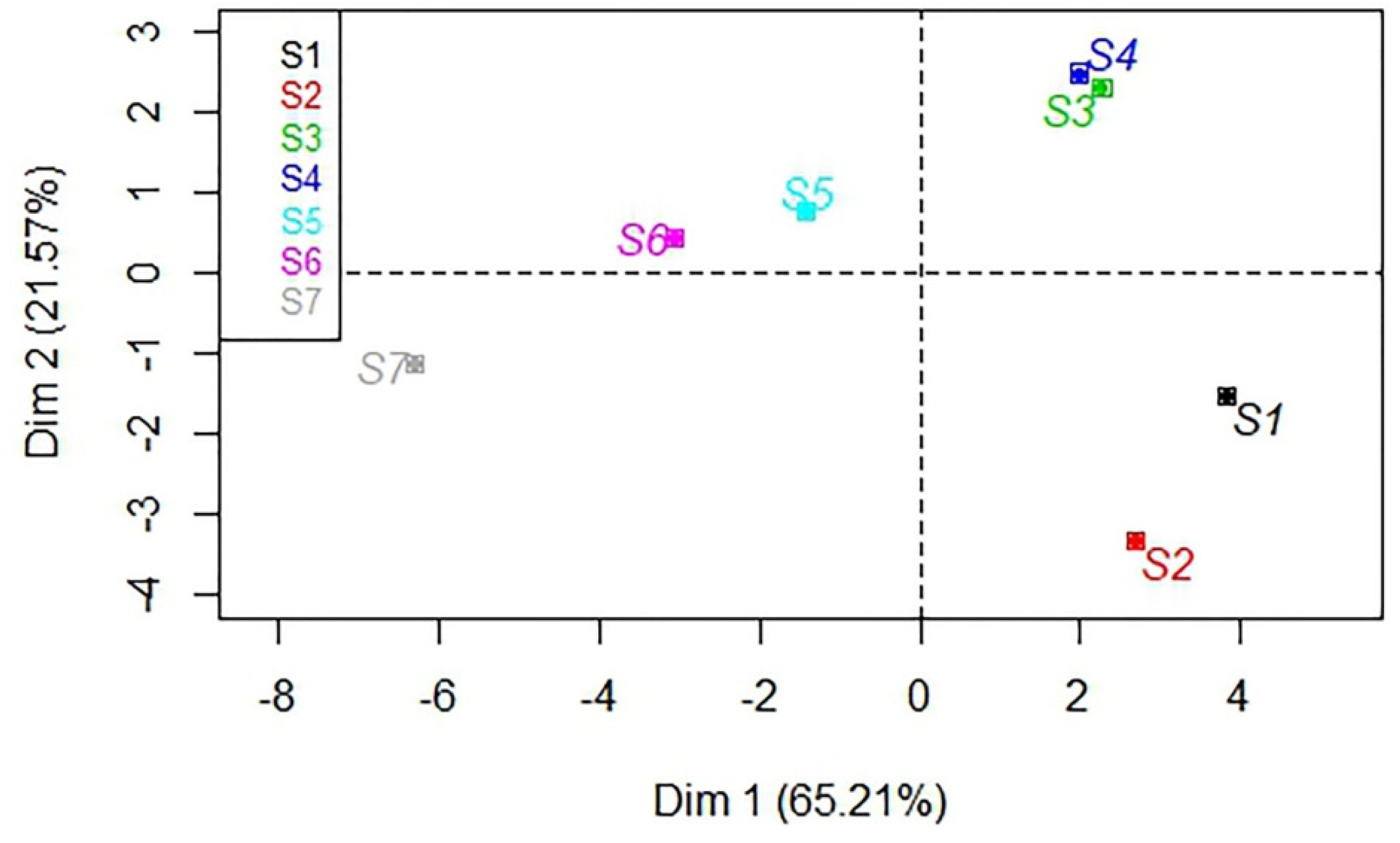
3.6. Principal Component Analysis
4. Discussion
4.1. Flowering and Quantitative Traits
4.2. Qualitative Traits
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abbott, L.K.; Murphy, M.V. Soil Biological Fertility: A Key to Sustainable Land Use in Agriculture; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; p. 264. [Google Scholar]
- Khalil, H.P.S.A.; Hossain, M.S.; Rosamah, E.; Azli, N.A.; Saddon, N.; Davoudpoura, Y.; Islam, M.N.; Dungani, R. The role of soil properties and it’s interaction towards quality plant fiber: A review. Renew. Sustain. Energy Rev. 2015, 43, 1006–1015. [Google Scholar] [CrossRef]
- Babalar, M.; Mumivand, H.; Hadian, J.; Tabatabaei, S.M.F. Effects of Nitrogen and Calcium Carbonate on Growth, Rosmarinic Acid Content and Yield of Satureja hortensis L. J. Agric. Sci. 2010, 2, 92–98. [Google Scholar] [CrossRef]
- Hassan, A. Effects of mineral nutrients on physiological and biochemical processes related to secondary metabolites production in medicinal herbs. Med. Arom. Plant. Sci. Biotechnol. 2012, 6, 105–110. [Google Scholar]
- Quan, M.; Liang, J. The influences of four types of soil on the growth, physiological and biochemical characteristics of Lycoris aurea (L’Her.). Herb. Sci. Rep. 2017, 7, 43284. [Google Scholar] [CrossRef] [PubMed]
- Mumivand, H.; Babalar, M.; Hadian, J.; Fakhr-Tabatabaei, M. Plant growth and essential oil content and composition of Saturn (Satureja hortensis L.) in response to calcium carbonate and nitrogen application rates. J. Med. Plants Res. 2011, 5, 1859–1866. [Google Scholar]
- Niu, Y.; Luo, Z.; Cai, L.; Coulter, J.A.; Zhang, Y.; Berti, M. Continuous monoculture of alfalfa and annual crops influence soil organic matter and microbial communities in the rainfed loess plateau of China. Agronomy 2020, 10, 1054. [Google Scholar] [CrossRef]
- Gohari, A.R.; Saeidnia, S.; Mahmoodabadi, M.K. An overview on saffron, phytochemicals, and medicinal properties. Pharmacogn. Rev. 2013, 7, 61–66. [Google Scholar] [CrossRef]
- Cardone, L.; Castronuovo, D.; Perniola, M.; Cicco, N.; Candido, V. Saffron (Crocus sativus L.), the king of spices: An overview. Sci. Hort. 2020, 272, 109560. [Google Scholar] [CrossRef]
- Shahandeh, H. Soil conditions for sustainable saffron production. In Saffron Science, Technology and Health; Koocheki, A., Khajeh-Hosseini, M., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 59–66. [Google Scholar] [CrossRef]
- International Trade Centre. Afghanistan’s National Export Strategy 2018–2022; Saffron Sector: Geneva, Switzerland, 2018. [Google Scholar]
- Azizbekova, N.S.H.; Milyaeva, E.L. Saffron in cultivation in Azerbaijan. In Saffron: Crocus sativus L.; Negbi, M., Ed.; CRC Press: London, UK, 1999; pp. 63–71. [Google Scholar]
- Husaini, A.M. Challenges of climate change: Omics based biology of saffron plants and organic agricultural biotechnology for sustainable saffron production. GM Crops Food. 2014, 5, 97–105. [Google Scholar] [CrossRef]
- Goliaris, A.H. Saffron cultivation in Greece. In Saffron: Crocus sativus L.; Negbi, M., Ed.; CRC Press: London, UK, 1999; pp. 73–85. [Google Scholar]
- Ait-Oubahou, A.; El-Otmani, M. Saffron cultivation in Morocco. In Saffron: Crocus sativus L.; Negbi, M., Ed.; CRC Press: London, UK, 1999; pp. 87–94. [Google Scholar]
- Zafferano. Available online: http://www.rosseterroir.it/zafferano/ (accessed on 25 July 2020).
- Fernandez, J.A. Biology, biotechnology and biomedicine of saffron. Recent Res. Dev. Plant. Sci. 2004, 2, 127–159. [Google Scholar]
- El Aymani, I.; El Gabardi, S.; Artib, M.; Chliyeh, M.; Selmaoui, K.; Ouazzani Touhami, A.; Benkirane, R.; Douira, A. Effect of the number of years of soil exploitation by saffron cultivation in Morocco on the diversity of endomycorrhizal fungi. Acta Phytopathol. Entomol. Hung. 2019, 54, 9–24. [Google Scholar] [CrossRef]
- Parray, J.A.; Kamili, A.N.; Reshi, Z.A.; Hamid, R.; Qadri, R.A. Screening of beneficial properties of rhizobacteria isolated from Saffron (Crocus sativus L.) rhizosphere. Afr. J. Microbiol. Res. 2013, 7, 2905–2910. [Google Scholar]
- Tammaro, F. Saffron (Crocus sativus L.) in Italy. In Saffron: Crocus sativus L.; Negbi, M., Ed.; CRC Press: London, UK, 1999; pp. 53–62. [Google Scholar]
- Kumar, R.; Singh, V.; Devi, K.; Sharma, M.; Singh, M.K.; Ahuja, P.S. State of art of saffron (Crocus sativus L.). Agronomy: A comprehensive review. Food Rev. Int. 2009, 25, 44–85. [Google Scholar] [CrossRef]
- Gresta, F.; Lombardo, G.M.; Ruberto, G.; Siracusa, L. Saffron, an alternative crop for sustainable agricultural systems: A review. Agron. Sustain. Dev. 2008, 28, 95–112. [Google Scholar] [CrossRef]
- Aghhavani Shajari, M.; Rezvani Moghaddam, P.; Ghorbani, R.; Koocheki, A. The possibility of improving saffron (Crocus sativus L.) flower and corm yield through the irrigation and soil texture managements. Sci. Hortic. 2020, 271, 109485. [Google Scholar] [CrossRef]
- Dhar, A.K. Saffron: Biology, utilization, agriculture, production and quality. J. Med. Arom. Pl. Sci. 2000, 22, 355–360. [Google Scholar]
- Mollafilabi, A. Experimental findings of production and echo physiological aspects of saffron (Crocus sativus L.). Acta Hort. 2004, 650, 195–200. [Google Scholar] [CrossRef]
- Kafi, M. Saffron ecophysiology. In Saffron (Crocus sativus) Production and Processing; Kafi, M., Koocheki, A., Rashed, M.H., Nassiri, M., Eds.; Science Publishers: Enfield, UK, 2006; pp. 39–58. [Google Scholar]
- McGimpsey, J.A.; Douglas, M.H.; Wallace, A.R. Evaluation of saffron (Crocus sativus L.) production in New Zealand. N. Z. J. Crop. Hort. Sci. 1997, 25, 159–168. [Google Scholar] [CrossRef]
- Gresta, F.; Lombardo, G.M.; Avola, G. Saffron stigmas production as affected by soil texture. Acta Hortic. 2010, 850, 149–152. [Google Scholar] [CrossRef]
- Turhan, H.; Kahriman, F.; Egesel, C.O.; Gul, M.K. The effects of different growing media on flowering and corm formation of saffron (Crocus sativus L.). Afr. J. Biotech. 2007, 6, 2328–2332. [Google Scholar]
- Aghhavani Shajari, M.; Rezvani Moghaddam, P.; Koocheki, A.; Fallahi, H.R.; Taherpour, K.R. Short Communication: Evaluation of the effects of soil texture on yield and growth of saffron (Crocus sativus L.). J. Saffron Agro. Tecnlol. 2015, 2, 311–322. [Google Scholar]
- D’Auria, M.; Mauriello, G.; Rana, G. Volatile organic compounds from saffron. Flavour Fragr. J. 2002, 19, 17–23. [Google Scholar] [CrossRef]
- ISO 3632-1: 2011. Spices Saffron (Crocus sativus L.); Food products SC7, Spices, culinary herbs and condiments; International Organization for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- Lage, M.; Cantrell, C.L. Quantification of saffron (Crocus sativus L.) metabolites crocins, picrocrocin and safranal for quality determination of the spice grown under different environmental Moroccan conditions. Sci. Hortic. 2009, 121, 366–373. [Google Scholar] [CrossRef]
- Soil Science Division Staff. Soil Survey Manual; USDA Handbook 18; Ditzler, C., Scheffe, K., Monger, H.C., Eds.; Government Printing Office: Washington, DC, USA, 2017; p. 125.
- Walkley, A.J.; Black, I.A. Estimation of soil organic carbon by the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis; Part 3, SSSA Book Series; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Madison: Indianapolis, IN, USA, 1996; pp. 961–1010. [Google Scholar] [CrossRef]
- Ministero per le Politiche Agricole. Metodi Ufficiali di Analisi Chimica del Suolo; D.M. del 13/09/99, Gazzetta Ufficiale n. 248 del 21/10/99; Ministero per le Politiche Agricole: Rome, Italy, 1999. [Google Scholar]
- Cuko, L.; Mitsopoulou, T.; Tsimidou, M.Z. A rapid procedure for the evaluation of saffron coloring strength using tristimulus colorimetry. Acta Hort. 2004, 650, 282–288. [Google Scholar]
- Konica Minolta. 2003. Available online: https://www.konicaminolta.eu/fileadmin/content/eu/Measuring Instruments/4 Learning Centre/C A/PRECISE COLORCOMMUNICATION/pcc english 13.pdf (accessed on 14 May 2020).
- Castronuovo, D.; Picuno, P.; Manera, C.; Scopa, A.; Sofo, A.; Candido, V. Biodegradable pots for Pointsettia cultivation: Agronomic and technical traits. Sci. Hort. 2015, 197, 150–156. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant. Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Chaudhari, P.R.; Ahire, D.V.; Ahire, V.D.; Chkravarty, M.; Maity, S. Soil bulk density as related to soil texture, organic matter content and available total nutrients of Coimbatore soil. Int. J. Sci. Res. Public 2013, 3, 1–8. [Google Scholar]
- Cardone, L.; Castonuovo, D.; Perniola, M.; Cicco, N.; Candido, V. Evaluation of corm origin and climatic conditions on saffron (Crocus sativus L.) yield and quality. J. Sci. Food Agric. 2019, 99, 5858–5869. [Google Scholar] [CrossRef]
- Renau-Morata, B.; Nebauer, S.G.; Sánchez, M.; Molina, R.V. Effect of corm size, water stress and cultivation conditions on photosynthesis and biomass partitioning during the vegetative growth of saffron (Crocus sativus L.). Ind. Crop. Prod. 2012, 39, 40–46. [Google Scholar] [CrossRef]
- Ghanbari, J.; Khajoei-Nejad, G.; van Ruth, S.M.; Aghighi, S. The possibility for improvement of flowering, corm properties, bioactive compounds, and antioxidant activity in saffron (Crocus sativus L.) by different nutritional regimes. Ind. Crop. Prod. 2019, 135, 301–310. [Google Scholar] [CrossRef]
- Koocheki, A.; Moghaddam, P.R.; Seyyedi, S.M. Depending on mother corm size, the removal of extra lateral buds regulates sprouting mechanism and improves phosphorus acquisition efficiency in saffron (Crocus sativus L.). Ind. Crops Prod. 2019, 141, 111779. [Google Scholar] [CrossRef]
- Rezvani Moghaddam, P.; Khorramdel, S.; Mollafilabi, A. Evaluation of soil physical and chemical characteristics impacts on morphological criteria and yield of saffron (Crocus sativus L.). J. Saffron Res. 2015, 3, 188–203. [Google Scholar]
- Finch, H.J.S.; Samuel, A.M.; Lane, G.P.F. Soils and Soil Management. In Lockhart & Wiseman’s Crop Husbandry Including Grassland, 9th ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 37–62. [Google Scholar]
- Liu, Y.; Zhang, Z.S.; He, Y.L.; Zhang, B.G.; Li, X.E. Quality of crude traditional Chinese drugs and ecological environment. World Sci. Technol. 2007, 9, 65–69. [Google Scholar]
- Khorramdel, S.; Gheshm, R.; Amin Ghafori, A.; Esmaielpour, B. Evaluation of soil texture and superabsorbent polymer impacts on agronomical characteristics and yield of saffron. J. Saffron Res. 2014, 1, 120–135. [Google Scholar]
- Micheni, A.; Kihanda, F.; Irungu, J. Soil organic matter (SOM): The basis for improved crop production in arid and semi-arid climate of eastern Kenya. In Managing Nutrient Cycles to Sustain Soil Fertility in Sub-Saharan Africa; Bationo, A., Ed.; Academy Science Publisher: Nairobi, Kenya, 2004; p. 608. [Google Scholar]
- Douglas, M.H.; Smallfield, B.M.; Wallace, A.R.; McGimpsey, J.A. Saffron (Crocus sativus L.): The effect of mother corm size on progeny multiplication, flower and stigma production. Sci. Hortic. 2014, 166, 50–58. [Google Scholar] [CrossRef]
- Koocheki, A.; Ebrahimian, E.; Seyyedi, S.M. How irrigation rounds and mother corm size control saffron yield, quality, daughter corms behavior and phosphorus uptake. Sci. Hortic. 2016, 213, 132–143. [Google Scholar] [CrossRef]
- Hossain, A.H.; Ishimine, Y. Growth, yield and quality of turmeric (Curcuma longa L.) cultivated on dark-red soil, gray, and red soil in Okinawa, Japan. Plant. Prod. Sci. 2005, 8, 482–486. [Google Scholar] [CrossRef]
- Al-Hamed, S.A.; Wahby, M.F.; Aboukarima, A.M.; El Marazky, M.S. Effect of soil and water characteristics on yield and properties of ‘Spunta’ potatoes. Chil. J. Agric. Res. 2017, 77, 250–256. [Google Scholar] [CrossRef]
- Sessitsch, A.; Weilharter, A.; Gerzabek, M.H.; Kirchmann, H.; Kandeler, E. Microbial population structures in soil particle size fractions of a long-term fertilizer field experiment. Appl. Environ. Microbiol. 2001, 67, 4215–4224. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Nat. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Bach, E.M.; Baer, S.G.; Meyer, C.K.; Six, J. Soil texture affects soil microbial and structural recovery during grassland restoration. Soil Biol. Biochem. 2010, 42, 2182–2191. [Google Scholar] [CrossRef]
- Li, L.; Xu, M.; Eyakub Ali, M.; Zhang, W.; Duan, Y.; Li, D. Factors affecting soil microbial biomass and functional diversity with the application of organic amendments in three contrasting cropland soils during a field experiment. PLoS ONE 2018, 13, e0203812. [Google Scholar] [CrossRef]
- Chamkhi, I.; Abbas, Y.; Tarmoun, K.; Aurag, J.; Sbabou, L. Morphological and molecular characterization of arbuscular mycorrhizal fungal communities inhabiting the roots and the soil of saffron (Crocus sativus L.) under different agricultural management practices. Arch. Agron. Soil Sci. 2019, 65, 1035–1048. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- Chodak, M.; Niklińska, M. Effect of texture and tree species on microbial properties of mine soils. Appl. Soil Ecol. 2010, 46, 268–275. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Baath, E. The microbial PLFA composition as affected by pH in an arable soil. Soil Biol. Biochem. 2010, 42, 516–520. [Google Scholar] [CrossRef]
- Oehl, F.; Laczko, E.; Oberholzer, H.R.; Jansa, J.; Egli, S. Diversity and biogeography of arbuscular mycorrhizal fungi in agricultural soils. Biol. Fertil. Soils 2017, 53, 777–797. [Google Scholar] [CrossRef]
- Caser, M.; Demasi, S.; Victorino, Í.M.M.; Donno, D.; Faccio, A.; Lumini, E.; Bianciotto, V.; Scariot, V. Arbuscular mycorrhizal fungi modulate the crop performance and metabolic profile of saffron in soilless cultivation. Agronomy 2019, 9, 232. [Google Scholar] [CrossRef]
- Bonomelli, C.; Gil, P.; Schaffer, B. Effect of Soil Type on Calcium Absorption and Partitioning in Young Avocado (Persea Americana Mill.) Trees. Agronomy 2019, 9, 837. [Google Scholar] [CrossRef]
- Zeka, K.; Ruparelia, K.C.; Continenza, M.A.; Stagos, D.; Vegliò, F.; Arroo, R.R.J. Petals of Crocus sativus L. as a potential source of the antioxidants crocin and kaempferol. Fitoterapia 2015, 107, 128–134. [Google Scholar] [CrossRef]
- Lahmass, I.; Ouahhoud, S.; Elmansuri, M.; Sabouni, A.; Elyoubi, M.; Benabbas, R.; Choukri, M.; Saalaoui, E. Determination of antioxidant properties of six by-products of Crocus sativus L. (saffron) plant products. Waste Biomass Valori. 2018, 9, 1349–1357. [Google Scholar] [CrossRef]
- Erden, K.; Özel, A. Influence of delayed harvest on yield and some quality parameters of saffron (Crocus sativus L.). J. Agric. Biol. Sci. 2016, 11, 313–316. [Google Scholar]
- Macchia, M.; Ceccarini, L.; Molfetta, I.; Cioni, P.L.; Flamini, G. Studies on saffron (Crocus sativus L.) from Tuscan Maremma (Italy): Effects of geographical origin, cultivation environment and drying method on volatile emission. J. Food Sci. Technol. 2013, 48, 2370–2375. [Google Scholar]
- Sereshti, H.; Ataolahi, S.; Aliakbarzadeh, G.; Zarre, S.; Poursorkh, Z. Evaluation of storage time effect on saffron chemical profile using gas chromatography and spectrophotometry techniques coupled with chemometrics. J. Food. Sci. Technol. 2018, 55, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Seljasen, R.; Lea, P.; Torp, T.; Riley, H.; Berentsen, E.; Thomsen, M.; Bengtsson, G.B. Effects of genotype, soil type, year and fertilisation on sensory and morphological attributes of carrots (Daucus carota L.). J. Sci. Food Agric. 2012, 92, 1786–1799. [Google Scholar] [CrossRef] [PubMed]
- Arnó, J.; Rosell, J.R.; Blanco, R.; Ramos, M.C.; Martínez-Casasnovas, J.A. Spatial variability in grape yield and quality influenced by crop and soil nutrition characteristics. Precis. Agric. 2012, 13, 393–410. [Google Scholar] [CrossRef]
- Rezaee, R.; Hosseinzadeh, H. Safranal: From an aromatic natural product to a rewarding pharmacological agent. Iran J. Basic. Med. Sci. 2013, 16, 12–26. [Google Scholar]
- Singh, S.; Tewari, G.; Pande, C.; Singh, C. Variation in essential oil composition of Ocimum americanum L. from north-western Himalayan region. J. Essent. Oil Res. 2013, 25, 278–290. [Google Scholar] [CrossRef]
- Mosleh, Z.; Salehi, M.H.; Rafieiolhossaini, M. Effect of different soil series and manure application on agro-morphological characteristics, essential oil and chamazulene content of German chamomile. J. Essent. Oil Bear. Plants 2013, 16, 730–739. [Google Scholar] [CrossRef]
- Amiri, N.; Yadegari, M.; Hamedi, B. Essential oil composition of Cirsium arvense L. produced in different climate and soil properties. Rec. Nat. Prod. 2018, 12, 251–262. [Google Scholar] [CrossRef]
- Moretti, M.D.L.; Peana, A.T.; Passino, G.S.; Solinas, V. Effects of soil properties on yield and composition of Rosmarinus officinalis essential oil. J. Essent. Oil Res. 1998, 10, 261–267. [Google Scholar] [CrossRef]
- Ormeno, E.; Fernandez, C. Effect of soil nutrient on production and diversity of volatile terpenoids from plants. Curr. Bioact. Compd. 2012, 8, 71–79. [Google Scholar] [PubMed]
- Baricevic, D.; Zupancic, A. The impact of drought stress and/or nitrogen fertilization in some medicinal plants. J. Herbs Spi. Med. Plant. 2002, 9, 53–64. [Google Scholar] [CrossRef]
- Pandey, V.; Patel, A.; Patra, D.D. Amelioration of mineral nutrition, productivity, antioxidant activity and aroma profile in marigold (Tagetes minuta L.) with organic and chemical fertilization. Ind. Crop. Prod. 2015, 76, 378–385. [Google Scholar] [CrossRef]
- Rezaian, S.; Paseban, M. The effect of micronutrients and manure fertilizers on the quantity and quality of Khorasan saffron. Acta Hortic. 2006, 739, 25–33. [Google Scholar] [CrossRef]
- Rabani-Foroutagheh, M.; Hamidoghlia, Y.; Mohajeri, S.A. Effect of split foliar fertilisation on the quality and quantity of active constituents in saffron (Crocus sativus L.). J. Sci. Food Agric. 2013, 94, 1872–1878. [Google Scholar] [CrossRef]
- Pardo, J.E.; Zalacain, A.; Carmona, M.; Lopez, E.; Alvaruiz, A.; Alonso, G.L. Influence of the type of dehydration process on the sensory properties of saffron spice. Ital. J. Food Sci. 2002, 4, 413–422. [Google Scholar]
- Alonso, G.L.; Sanchez-Fernandz, M.A.; Seaz, J.R.; Zalacain, A.; Salinas, M.R. Evaluation of the colour of spanish saffron using tristimulus colorimetry. Ital. J. Food Sci. 2003, 15, 249–258. [Google Scholar]
- Arias, R.; Lee, T.C.; Logendra, L.; Janes, H. Correlation of lycopene measured by HPLC with the L*, a*, b* color readings of a hydroponic tomato and the relationship of maturity with color and lycopene content. J. Agric. Food Chem. 2000, 48, 1697–1702. [Google Scholar] [CrossRef]
- Khalili, M.R.; Asadi, M.E.; Torkashvand, A.M.; Pazira, E. Regression Analysis for Yield Comparison of saffron as affected by physicochemical properties of the soil, case study in Northeast of Iran. Agric. Res. 2020. [Google Scholar] [CrossRef]
| Soil Type | Sand (%) | Silt (%) | Clay (%) | pH (H2O) | EC 1 (µS cm−1) | Organic Carbon 2 | Organic Matter (g kg−1) | Total Lime (g kg−1) | Active Lime (g kg−1) | USDA Classification | Bulk Density (g kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 29.44 | 30.24 | 40.32 | 7.46 | 186.0 | 7.32 | 12.63 | 13.38 | 13.75 | Clay | 1.26 |
| S2 | 33.22 | 32.19 | 34.59 | 6.41 | 135.5 | 5.54 | 9.56 | 13.39 | 17.50 | Clay–loam | 1.28 |
| S3 | 52.56 | 27.85 | 19.59 | 7.64 | 272.0 | 5.03 | 8.67 | 85.86 | 26.25 | Loam | 1.38 |
| S4 | 60.31 | 21.36 | 18.33 | 7.30 | 333.0 | 3.17 | 5.46 | 85.84 | 21.25 | Sandy–loam | 1.41 |
| S5 | 69.26 | 14.05 | 16.69 | 8.11 | 169.2 | 1.18 | 2.04 | 145.3 | 23.75 | Sandy–loam | 1.45 |
| S6 | 81.67 | 9.32 | 9.01 | 7.61 | 254.0 | 1.19 | 2.05 | 92.43 | 26.25 | Loamy–sand | 1.51 |
| S7 | 90.61 | 2.27 | 7.12 | 8.10 | 86.1 | 0.00 | 0.00 | 123.33 | 26.75 | Sandy | 1.55 |
| Treatments 1 | Stamen Length (mm) | Stamen Fresh Weight (g) | Stamen Dry Weight (g) | Tepal Fresh Weight (g) | Tepal Dry Weight (g) | By-Products Dry Weight (g) | L* | a* | b* |
|---|---|---|---|---|---|---|---|---|---|
| Soil type (S) | |||||||||
| S1 | 20.8 a | 0.0405 a | 0.0076 c | 0.316 a | 0.039 a | 0.0466 ab | 48.6 c | 26.2 a | −19.8 a |
| S2 | 20.6 a | 0.0360 b | 0.0042 d | 0.308 b | 0.037 ab | 0.0412 c | 51.9 ab | 25.4 a | −20.7 a |
| S3 | 19.5 b | 0.0348 c | 0.0086 b | 0.299 c | 0.034 b | 0.0426 c | 49.6 bc | 25.3 a | −19.3 a |
| S4 | 20.4 a | 0.0389 ab | 0.0107 a | 0.309 b | 0.037 ab | 0.0477 a | 51.6 ab | 25.8 a | −20.4 a |
| S5 | 19.3 b | 0.0311d | 0.0085 b | 0.300 c | 0.036 ab | 0.0445 b | 51.2 ab | 25.4 a | −19.6 a |
| S6 | 20.6 a | 0.0343 c | 0.0056 cd | 0.299 c | 0.033 b | 0.0386 d | 52.7 a | 25.4 a | −19.4 a |
| S7 | 18.5 c | 0.0238 e | 0.0072 c | 0.279 d | 0.029 c | 0.0362 d | 51.7 ab | 26.1 a | −20.2 a |
| Significance2 | ** | *** | *** | *** | ** | ** | ** | NS | NS |
| Years (Y) | |||||||||
| 2017–2018 | 19.0 | 0.0247 | 0.0074 | 0.299 | 0.033 | 0.0405 | 51.5 | 25.1 | −19.3 |
| 2018–2019 | 21.0 | 0.0437 | 0.0076 | 0.304 | 0.038 | 0.0445 | 50.6 | 26.2 | −20.5 |
| Significance | NS | *** | NS | NS | ** | ** | NS | NS | NS |
| Interaction S × Y | |||||||||
| Significance | NS | ** | ** | ** | * | * | NS | NS | NS |
| Treatments 1 | Days to Flower (d) | Flowering Interval (d) | Stigma Length (mm) | Stigma Fresh Weight (g) | Stigma Dry Weight (g) | Flowers (n corm−1) | Flowers (n m−2) | Stigma Yield (g m−2) |
|---|---|---|---|---|---|---|---|---|
| Soil type (S) | ||||||||
| S1 | 59.3 a | 17.2 a | 37.7 a | 0.0426 a | 0.0071 a | 1.3 a | 279.2 b | 2.0 a |
| S2 | 59.7 a | 12.5 c | 36.2 ab | 0.0396 ab | 0.0066 ab | 1.2 a | 269.8 b | 1.8 ab |
| S3 | 59.7 a | 12.5 c | 35.9 ab | 0.0378 ac | 0.0063 ac | 1.4 a | 312.5 ab | 2.0 a |
| S4 | 59.7 a | 13.7 bc | 35.6 ab | 0.0378 ac | 0.0063 ac | 1.5 a | 328.1 a | 2.0 a |
| S5 | 61.2 a | 15.7 ab | 33.4 ac | 0.0354 bc | 0.0059 bc | 1.3 a | 280.2 b | 1.7 ab |
| S6 | 61.2 a | 16.5 ab | 31.9 bc | 0.0330 c | 0.0055 c | 1.2 a | 265.6 b | 1.5 b |
| S7 | 61.5 a | 14.3 ac | 28.8 c | 0.0234 d | 0.0039 d | 0.9 b | 190.6 c | 0.8 c |
| Significance2 | NS | * | ** | ** | ** | * | ** | ** |
| Years (Y) | ||||||||
| 2017–2018 | 64.0 | 16.1 | 31.9 | 0.0378 | 0.0063 | 1.2 | 271.4 | 1.7 |
| 2018–2019 | 56.6 | 13.2 | 36.4 | 0.0336 | 0.0056 | 1.3 | 278.9 | 1.6 |
| Significance | ** | * | ** | ** | * | NS | NS | NS |
| Interaction S × Y | ||||||||
| Significance | ** | ** | ** | ** | * | NS | ** | ** |
| Treatments 1 | Qualitative Traits | Color Coordinates | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A1%1cm (440 nm)3 | ISO Reference (Crocetin Esters) 4 | A1%1cm (257 nm)5 | ISO Reference (Picrocrocin)6 | A1%1cm (330 nm)7 | ISO Reference (Safranal)8 | L* | a* | b* | |
| Soil type (S) | |||||||||
| S1 | 290.5 a | I | 107.9 a | I | 23.6 c | I | 36.5 c | 38.6 a | 36.9 c |
| S2 | 287.6 a | I | 105.4 a | I | 24.1 ac | I | 36.9 c | 36.8 c | 37.5 c |
| S3 | 289.1 a | I | 109.2 a | I | 26.6 a | I | 37.7 bc | 37.2 bc | 38.5 bc |
| S4 | 275.6 b | I | 106.4 a | I | 25.4 ab | I | 37.7 bc | 38.2 ab | 38.5 bc |
| S5 | 271.7 b | I | 105.9 a | I | 24.8 b | I | 38.1 bc | 37.3 bc | 39.8 ab |
| S6 | 250.6 c | I | 96.2 b | I | 24.7 b | I | 38.9 b | 37.8 ac | 40.2 ab |
| S7 | 247.4 c | I | 95.1 b | I | 24.9 b | I | 40.9 a | 38.9 a | 41.7 a |
| Significance2 | ** | * | * | ** | ** | ** | |||
| Years (Y) | |||||||||
| 2017–2018 | 266.3 | I | 103.6 | I | 21.2 | I | 40.2 | 38.8 | 41.0 |
| 2018–2019 | 280.1 | I | 103.9 | I | 28.6 | I | 36.5 | 36.8 | 38.0 |
| Significance | ** | NS | ** | * | NS | NS | |||
| Interaction S × Y | |||||||||
| Significance | * | * | * | * | NS | NS | |||
| Treatments 1 | Leaf Length (cm) | Leaf Fresh Weight (g) | Leaf Dry Weight (g) | Leaf (n plant−1) | Leaf Area (cm2 plant−1) |
|---|---|---|---|---|---|
| Soil type (S) | |||||
| S1 | 49.8 a | 0.557 a | 0.152 a | 27.6 a | 192.4 a |
| S2 | 43.4 b | 0.521 bc | 0.137 bc | 22.9 ac | 180.8 b |
| S3 | 43.9 b | 0.499 c | 0.131 c | 26.1 ab | 140.3 d |
| S4 | 42.6 b | 0.542 ab | 0.143 ab | 23.8 ac | 160.8 c |
| S5 | 34.4 c | 0.319 d | 0.089 d | 20.7 c | 98.6 e |
| S6 | 34.2 c | 0.318 d | 0.081 d | 20.7 c | 107.9 e |
| S7 | 26.9 d | 0.234 e | 0.066 e | 21.1 bc | 74.4 f |
| Significance2 | *** | *** | *** | * | *** |
| Years (Y) | |||||
| 2017–2018 | 42.3 | 0.434 | 0.111 | 19.9 | 145.2 |
| 2018–2019 | 36.4 | 0.421 | 0.117 | 26.7 | 127.5 |
| Significance | * | NS | NS | *** | ** |
| Interaction S × Y | |||||
| Significance | *** | *** | *** | *** | *** |
| Treatments 1 | Daughter Corm (n Mother Corm−1) | Daughter Corm Mean Weight (g) | Total Daughter Corm Weight (g Plant−1) | Daughter Corm Horizontal Diameter (cm) | Daughter Corm Yield (kg m−2) | Growth Cycle (d) |
|---|---|---|---|---|---|---|
| Soil type (S) | ||||||
| S1 | 3.2 a | 13.5 a | 35.0 ab | 2.9 ab | 7.7 ab | 244.5 b |
| S2 | 2.5 b | 10.2 ab | 24.9 cd | 2.9 ab | 5.5 cd | 244.5 b |
| S3 | 3.4 a | 14.6 a | 35.7 ab | 3.2 a | 7.8 a | 245.0 b |
| S4 | 3.4 a | 14.4 a | 36.7 a | 3.1 a | 8.0 a | 245.0 b |
| S5 | 3.5 a | 9.7 ab | 29.1 bc | 2.7 ab | 6.4 bc | 248.5 ab |
| S6 | 3.6 a | 9.5 ab | 25.7 cd | 2.6 ab | 5.6 cd | 248.5 ab |
| S7 | 3.4 a | 6.3 b | 21.4 d | 2.3 b | 4.7 d | 251.5 a |
| Significance2 | * | * | ** | * | *** | * |
| Years (Y) | ||||||
| 2017–2018 | 2.2 | 15.2 | 31.7 | 3.3 | 6.93 | 252.3 |
| 2018–2019 | 4.3 | 7.1 | 28.0 | 2.4 | 6.12 | 241.3 |
| Significance | *** | *** | NS | ** | NS | * |
| Interaction S × Y | ||||||
| Significance | ** | ** | ** | * | ** | * |
| Variables | Principal Components | |
|---|---|---|
| 1 | 2 | |
| Sand | −0.9324 | 0.3465 |
| Silt | 0.9699 | −0.1664 |
| Clay | 0.8446 | −0.4923 |
| pH | −0.8048 | 0.2333 |
| Total lime | −0.7271 | 0.5592 |
| Organic matter | 0.9207 | −0.2561 |
| Electrical conductivity | 0.4197 | 0.7920 |
| By-products weight | 0.8051 | 0.2875 |
| Leaf area | 0.9662 | −0.1156 |
| Daughter corm number | −0.4839 | 0.7433 |
| Daughter corm weight | 0.8518 | 0.4890 |
| Daughter corm diameter | 0.8870 | 0.4031 |
| Stigma yield | 0.7673 | 0.5823 |
| Daughter corm yield | 0.7456 | 0.5973 |
| L* | −0.9122 | 0.1132 |
| Color | 0.9505 | −0.0609 |
| Taste | 0.8936 | 0.2126 |
| Aroma | −0.0719 | 0.7902 |
| Eigenvalue | 11.73 | 3.88 |
| Total variance (%) | 65.20 | 21.57 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardone, L.; Castronuovo, D.; Perniola, M.; Scrano, L.; Cicco, N.; Candido, V. The Influence of Soil Physical and Chemical Properties on Saffron (Crocus sativus L.) Growth, Yield and Quality. Agronomy 2020, 10, 1154. https://doi.org/10.3390/agronomy10081154
Cardone L, Castronuovo D, Perniola M, Scrano L, Cicco N, Candido V. The Influence of Soil Physical and Chemical Properties on Saffron (Crocus sativus L.) Growth, Yield and Quality. Agronomy. 2020; 10(8):1154. https://doi.org/10.3390/agronomy10081154
Chicago/Turabian StyleCardone, Loriana, Donato Castronuovo, Michele Perniola, Laura Scrano, Nunzia Cicco, and Vincenzo Candido. 2020. "The Influence of Soil Physical and Chemical Properties on Saffron (Crocus sativus L.) Growth, Yield and Quality" Agronomy 10, no. 8: 1154. https://doi.org/10.3390/agronomy10081154
APA StyleCardone, L., Castronuovo, D., Perniola, M., Scrano, L., Cicco, N., & Candido, V. (2020). The Influence of Soil Physical and Chemical Properties on Saffron (Crocus sativus L.) Growth, Yield and Quality. Agronomy, 10(8), 1154. https://doi.org/10.3390/agronomy10081154








