Pollination Is Sufficient, Even with Low Bee Diversity, in Pumpkin and Winter Squash Fields
Abstract
1. Introduction
2. Materials and Methods
2.1. Natural Open Pollination vs. Supplemental Pollination
2.2. Pumpkin and Winter Squash Sites in Farmers’ Fields
2.3. Measurement of Pollen Deposition on Stigmas
2.4. Hourly Bee Counts on 100 Flowers
2.5. Statistical Methods
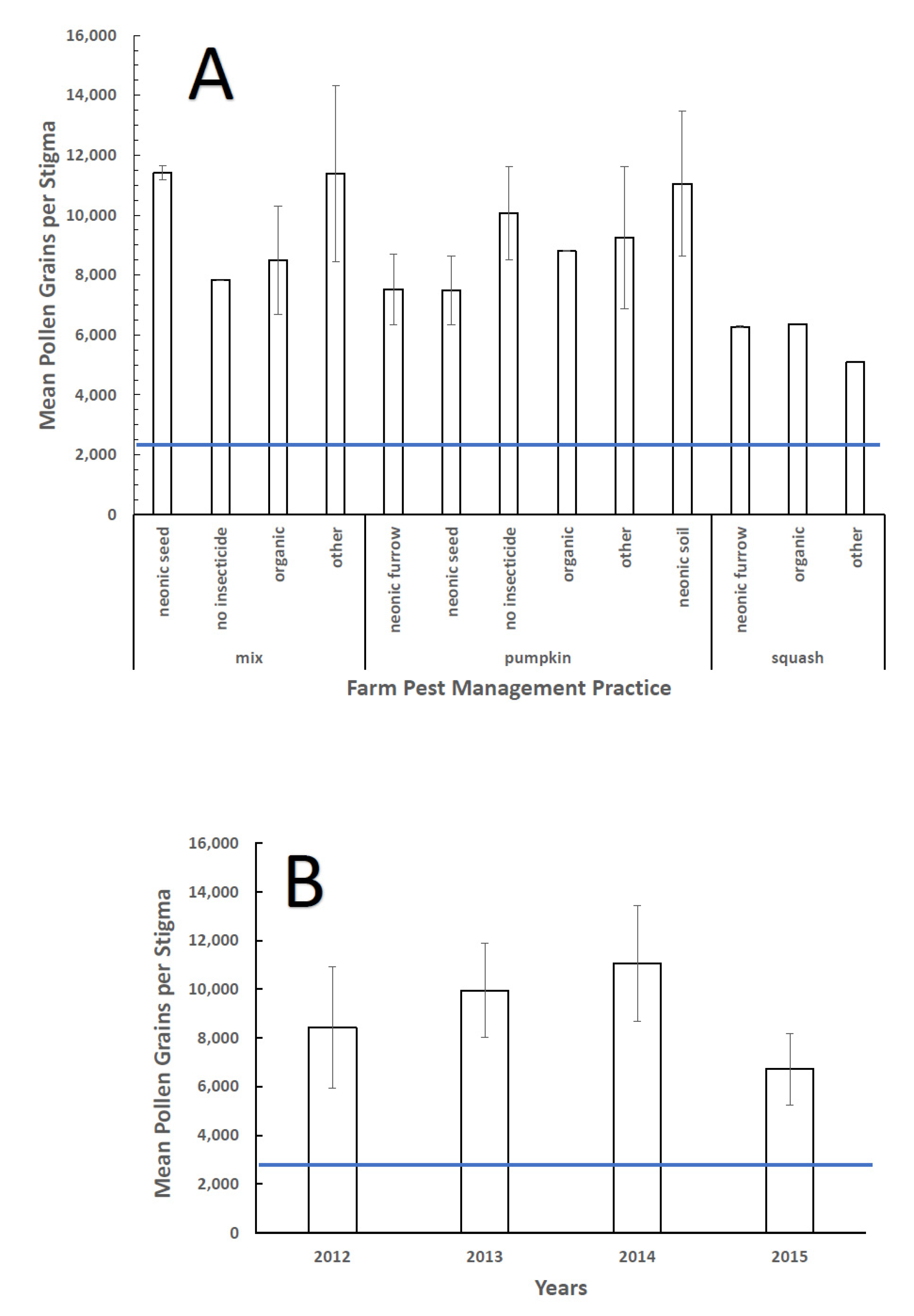
3. Results
3.1. Natural Open vs. Supplemental Pollination
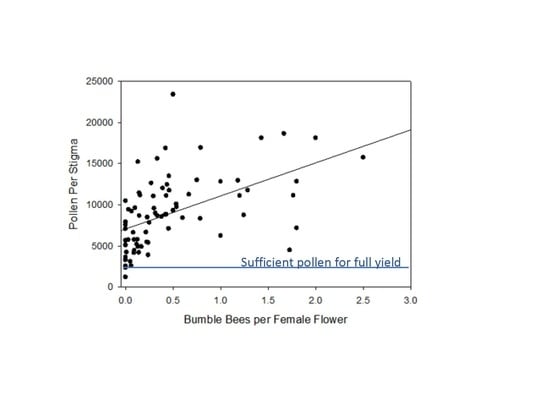
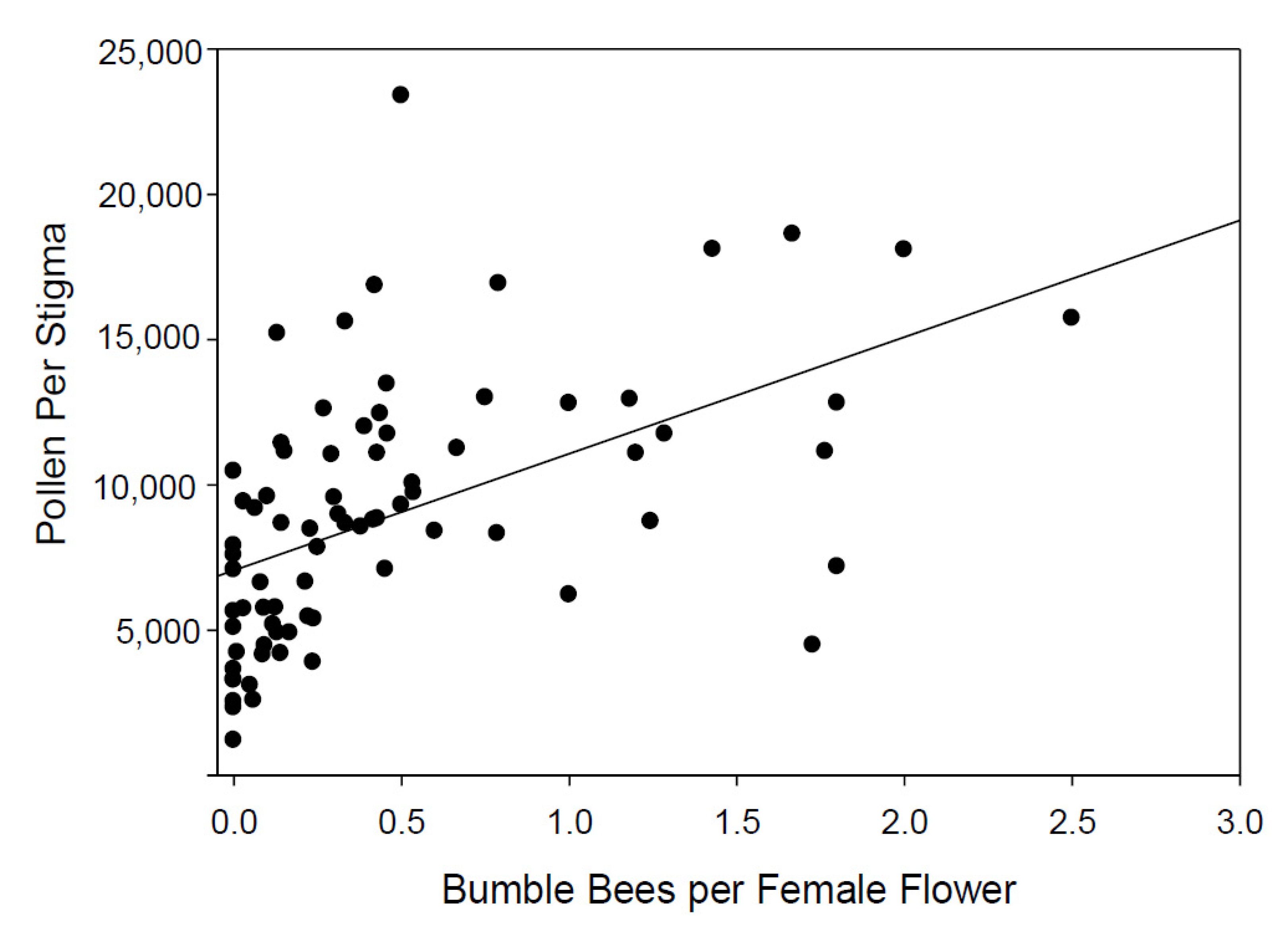
3.2. Measurement of Pollen Deposition on Stigmas of Pumpkin and Winter Squash Flowers on Fields across Connecticut and Relationship to the Hourly Counts of Bees on 100 Flowers
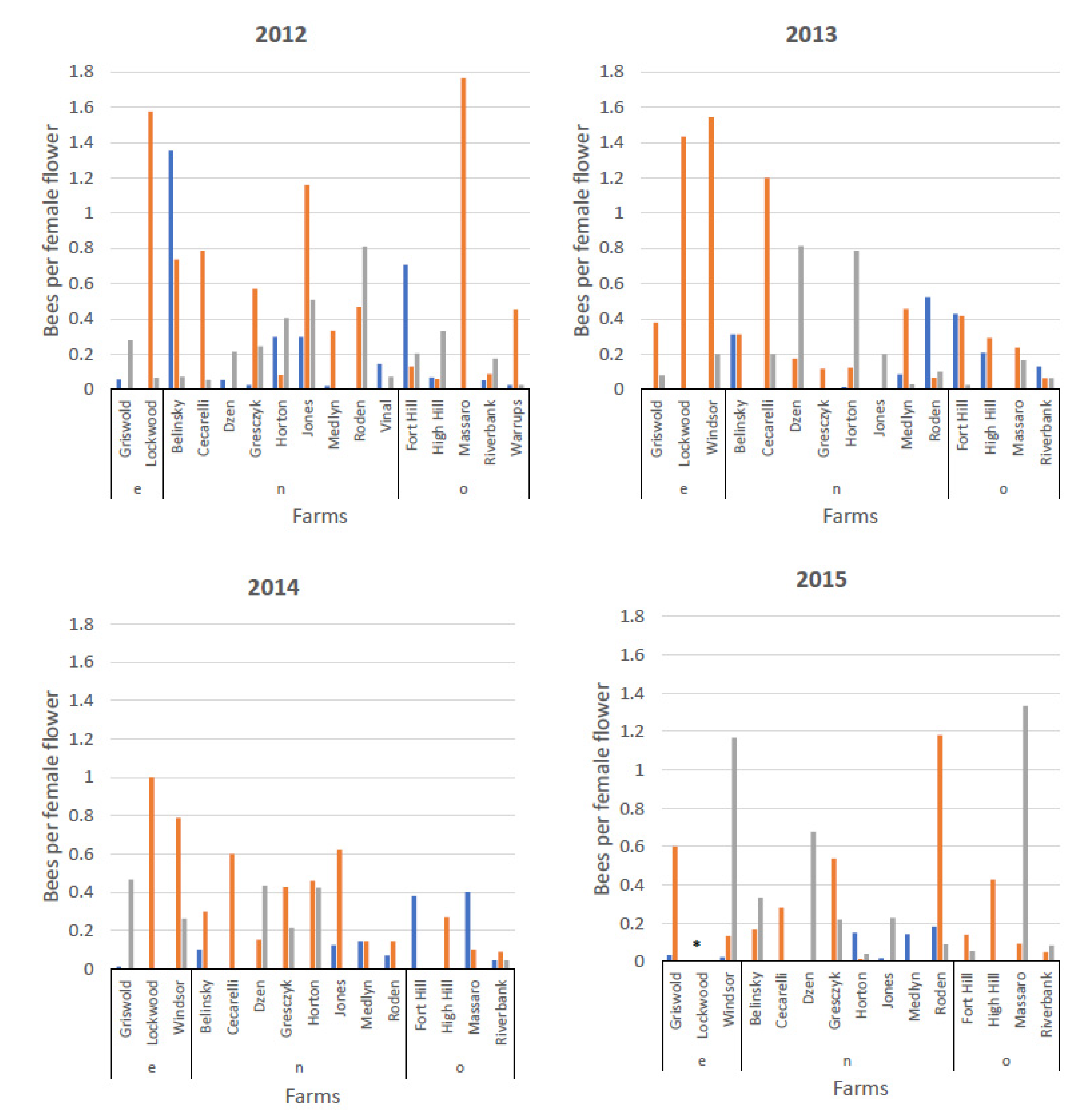
3.3. Visitation by Bee Species to Male and Female Flowers and Distribution of Bee Species on Female Flowers by Farm
4. Discussion
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Quick Stats, National Agricultural Statistics Service. Available online: https://quickstats.nass.usda.gov/ (accessed on 11 May 2020).
- Stapleton, S.C.; Wien, H.C.; Morse, R.A. Flowering and fruit set of pumpkin cultivars under field conditions. HortScience 2000, 35, 1074–1077. [Google Scholar] [CrossRef]
- Loy, J.B. Morpho-physiological aspects of productivity and quality in squash and pumpkins (Cucurbita spp.). Crit. Rev. Plant. Sci. 2004, 23, 337–363. [Google Scholar] [CrossRef]
- Shuler, R.E.; Roulston, T.H.; Farris, G.E. Farming practices influence wild pollinator populations on squash and pumpkin. J. Econ. Entomol. 2005, 98, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Julier, H.E.; Roulston, T.H. Wild bee abundance and pollination service in cultivated pumpkins: Farm management, nesting behavior and landscape effects. J. Econ. Entomol. 2009, 102, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Artz, D.R.; Hsu, C.L.; Nault, B.A. Influence of honey bee, Apis mellifera, hives and field size on foraging activity of native bee species in pumpkin fields. Environ. Entomol. 2011, 40, 1144–1158. [Google Scholar] [CrossRef] [PubMed]
- Artz, D.R.; Nault, B.A. Performance of Apis mellifera, Bombus impatiens, and Peponapis pruinosa (Hymenoptera: Apidae) as pollinators of pumpkin. J. Econ. Entomol. 2011, 104, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.D.; Reiners, S.; Nault, B.A. Pollination services provided by bees in pumpkin fields supplemented with either Apis mellifera or Bombus impatiens or not supplemented. PLoS ONE 2013, 8, e69819. [Google Scholar] [CrossRef]
- Phillips, B.W.; Gardiner, M.M. Use of video surveillance to measure the influences of habitat management and landscape composition on pollinator visitation and pollen deposition in pumpkin (Cucurbita pepo) agroecosystems. PeerJ 2015, 3, e1342. [Google Scholar] [CrossRef]
- McGrady, C.M.; Troyer, R.; Fleischer, S.J. Wild bee visitation rates exceed pollination thresholds in commercial Cucurbita agroecosystems. J. Econ. Entomol. 2019, 113, 562–574. [Google Scholar] [CrossRef]
- Winfree, R.; Reilly, J.R.; Bartomeus, I.; Cariveau, D.P.; Williams, N.M.; Gibbs, J. Species turnover promotes the importance of bee diversity for crop pollination at regional scales. Science 2018, 359, 791–793. [Google Scholar] [CrossRef]
- Cane, J.H.; Sampson, B.J.; Miller, S.A. Pollination value of male bees: The specialist bee Peponapis pruinosa (Apidae) at summer squash (Cucurbita pepo). Environ. Entomol. 2011, 40, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Brochu, K.K.; van Dyke, M.T.; Milano, N.J.; Petersen, J.D.; McArt, S.H.; Nault, B.A.; Kessler, A.; Danforth, B.N. Pollen defenses negatively impact foraging and fitness in a generalist bee (Bombus impatiens: Apidae). Sci. Rep. 2020, 10, 3112. [Google Scholar] [CrossRef] [PubMed]
- Hurd, P.; Linsley, E. The squash and gourd bees—Genera Peponapis Robertson and Xenoglossa Smith—Inhabiting America north of Mexico (Hymenoptera: Apoidea). Hilgardia 1964, 35, 375–477. [Google Scholar] [CrossRef]
- Knapp, J.L.; Osborne, J.L. Cucurbits as a model system for crop pollination management. J. Pollinat. Ecol. 2019, 25, 89–102. [Google Scholar]
- Walters, S.A.; Taylor, B.H. Effects of honey bee pollination on pumpkin fruit and seed yield. HortScience 2006, 41, 370–373. [Google Scholar] [CrossRef]
- Riggs, D.I.M.; Robinson, R.W.; Howell, J.C.; Reiners, S.; McClurg, C.A.; Rouse, R.; Wien, H.C.; Glatz, R.J.; Morse, R.A.; Zitter, T.A.; et al. Pumpkin Production Guide; Riggs, D.I.M., Ed.; Plant and Life Sciences Publishing: Ithaca, NY, USA, 2003; p. 150. [Google Scholar]
- Cady, S.W. Pollination and Flowering Patterns of Field-Grown Pumpkin (Cucurbita pepo). Master’s Thesis, Cornell University, Ithaca, NY, USA, 1995. [Google Scholar]
- Vidal, M.G.; Jong, D.; Wien, H.C.; Morse, R.A. Pollination and fruit set in pumpkin (Cucurbita pepo) by honey bees. Braz. J. Bot. 2010, 33, 106–113. [Google Scholar] [CrossRef]
- Pfister, S.C.; Eckerter, P.W.; Schirmel, J.; Cresswell, J.E.; Entling, M.H. Sensitivity of commercial pumpkin yield to potential decline among different groups of pollinating bees. R. Soc. Open Sci. 2017, 4. [Google Scholar] [CrossRef]
- Tepedino, V.J. The pollination efficiency of the squash bee (Peponapis pruinosa) and the honey bee (Apis mellifera) on summer squash (Cucurbita pepo). J. Kans. Entomol. Soc. 1981, 54, 359–377. [Google Scholar]
- Nepi, M.; Pacini, E. Pollination, pollen viability and pistil receptivity in Cucurbita pepo. Ann. Bot. 1993, 72, 527–536. [Google Scholar] [CrossRef]
- Willis, D.S. The Pollination System of Cucurbita pepo and Peponapis pruinosa in Southern Ontario. Master’s Thesis, University of Guelph, Guelph, ON, Canada, 1993. [Google Scholar]
- Chan, D.S.W.; Prosser, R.S.; Rodríguez-Gil, J.L.; Raine, N.E. Assessment of risk to hoary squash bees (Peponapis pruinosa) and other ground-nesting bees from systemic insecticides in agricultural soil. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Skidmore, A.R.; Short, C.A.; Dills, C.; Goodell, K.; Bessin, R.T. Preference of Peponapis pruinosa (Hymenoptera: Apoidea) for tilled soils regardless of soil management system. Environ. Entomol. 2019, 48, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D.; Lye, G.C.; Darvill, B. Decline and conservation of bumble bees. Annu. Rev. Entomol. 2008, 53, 191–208. [Google Scholar] [CrossRef]
- Stoner, K.A. Current pesticide risk assessment protocols do not adequately address differences between honey bees (Apis mellifera) and bumble bees (Bombus spp.). Front. Environ. Sci. 2016, 4, 79. [Google Scholar] [CrossRef]
- Gradish, A.E.; van der Steen, J.; Scott-Dupree, C.D.; Cabrera, A.R.; Cutler, G.C.; Goulson, D.; Klein, O.; Lehmann, D.M.; Luckmann, J.; O’Neill, B.; et al. Comparison of pesticide exposure in honey bees (Hymenoptera: Apidae) and bumble bees (Hymenoptera: Apidae): Implications for risk assessments. Environ. Entomol. 2019, 48, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Carvell, C.; Bourke, A.F.G.; Dreier, S.; Freeman, S.N.; Hulmes, S.; Jordan, W.C.; Redhead, J.W.; Sumner, S.; Wang, J.; Heard, M.S. Bumblebee family lineage survival is enhanced in high-quality landscapes. Nature 2017, 543, 547–549. [Google Scholar] [CrossRef]
- Kleijn, D.; Winfree, R.; Bartomeus, I.; Carvalheiro, L.G.; Henry, M.; Isaacs, R.; Klein, A.-M.; Kremen, C.; M’gonigle, L.K.; Rader, R. Delivery of crop pollination services is an insufficient argument for wild pollinator conservation. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Bartomeus, I.; Ascher, J.S.; Gibbs, J.; Danforth, B.N.; Wagner, D.L.; Hedtke, S.M.; Winfree, R. Historical changes in northeastern US bee pollinators related to shared ecological traits. Proc. Natl. Acad. Sci. USA 2013, 110, 4656–4660. [Google Scholar] [CrossRef]
- Cameron, S.A.; Lozier, J.D.; Strange, J.P.; Koch, J.B.; Cordes, N.; Solter, L.F.; Griswold, T.L. Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. USA 2011, 108, 662–667. [Google Scholar] [CrossRef]
- Jacobson, M.M.; Tucker, E.M.; Mathiasson, M.E.; Rehan, S.M. Decline of bumble bees in northeastern North America, with special focus on Bombus terricola. Biol. Conserv. 2018, 217, 437–445. [Google Scholar] [CrossRef]
| Year | Farm | Date | Open Pollen Deposition on Stigma (Mean ± sd) | No. Stigmas Open/Supplemental Pollination | % Fruit Set Open Pollination | % Fruit Set Supplemental | % Harvested Open Pollination | % Harvested Supplemental |
|---|---|---|---|---|---|---|---|---|
| 2012 | Lockwood | 7 August | 11/11 | 27 | 9 | |||
| 2012 | Lockwood | 14 August | 5/6 | 60 | 33 | |||
| 2012 | Lockwood | 21 August | 7/6 | 71 | 67 | |||
| 2013 | Griswold | 24 July | 15,604 ± 4924 | 19/19 | 42 | 47 | 42 | 37 |
| 2013 | Griswold | 1 August | 16,856 ± 4258 | 26/27 | 35 | 41 | 35 | 37 |
| 2013 | Lockwood | 6 August | 7175 ± 2080 | 14/14 | 71 | 71 | 0 | 0 |
| 2013 | Lockwood | 19 August | 15,728 ± 6413 | 30/30 | 50 | 60 | 10 | 33 |
| 2013 | Lockwood | 27 August | 6770 ± 3444 | 13/13 | 8 | 17 | 0 | 8 |
| 2013 | Windsor | 5 August | 12,814 ± 4155 | 20/20 | 75 | 55 | 15 | 5 |
| 2013 | Windsor | 14 August | Not available | 7/3 | 71 | 100 | 43 | 67 |
| 2013 | Windsor | 22 August | 11,750 ± 3755 | 10/10 | 20 | 20 | 0 | 10 |
| 2014 | Griswold | 23 July | 2326 ± 1152 | 19/20 | 95 | 100 | 84 | 95 |
| 2014 | Lockwood | 15 August | 12,795 ± 4342 | 21/20 | 100 | 95 | 90 | 75 |
| 2014 | Windsor | 25 July | 16,919 ± 2780 | 20/20 | 50 | 65 | 50 | 55 |
| Mean ± sd over trials | 11,874 ± 4945 | 56 ± 29 | 61 ± 29 | 38 ± 31 | 38 ± 30 |
| Site | Year | Pollination | No. Fruit | Mean Fruit wt. (kg) | Mean Fruit Diameter (cm) | Mean Weight of Seeds (>1.5 cm) | Mean No. Seeds (>1.5 cm) |
|---|---|---|---|---|---|---|---|
| Lockwood | 2012 | O | 11 | 6.20 ± 1.75 | 26.49 ± 3.23 | 72.94 ± 22.07 | 599 ± 73 |
| S | 8 | 6.20 ± 2.19 | 26.41 ± 4.66 | 61.42 ± 25.75 | 561 ± 114 | ||
| Griswold | 2013 | O | 16 | 8.74 ± 2.27 | 30.55 ± 2.93 | 80.98 ± 9.57 | 488 ± 74 |
| S | 19 | 9.03 ± 2.12 | 31.30 ± 3.11 | 78.78 ± 18.78 | 445 ± 104 | ||
| Lockwood | 2013 | O | 3 | 8.95 ± 0.97 | 31.59 ± 2.16 | 97.37 ± 13.87 | 570 ± 2 |
| S | 6 | 6.68 ± 0.73 | 28.49 ± 1.22 | 84.97 ± 8.35 | 553 ± 30 | ||
| Windsor | 2013 | O | 6 | 7.12 ± 1.64 | 28.40 ± 2.54 | 79.64 ± 17.51 | 580 ± 36 |
| S | 4 | 5.68 ± 2.21 | 25.23 ± 3.73 | 54.11 ± 9.51 | 543 ± 91 | ||
| Griswold | 2014 | O | 16 | 7.09 ± 1.47 | 28.84 ± 2.39 | 97.06 ± 21.32 | 555 ± 101 |
| S | 19 | 7.42 ± 1.62 | 29.27 ± 2.62 | 98.11 ± 13.56 | 564 ± 52 | ||
| Lockwood | 2014 | O | 18 | 4.41 ± 1.19 | 24.09 ± 2.69 | 76.88 ± 18.11 | 543 ± 79 |
| S | 15 | 4.62 ± 1.92 | 24.18 ± 3.68 | 70.93 ± 24.77 | 506 ± 90 | ||
| Windsor | 2014 | O | 10 | 5.12 ± 1.17 | 24.71 ± 2.69 | 86.96 ± 24.28 | 528 ± 100 |
| S | 11 | 4.60 ± 1.63 | 23.92 ± 3.55 | 72.78 ± 28.67 | 482 ± 141 | ||
| Mean over trials | O | 6.8 ± 1.7 | 27.81 ± 2.84 | 84.55 ± 9.64 | 552 ± 37 | ||
| S | 6.3 ± 1.6 | 26.98 ± 2.79 | 74.44 ± 14.65 | 522 ± 46 |
| Terms in the Model | Coefficient | Std. Error | t | p |
|---|---|---|---|---|
| Constant | 7149.935 | 737.70 | 9.692 | <0.001 |
| Bumble bees per female flower | 3937.689 | 798.43 | 4.932 | <0.001 |
| Honey bees per female flower | −1161.083 | 1277.63 | −0.909 | 0.366 |
| Squash bees per female flower | 1763.593 | 1323.03 | 1.333 | 0.187 |
| 2012 | ||||||
| Sex of Flower | Flowers Observed | BB per Flower | HB per Flower | SB per Flower | Other per Flower | Total |
| F | 1133 | 0.64 | 0.25 | 0.21 | 0.013 | 1.12 |
| M | 8466 | 0.38 | 0.09 | 0.13 | 0.008 | 0.60 |
| Total | 9604 | 0.40 | 0.11 | 0.14 | 0.008 | 0.65 |
| 2013 | ||||||
| Sex of Flower | Flowers Observed | BB per Flower | HB per Flower | SB per Flower | Other per Flower | Total |
| F | 704 | 0.50 | 0.26 | 0.09 | 0.008 | 0.86 |
| M | 10,296 | 0.23 | 0.03 | 0.06 | 0.003 | 0.322 |
| Total | 10,200 | 0.26 | 0.04 | 0.07 | 0.003 | 0.37 |
| 2014 | ||||||
| Sex of Flower | Flowers Observed | BB per Flower | HB per Flower | SB per Flower | Other per Flower | Total |
| F | 375 | 0.36 | 0.12 | 0.09 | 0.000 | 0.56 |
| M | 7325 | 0.22 | 0.03 | 0.07 | 0.001 | 0.32 |
| Total | 7700 | 0.22 | 0.04 | 0.07 | 0.001 | 0.34 |
| 2015 | ||||||
| Sex of Flower | Flowers Observed | BB per Flower | HB per Flower | SB per Flower | Other per Flower | Total |
| F | 893 | 0.25 | 0.25 | 0.04 | 0.010 | 0.55 |
| M | 8859 | 0.15 | 0.05 | 0.05 | 0.008 | 0.26 |
| Total | 9752 | 0.17 | 0.09 | 0.05 | 0.009 | 0.31 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoner, K.A. Pollination Is Sufficient, Even with Low Bee Diversity, in Pumpkin and Winter Squash Fields. Agronomy 2020, 10, 1141. https://doi.org/10.3390/agronomy10081141
Stoner KA. Pollination Is Sufficient, Even with Low Bee Diversity, in Pumpkin and Winter Squash Fields. Agronomy. 2020; 10(8):1141. https://doi.org/10.3390/agronomy10081141
Chicago/Turabian StyleStoner, Kimberly A. 2020. "Pollination Is Sufficient, Even with Low Bee Diversity, in Pumpkin and Winter Squash Fields" Agronomy 10, no. 8: 1141. https://doi.org/10.3390/agronomy10081141
APA StyleStoner, K. A. (2020). Pollination Is Sufficient, Even with Low Bee Diversity, in Pumpkin and Winter Squash Fields. Agronomy, 10(8), 1141. https://doi.org/10.3390/agronomy10081141