Occurrence of Resistance to ALS Inhibitors in European Cyperus esculentus L.: Characterisation and Implications for Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Whole-Plant Bioassay
2.3. ALS Gene Assembly and Primer Design
2.4. ALS Gene Expression Analyses and Sequencing
3. Results and Discussion
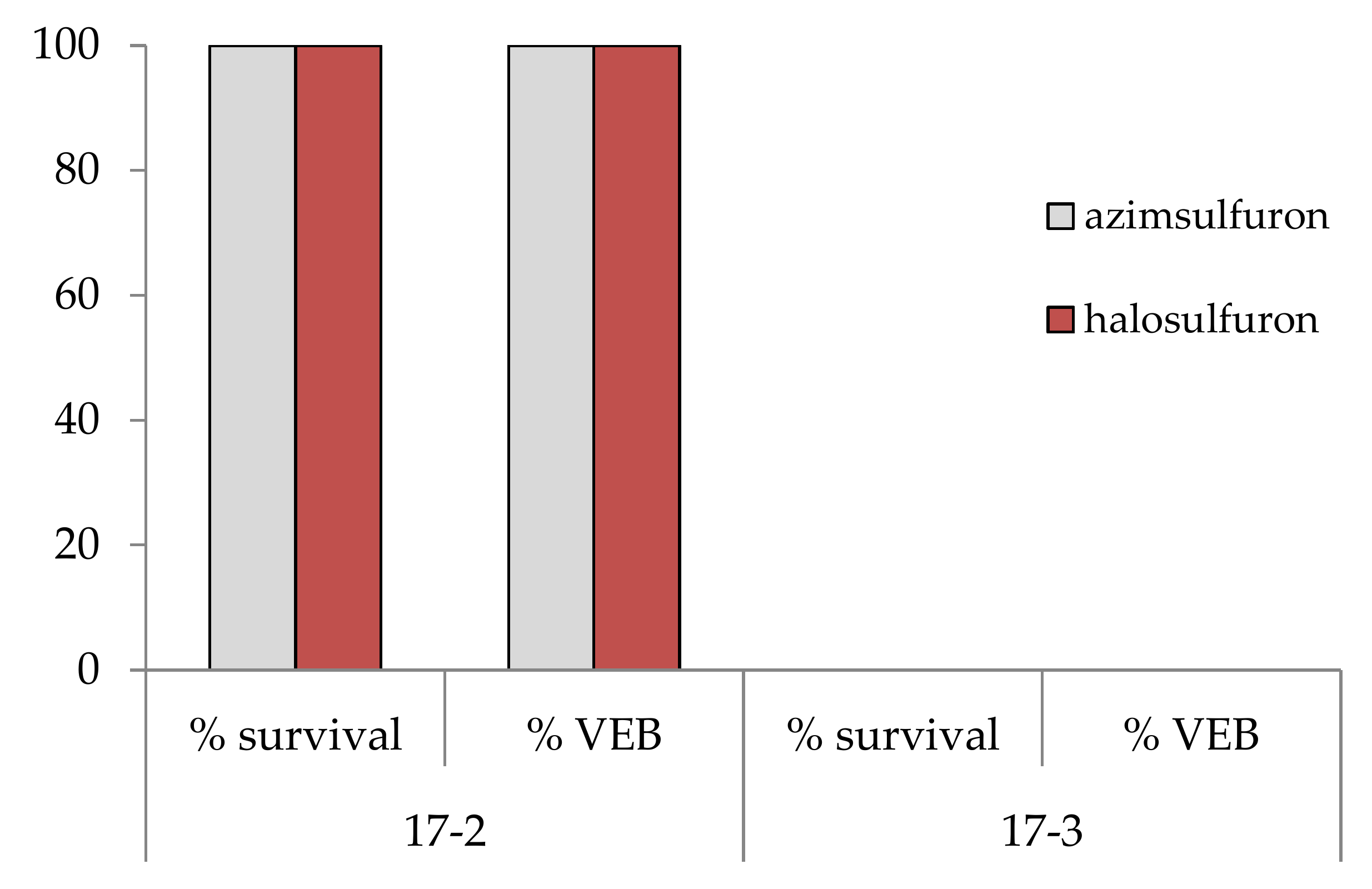
3.1. Herbicide Cross-Resistance
3.2. ALS Gene Mutation
3.3. Implications for C. esculentus Management
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EPPO 2020. EPPO Lists of Invasive Alien Plants. Available online: https://www.eppo.int/ACTIVITIES/invasive_alien_plants/iap_lists#a1 (accessed on 8 June 2020).
- Li, M.-R.; Wedin, D.A.; Tieszen, L.L. C3 and C4 photosynthesis in Cyperus (Cyperaceae) in temperate eastern north America. Can. J. Bot. 1999, 77, 209–218. [Google Scholar] [CrossRef]
- Follak, S.; Belz, R.; Bohren, C.; De Castro, O.; Del Guacchio, E.; Pascual-Seva, N.; Schwarz, M.; Verloove, F.; Essl, F. Biological flora of Central Europe: Cyperus esculentus L. Perspect. Plant. Ecol. Evol. Syst. 2016, 23, 33–51. [Google Scholar] [CrossRef]
- Gifford, E.M.; Bayer, D.E. Developmental anatomy of Cyperus esculentus (Yellow nutsedge). Int. J. Plant. Sci. 1995, 156, 622–629. [Google Scholar]
- Rodrigues, A.C.; Maranhão Estelita, M.E. Morphoanatomy of the stem in Cyperaceae. Acta Bot. Bras. 2009, 23, 889–901. [Google Scholar] [CrossRef]
- Jordan-Molero, J.E.; Stoller, E.W. Seasonal development of yellow and purple nutsedges (Cyperus esculentus and C. rotundus) in Illinois. Weed Sci. 1978, 26, 614–618. [Google Scholar] [CrossRef]
- Ransom, C.V.; Rice, C.A.; Shock, C.C. Yellow nutsedge (Cyperus esculentus) growth and reproduction in response to nitrogen and irrigation. Weed Sci. 2009, 57, 21–25. [Google Scholar] [CrossRef]
- Bohren, C.; Wirth, J. La propagation du souchet comestible (Cyperus esculentus L.) concerne tout le monde. Rech. Agron. Suisse 2015, 6, 384–391. [Google Scholar]
- Lapham, J.; Drennan, D.S.H. The fate of yellow nutsedge (Cyperus esculentus) seed and seedlings in soil. Weed Sci. 1990, 38, 125–128. [Google Scholar] [CrossRef]
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 1982. [Google Scholar]
- Pellizzari, M.; Verloove, F. The genus Cyperus in the eastern Po Plain (Italy): Historical and recent data. Webbia 2017, 72, 127–137. [Google Scholar]
- Milan, M.; Pozzi, T.; Muscara, A.; Fogliatto, S.; De Palo, F.; Vidotto, F.; Ferrero, A. Chemical control of Cyperus esculentus in corn. In Proceedings of the Giornate Fitopatologiche, Chianciano Terme, Siena, Italy, 6–9 March 2018; Volume 1, pp. 531–540. [Google Scholar]
- Bohren, C.; Wirth, J. Implementation of control strategies against yellow nutsedge (Cyperus esculentus L.) into practice. Julius-Kühn-Archiv 2018, 458, 188–196. [Google Scholar]
- Mascanzoni, E.; Perego, A.; Marchi, N.; Scarabel, L.; Panozzo, S.; Ferrero, A.; Acutis, M.; Sattin, M. Epidemiology and agronomic predictors of herbicide resistance in rice at a large scale. Agron. Sust. Dev. 2018, 38, 68. [Google Scholar] [CrossRef]
- Armel, G.R.; Wilson, H.P.; Richardson, R.J.; Whaley, C.M.; Hines, T.E. Mesotrione combinations with atrazine and betazon for yellow and purple nutsedge (Cyperus esculentus and C. rotundus) control in corn. Weed Technol. 2008, 22, 391–396. [Google Scholar] [CrossRef]
- Nelson, K.A.; Renner, K.A. Yellow nutsedge (Cyperus esculentus) control and tuber production with glyphosate and ALS-inhibiting herbicides. Weed Technol. 2002, 16, 512–519. [Google Scholar] [CrossRef]
- GIRE. Italian Herbicide Resistance Working Group. Database of Herbicide Resistance in Italy. Available online: www.resistenzaerbicidi.it (accessed on 6 May 2020).
- Panozzo, S.; Scarabel, L.; Tranel, P.J.; Sattin, M. Target-site resistance to ALS inhibitors in the polyploid species Echinochloa crus-galli. Pestic. Biochem. Physiol. 2013, 105, 93–101. [Google Scholar] [CrossRef]
- Scarabel, L.; Cenghialta, C.; Manuello, D.; Sattin, M. Monitoring and management of imidazolinone-resistant red rice (Oryza sativa L.,var. sylvatica) in Clearfield® Italian paddy rice. Agronomy 2012, 2, 371–383. [Google Scholar] [CrossRef]
- Tranel, P.J.; Wright, T.R. Resistance of weeds to ALS-inhibiting herbicides: What have we learned? Weed Sci. 2002, 50, 700–712. [Google Scholar] [CrossRef]
- Délye, C.; Jasieniuk, M.; Le Corre, V. Deciphering the evolution of herbicide resistance in weeds. Trends Genet. 2013, 29, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: www.weedscience.org (accessed on 6 May 2020).
- Qin, Y.; Powles, S.B. Resistance to AHAS inhibitor herbicides: Current understanding. Pest. Manag. Sci. 2014, 70, 1340–1350. [Google Scholar]
- Murphy, B.P.; Tranel, P.J. Target-site mutations conferring herbicide resistance. Plants 2019, 8, 382. [Google Scholar] [CrossRef]
- Tehranchian, P.; Norsworthy, J.K.; Nandula, V.; McElroy, S.; Chen, S.; Scott, R.C. First report of resistance to acetolactate-synthase-inhibiting herbicides in yellow nutsedge (Cyperus esculentus): Confirmation and characterization. Pest. Manag. Sci. 2015, 71, 1274–1280. [Google Scholar] [CrossRef]
- Günnigmann, A.; Becker, D. Permit—A new herbicide for control of Cyperus esculentus in maize. Julius-Kühn-Archiv 2016, 452, 347–354. [Google Scholar]
- Scarabel, L.; Locascio, A.; Furini, A.; Sattin, M.; Varotto, S. Characterisation of ALS genes in the polyploid species Schoenoplectus mucronatus and implications for resistance management. Pest. Manag. Sci. 2010, 66, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Keeley, P.E.; Carter, C.H.; Thullen, R.J. Influence of glyphosate on resprouting of parent tubers of Cyperus esculentus. Weed Sci. 1985, 34, 25–29. [Google Scholar] [CrossRef]
- Grichar, W.J.; Colburn, A.E.; Baumann, P.A. Yellow nutsedge (Cyperus esculentus) control in peanut (Arachis hipogaea) as influenced by method of metolachlor application. Weed Technol. 1996, 10, 278–281. [Google Scholar] [CrossRef]
- Keller, M.; Total, R.; Krauss, J.; Neuweiler, R. Validation of yellow nutsedge (Cyperus esculentus) control strategies in maize in an on-farm, large-scale field trial. Julius-Kühn-Archiv 2018, 458, 197–203. [Google Scholar]
- Pereira, W.; Crabtree, G.; William, R.D. Herbicide action on purple and yellow nutsedge (Cyperus rotundus and C. esculentus). Weed Technol. 1987, 1, 92–98. [Google Scholar] [CrossRef]
- Ghafar, Z.; Watson, A.K. Effect of corn (Zea mays) population on the growth of yellow nutsedge (Cyperus esculentus). Weed Sci. 1983, 5, 588–592. [Google Scholar] [CrossRef]
- Lotz, L.A.P.; Groeneveld, R.M.W.; Habekotté, B.; Van Oene, H. Reduction of growth and reproduction of Cyperus esculentus by specific crops. Weed Res. 1991, 31, 153–160. [Google Scholar] [CrossRef]
- Johnson, W.C., III; Davis, R.F.; Mullinix, B.G., Jr. An integrated system of summer solarization and fallow tillage for Cyperus esculentus and nematode management in the southeastern coastal plain. Crop. Prot. 2007, 26, 1660–1666. [Google Scholar] [CrossRef]
- Stoller, E.W.; Wax, L.M. Yellow nutsedge shoot emergence and tuber longevity. Weed Sci. 1973, 21, 76–81. [Google Scholar] [CrossRef]
- Horowitz, M.; Roger, Y.; Herlinguer, G. Solarization for weed control. Weed Sci. 1983, 31, 170–179. [Google Scholar] [CrossRef]

| NCBI Accession Number. | Weed Species | Sequence Length (bp) | Position Referred to Consensus Sequence (from 1 to 1730 bp) | % Similarity With Consensus Sequence | Identical Sites (%) |
|---|---|---|---|---|---|
| KM624613.1 | Cyperus esculentus | 716 | from 958 to 1673 | 94.4% | 640 bp (94%) |
| EF061294.2 | Cyperus difformis | 1709 | from 22 to 1730 | 94.7% | 1606 bp (94%) |
| KM235318.1 | Cyperus difformis | 1706 | from 1 to 1706 | 94.7% | 1603 bp (94%) |
| KT150718.1 | Cyperus compressus | 668 | from 995 to 1662 | 94.4% | 595 bp (89.1%) |
| KT150720.1 | Cyperus compressus | 668 | from 995 to 1662 | 94.4% | 595 bp (89.1%) |
| Primer | Sequence 5′ –> 3′ | Position (bp) in Consensus Sequence (From-To) |
|---|---|---|
| 1_Fw | CAGCACCAAATGTGGGC | 1180–1196 |
| 1_Rev | TCATTGGCAAGACGTGCTC | 1654–1675 |
| 2_Fw | CCTCGTCAGTGGCTATCCAG | 1222–1241 |
| 2_Rev | TGCGGTACGATCACATCC | 1632–1649 |
| 3_Fw | GCCACTTCAGGCCGTC | 445–460 |
| 3_Rev | GAGCGACTAGCAAATGCCTC | 868–887 |
| 4_Fw | CCTCGCATCATCAAAGAGGC | 415–434 |
| 4_Rev | CAGTATTTGGTTCATGCCTTGC | 978–992 |
| 5_Fw | GATGTTCTCGTTGAGGTTCTCGAGAG | 22–47 |
| 6_Fw | ATGTCTTCGCCTATCCAGGCGGAGC | 62–86 |
| 5_Rev | GAC GGC CTG AAG TGG C | 445–460 |
| HRAC Group | Active Ingredient | Year of Registration | Application | Crop | References |
|---|---|---|---|---|---|
| G | glyphosate | 1977 | False seed bed, Pre-sowing, post-harvesting | R, M, S | [16] |
| C3 | bentazon | 1973 | post-emergence | R, M, S | [29,31] |
| K3 | S-metolachlor | 2001 | pre-emergence | M, S | [12,29,31] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarabel, L.; Farinati, S.; Sattin, M. Occurrence of Resistance to ALS Inhibitors in European Cyperus esculentus L.: Characterisation and Implications for Management. Agronomy 2020, 10, 1133. https://doi.org/10.3390/agronomy10081133
Scarabel L, Farinati S, Sattin M. Occurrence of Resistance to ALS Inhibitors in European Cyperus esculentus L.: Characterisation and Implications for Management. Agronomy. 2020; 10(8):1133. https://doi.org/10.3390/agronomy10081133
Chicago/Turabian StyleScarabel, Laura, Silvia Farinati, and Maurizio Sattin. 2020. "Occurrence of Resistance to ALS Inhibitors in European Cyperus esculentus L.: Characterisation and Implications for Management" Agronomy 10, no. 8: 1133. https://doi.org/10.3390/agronomy10081133
APA StyleScarabel, L., Farinati, S., & Sattin, M. (2020). Occurrence of Resistance to ALS Inhibitors in European Cyperus esculentus L.: Characterisation and Implications for Management. Agronomy, 10(8), 1133. https://doi.org/10.3390/agronomy10081133





